Interleukin-18 Suppresses Angiogenesis and Lymphangiogenesis in Implanted Lewis Lung Cancer
2010-01-08ShengYangXiangqiChenHuishanLuZhiyingLiTingyanLin
Sheng Yang,Xiang-qi Chen,Hui-shan Lu,Zhi-ying Li,Ting-yan Lin*
1Department of Oncology,2Department of Respiratory,the Union Hospital,Fu Jian Medical University,Fuzhou 350001,China;
INTRODUCTION
According to WHO data and statistics,lung cancer is the leading cause of cancer deaths worldwide[1].Metastatic tumors are very common in the late stages of lung cancer[2].The spread of metastases may occur via blood or lymphatics or through both routes.Basic and clinical studies indicate that suppression of angiogenesis and lymphangiogenesis can inhibit tumor progression and metastasis[3].Interleukin 18 (IL-18)is a product of activated macrophages or Kupffer cells,which was cloned in 1995.It can induce interferon-γ (IFN-γ)production in T cells,B cells and natural killer cells.It can up-regulate natural killer cell activity as well as the cytotoxicity of cytotoxic T-lymphocytes (CTLs),and plays an important role in the induction of Th1 response.IL-18 is a potential player in immunological regulation,antitumor effects and anti-inffection[4,5].Our study investigated the effect of IL-18 on angiogenesis and lymphangiogenesis in implanted Lewis lung cancer.
MATERIALS AND METHODS
Lewis lung cancer cell lines were a generous gift from College of Pharmacy,Fujian Medical University.Male C57BL/6 mice of 6 weeks of age were purchased from the Shanghai SLAC Laboratory Animal Co.,China.IL-18 was obtained from Sigma(USA).VEGF-A,VEGF-C,VEGFR-3 antibody and SABC immunohistochemistry assay kit were purchased from Wuhan Boster Biological Technology,Ltd.(China).VIII-RAg antibody and Max VisionTMimmunohistochemistry assay kit were purchased from Fuzhou Maxim Biological Technology,Ltd.(China).
Establishment of Lewis Lung Cancer Model[6,7]
Sixteen syngeneic C57BL/6 mice were fed in specific pathogen-free (SPF) enviroment and implanted with 0.1ml single cell suspension of Lewis lung cancer cells (2×107cells/ml)subcutaneously in the right axillary’s region.One week after the tumor inoculation,the tumor-bearing mice were randomly divided into IL-18-treated group (group A)and control group (group B)(eight mice per group).The mice in the IL-18-treated group were intraperitoneal injected with IL-18 (1 μg/100 μl),while the mice in the control group were injected with normal saline(100 μl),once daily on days 7-13.The health conditions and mental status of mice were observed.
Calculation of Tumor Volume and Inhibition Rate
The sizes of subcutaneous nodules were measured on days 7,9,11,13,15,17 and 19.The tumor volume (V)was calculated according to the formula: V=0.52AB2(A is the largest diameter and B is the smallest diameter).Nineteen days after gene therapy,the mice were anesthetized with 10% chloral hydrate anesthesia (3 ml/kg,intraperitoneal,i.p.)and killed,and then the tumors were collected.The tumor inhibition rate was calculated as (Mean tumor weight of control group-Mean tumor weight of IL-18-treated group)/Mean tumor weight of control group × 100%.The tumor tissues were then fixed in 10% buffered formalin and embedded in paraffin wax.The paraffinembedded tissues were cut into 5 μm thick slides for analysis.
Immunohistochemistry (IHC)
The tumor tissues were analyzed using MaxVisonTMor SABC methods.VEGF-A,VEGF-C,MVD and LMVD levels were observed under light microscope.VEGF-A and VEGF-C staining were scored according to Fromowitz’s method[8].(1)0,no stain in cytoplasm; 1,light brown stain in cytoplasm;3,brown stain in cytoplasm; 4,dark brown stain or brownish black particles in cytoplasm had a.(2)0,positive cells <5%; 1,6%-25% positive cells; 2,26%-50% positive cells; 3,51%-75% positive cells; 4,>75% positive cells.(1)plus (2)was the final scores.A core of 0-1 was considered as no expression,2-3 was weakly positivity,4-5 was mild positivity,and 6-7 was intense positivity.A total of 5 fields from each lung sample were screened randomly(×400).The mean value was accepted as the representative of the sample.MVD was calculated according to Weidner’s method[9].Areas of highest neovascularization were found by scanning the tumor sections at low power.After the area of highest neovasculari- zation was identified,individual microvessel counts were made at high power.Any highlighted endothelial cell or endothelial cell cluster clearly separated from adjacent microvessels,tumor cells,and other connective tissue elements was considered a single,countable microvessel.Even those distinct clusters of brown-staining endothelial cells,which might be from the same microvessel snaking its way in and out of the section,were considered distinct and countable as separate microvessels.Vessel lumens,although usually present,were not necessary for a structure to be defined as microvessels,and red cells were not used to define a vessel lumen.Results were expressed as an average of 5 fields MVD.Beasley et al.[10,11]described LMVD counting method.Areas of highest neovascularization were found by scanning the tumor sections at low power(×100).After the area of highest lymphatic microvessel was identified,lymphatic microvessels were identified at high power (×400).A total of 5 fields from each lung sample were screened randomly; the mean value was accepted as the representative of the sample.Macrophages and phogocytes reacting to antibody were excluded according to morphology.
HE Staining to Determine Lung Metastasis[12]
On the d19,the mice were killed and the lungs and primary tumors were removed and weighed.The number of metastasized pulmonary colonies was counted and lung tissues were fixed in formalin for further analysis.The inhibitory rate of lung metastasis(%)was calculated using the equation [1- (NIL-18-treatedgroup/Ncontrolgroup)]×100%,where N is the number of metastasized pulmonary colonies.Transfer rate was calculated using the equation (Number of metastatic animals / Total animals)×100%.
Statistical Analysis
Statistical analyses were performed with SAS software.Quantitative data which followed normal distribution were presented as¯±s,otherwise were presented as median (Q25-Q75).Qualitative data were expressed as case number or percentage.Mean tumor volume comparisons between groups were done using repeated-measures ANOVA.Mean tumor volume were examined using the Pearson χ2test.The correlation between mean tumor weigh,LMVD and MVD was examined using Student's t test.VEGF-A,VEGF-C and the number of metastasized pulmonary colonies were compared using the Wilcoxon signed-ranks test.Lung transfer rate was calculated by Fisher’s exact test.P<0.05 was considered statistically significant.
RESULTS
Effect of IL-18 on Tumor Growth in Mice
One week after hypodermic inoculation of Lewis cells,tumor formation was found in all mice.The sizes of subcutaneous nodules were all larger than 0.5 cm,the tumor formation rate was 100%.The growth speed in IL-18 treated group(group A)was obviously slower than that of control group(group B)(Figure 1).The mean tumor volumes after 19 days between the two groups had statistical significance (F=11.902,P<0.01).The mean tumor weights between the two groups had statistical significance (t=4.195,P<0.01),the tumor inhibition rate was 75% (Table 1).
During the experiment,all mice were in good health condition and did not have hemorrhage or diarrhea.The treatment group mice had statistically significant increase in weight (t=3.44,P<0.05).
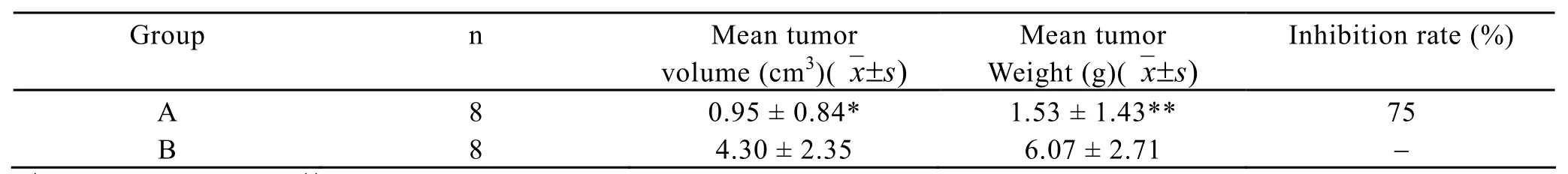
Table 1.Comparison of mean tumor volume,tumor weight,and inhibition rate between the two groups
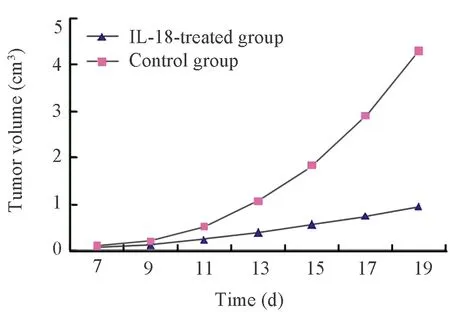
Figure 1.Tumor growth curve
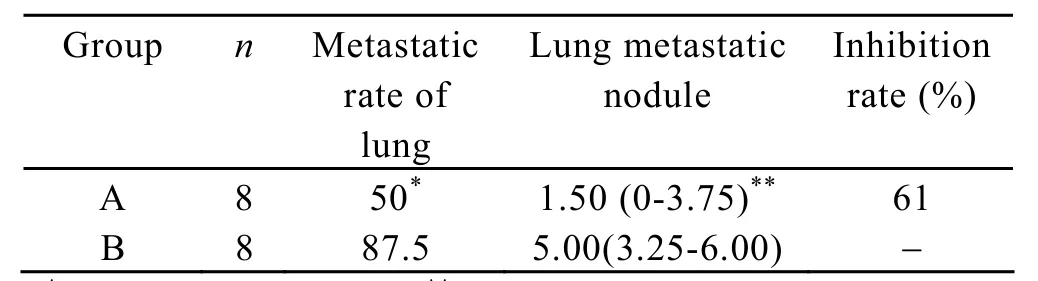
Table 2.Effect of IL-18 on lung colonization of Lewis lung carcinoma cells.Medain (Q25-Q75)
Effects of IL-18 on Metastasis of Lewis Lung Carcinoma in Mice
The number of pulmonary metastatic colonies in group A was significantly lower than that of group B,there was statistical difference (P<0.05; Table 2).The inhibitory rate of colony formation was approximately 61%.The transfer rate in group A was lower than that of group B,there was no statistical difference (P>0.05;Table 2).
Effects of IL-18 on VEGF-A,VEGF-C,MVD and LMVD in Tumor Tissues
VEGF-A and VEGF-C expressions in group A were significantly lower than that of group B (Figure 2-5),there was statistical difference (Z=2.23,Z=2.37,P<0.05; Table 3).MVD in group A and B were 16.00± 4.00,25.00±4.78 (Figure 6,7),there was statistical difference (t=4.08,P<0.01; Table 3).LMVD in group A was lower than that of group B (Figure 8,9),there is no statistical difference (t=2.40,P>0.05; Table 3).

Figure 2.VEGF expression of tumor tissues in group A(400×)
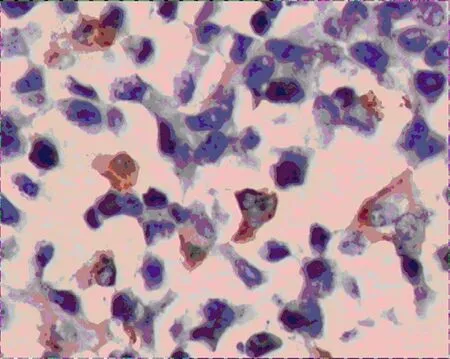
Figure 3.VEGF expression of tumor tissues in group B(400×)

Figure 4.VEGF-C expression of tumor tissues in group A (400×)
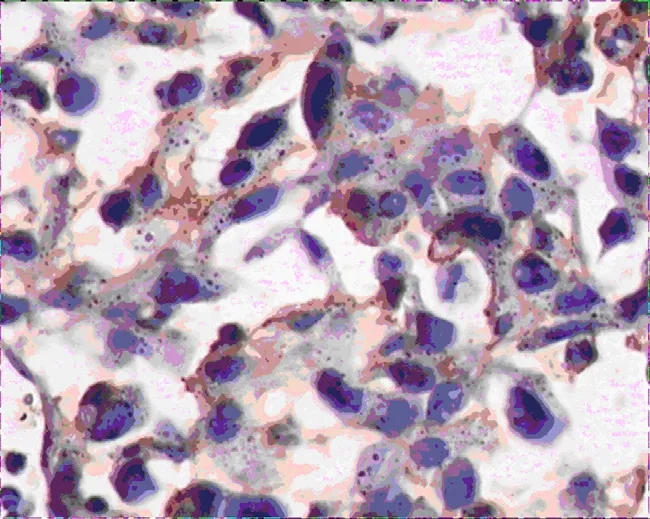
Figure 5.VEGF-C expression of tumor tissues in group B(400×)
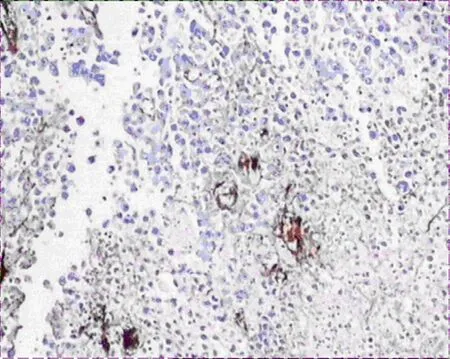
Figure 6.MVD expression of tumor tissues in group A(400×)
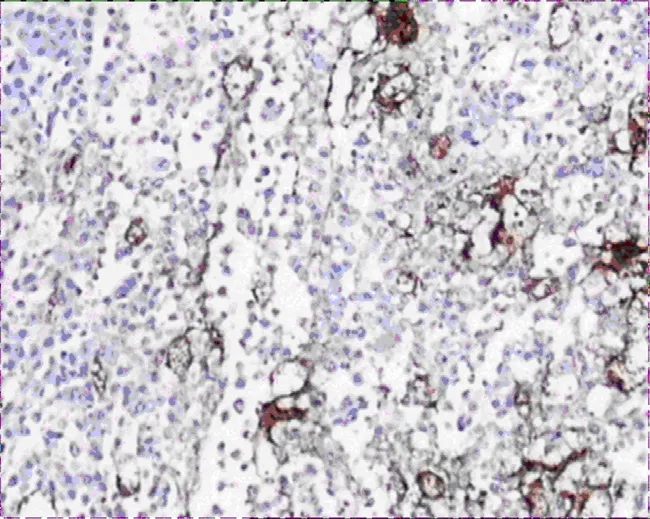
Figure 7.MVD expression of tumor tissues in group B(400×)
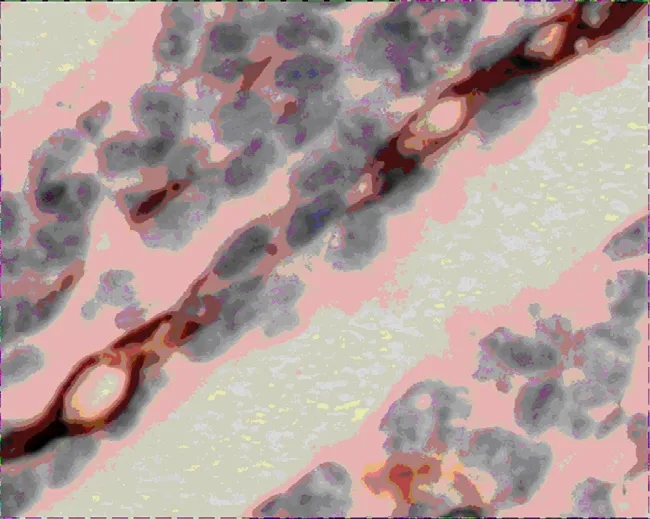
Figure 8.LMVD expression of tumor tissues in group A(400×)
Figure 9.LMVD expression of tumor tissues in group B(400×)
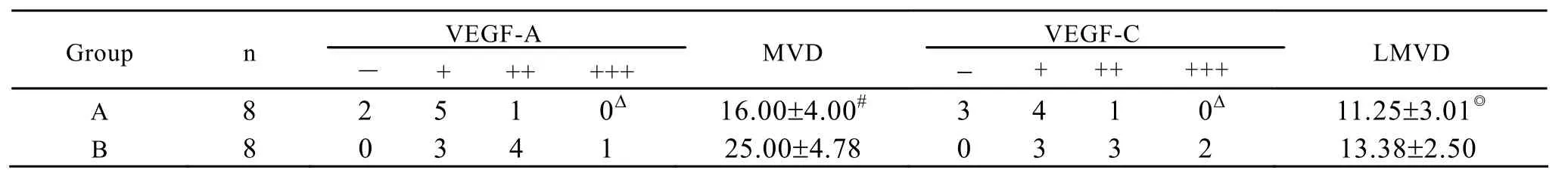
Table 3.Effect of IL-18 on VEGF-A,VEGF-C expression and MVD,LMVD in mice tumor tissues
DISCUSSION
The occurrence,development and metastasis of solid tumors are often accompanied by the increase of new vessels and lymphatic vessels,while tumor metastasis is closely linked with the blood vessels and lymphatic vessels in tumor[13,14].The metastasis of malignant tumors is mainly through blood and lymphatic channel,and vascular system and lymphatic system form a mutual transportation network structure.Tumor cells can pass freely between the two pipeline systems.Block of one channel is not enough to control the distant metastasis of tumor cells,thus blocking both the blood and lymph node metastasis is the most effective strategy for tumor metastasis[15].
Many studies confirm that tumor growth is closely related to tumor invasion.Tumor angiogenesis is the prerequisite for tumor growth and hematogeneous metastasis.Angiogenesis is regulated by angiogenic factors.VEGF-A,also known as vascular perme- ability factor(VPF),plays a fundamental role in physiological and pathophysiological forms of angiogenesis and regulation of endothelial cell differentiation[16,17].VEGF-A can enhance vascular permeability and plays an important role in nutrition transportation of tumor before angiogenesis.A multivariate analysis demonstrated that MVD is associated with a poor prognosis in tumor patients.So MVD level represents tumor angiogenesis condition and drug sensitivity,and is regarded as an important index in predicting tumor metastasis,recurrence and prognosis[18,19].Our study revealed that IL-18 could suppress angiogenesis through decreasing VEGF-A and MVD expression of Lewis lung carcinoma in mice.Cao et al[20]demonstrated that Systemic and intralesional administrations of IL-18 produced a significant suppression of the growth of murine T241 fibrosarcoma in syngeneic C57Bl6/J and immunodeficient SCID mice.The antitumor effect appeared to be potent because an average of >75% inhibition of primary tumor growth was observed.In cell culture,murine T241 fibrosarcoma cells are insensitive to recombinant IL-18 at concentrations sufficient to significantly inhibit endothelial cell proliferation.Immunohistochemical studies of tumor tissues revealed hypovascularization of the IL-18- treated tumors.These results suggest that IL-18 may participate in the regulation of tumor angiogenesis.The same results also occurred in breast cancer,implanted liver carcinoma and melanoma[21,22].
Many studies have shown that the occurrence and development of tumor lymphangiogenesis is the important factor to affect tumor growth and invasion and metastasis.On the one hand,it can provide nutrition for tumor growth and maintain tumor microenvironment and pressure balance,on the other hand,the proliferation of tumor cells also provides more opportunities for the contact between the lymphatic endothelium and lymphatic invasion[23].Three experimental researches[24-26]have directly proved that tumors cells could express regulating factor and induce the formation of lymphatics within tumors and lead to the spread of the tumor cells to lymph nodes.Therefore,any factors on the incidence of lymphatic vessels and development may influence tumor growth and lymphatic metastasis.The VEGF family of ligands and receptors is intimately involved in tumor angiogenesis,lymphangiogenesis and metastasis.VEGFR-3 is a member of VEGF family,which can regulate the growth and maintenance of the lymphatic vessels.In vitro,VEGF-C can bind to VEGF receptor-3.VEGF-C/VEGFR-3 coexpression suggests an autocrine/paracrine loop responsible for a high proliferation rate in tumor cells through MEK/ERK and PI3/kinase/Akt pathway.As VEGF-C/VEGFR-3 coexpression is very frequent in metastatic lymph nodes tumor cells,VEGF-C is regarded as the most important and specific regulating factor[27].Our studies revealed that VEGF-C was highly expressed in control group and obviously decreased in IL-18-treated group,with significant difference.LMVD in IL-18-treated group was lower than that of control group,though with no significant difference,indicating a certain suppression of tumor lymphangiogenesis.The mechanism of the anti-lymphangiogenesis effect of IL-18 may involve the suppression of VEGF-C expression in tumor cells,suppressing NF-kappa B nuclear translocation or inducing lymphatic endothelium apoptosis[28].
In our experiment,the tumor volume and weight in IL-18-treated group was lower than control group,with significant difference(P<0.01).The tumor inhibition rate was 75%.The inhibitory rate of colony formation was approximately 61%.The transfer rate in group A was lower than that of group B,with no statistical difference(P>0.05).In conclusion,IL-18 can suppress tumor growth and metastasis of Lewis lung tumor through down-regulating VEGF-A and VEGF-C expression.
Lung tumor blood vessel and lymphatic tissue formations are independent but coordinated processes,which are affected by many factors.Comprehensive study of the expressions of a variety of factors in tumor tissues,exploring the interaction between the various factor,and the different roles of the various factors at different stages of tumor progression have great value on progress,prognosis and treatment of lung cancer[29].At present,using antiangiogenesis combined with anti-lymphangiogenesis in the treatment of lung cancer is still in the early stage of animal experiments and has a long way to go to achieve clinical use.Nevertheless we believe that therapeutic approach will bring a new leap forward in comprehensive therapy of lung cancer[30,31].
[1]Alberg AJ,Nonemaker J.Who is at high risk for lung cancer? Population-level and individual-level perspectives[J].Semin respire Crit Care Med 2008;29: 223-32.
[2]Goeckenjan G.Lung cancer-historical development,current status,future prospects[J].Pneumologie 2010;64: 555-9.
[3]Zhang D,Li b,shi J,et al.Suppression of tumor growth and metastasis by simultaneously blocking vascular endotheial growth factor (VEGF)-A and VEGF-C with a receptor-immunoglobulin fusion protein[J].Cancer Res 2010; 70: 2495-503.
[4]Kinoshita M,Kuranaga N,Matsumoto A,et al.Multiple interleukin-18 injections promote both mouse Th1 and Th2 responses after sublethal Escherichia coli infection[J].Clin Exp Immunol 2006;143: 41-9.
[5]Bachmann M,Dragoi C,Poleganov MA,et al.Interleukin-18 directly activates T-bet expression and function via p38 mitogen-activated protein kinase and nuclear factor-kappaB in acute myeloid leukemiaderived predendritic KG-1 cells[J].Mol Cancer Ther 2007; 6: 723-31.
[6]Wigginton JM,Lee JK,Wiltrout TA,et al.Synergistic Engagement of an Ineffective Endogenous Anti Tumor Immune Response and Induction of IFN γ and Fas Ligand Dependent Tumor Eradication by Combined Administration of IL-18 and IL-2[J].J Immunol 2002; 169: 4467-74.
[7]Zhang YH,Wang C,Pang BS,et al.The effect of VEGF antisense oligonuleotides combined with low molecular weight heparin on the growth and metastasis of mice Lewis lung cancer[J].Zhonghua Yi Xue Za Zhi 2006; 86: 749-52.
[8]Fromowitz FB,Viola MV,Chao S,et al.Ras p21 expression in the progression of breast cancer[J].Human Patho 1987; 18:1268-74.
[9]Weidner N.Intratumor microvessel density as a prognostic factor in cancer[J].Am J Pathol 1995; 147:9-19.
[10]Beasley NJ,Prevo R,Banerji S,et al.Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer[J].Cancer Res 2002;625:1315-20.
[11]Van Trappen PO,Steele D,Lowe DG,et al.Expression of vascular endotheial growth factor(VEGF)-C and VEGF-D,and their receptor VBEGFR-3,during different stages of cervical carcinogenesis[J].J Pathol 2003; 201: 544-54.
[12]Pan Y,Song QL,Lin YH,et al.GLB prevents tumor metastasis of Lewis lung carcinoma by inhibiting tumor adhesion actions[J].Acta Pharmacol Sin 2005;26:881-6.
[13]Takanami I,Abiko T,Koizumi S.Expression of periostin in patients with non-small cell lung cancer:correlation with angiogenesis and lymphangiogenesis[J].Int J Biol Markers 2008; 23: 182-6.
[14]Lohela M,Bry M,Tammela T,et al.VEGFs and receptors involved in angiogenesis versus lymphangiogenesis[J].Curr Opin Cell Biol 2009; 21:154-65.
[15]Li J,Li BL,Zhang HQ,et al.Relationship between vascular endothelial growth factor C expression level and lymph nodemetastasis in non small cell lung cancer[J].Natl Med J China (in Chinese)2008; 88:2982-5.
[16]Tuder RM,Yun JH.Vascular endothelial growth factor of the lung: friend or foe[J].Curr Opin Pharmacol 2008; 8: 255-60.
[17]Delli CJ,Karam AK,Montgomery L.Vascular endothelial growth factor and its relationship to the prognosis and treatment of breast,ovarian,and cervical cancer[J].Angiogenesis 2010; 13: 43-58.
[18]Kadota K,Huang CL,Liu D,et al.The clinical significance of lymphangiogenesis and angiogenesis in non-small cell lung cancer patients[J].Eur J Cancer 2008; 44:1057-67.
[19]Kösem M,Tuncer I,Kotan C,et al.Significance of VEGF and microvascular density in gastric carcinoma[J].Hepatogastroenterology 2009; 56:1236-40.
[20]Cao R,Farnebo J,Kurimoto M,et al.Interleukin-18 acts as an angiogenesis and tumor suppressor[J].FASEB J 1999; 13: 2195-202.
[21]Han MY,Zheng S,Yu JM,et al.Study on interleukin-18 gene transfer into human breast cancer cells to prevent tumorigenicity[J].J Zhejiang Univ Sci 2004; 5: 472-6.
[22]Leng J,Zhang L,Yao H,et al.Antitumor effects of interleukin-18 gene-modified hepatocyte cell line on implanted liver carcinoma[J].Chin Med J(Engl)2003;116: 1475-9.
[23]Takizawa H,Kondo K,Fujino H,et al.The balance of VEGF-C and VEGFR-3 mRNA is a predictor of lymph node metastasis in non-small cell lung cancer[J].Br J Cancer 2006; 95:75-9.
[24]Skobe M,Hawighorst T,Jackson DG,et al.Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis[J].Nat Med 2001; 7:192-8.
[25]Stacker SA,Caesar C,Baldwin ME,et al.VEGF-D promotes the metastatic spread of tumor cells via the lymphatics[J].Nat Med 2001; 7:186-91.
[26]Mandriota SJ,Jussila L,JeltschM,et al.Vascular endothelial growth factor-C mediated lymphangiogenesis tumour metastasis[J].EMBO J 2001; 20:672-82.
[27]Saintigny P,Kambouchner M,Ly M,et al.Vascular endothelial growth factor-C and its receptor VEGFR-3 in non-small-cell lung cancer: concurrent expression in cancer cells from primary tumour and metastatic lymph node[J].Lung Cancer 2007; 58:205-13.
[28]Tsai PW,Shiah SG,Lin MT,et al.Up-regulation of vascular endothelial growth factor C in breast cancer cells by heregulin-beta 1.A critical role of p38/nuclear factor-kappa B signaling pathway[J].J Biol chem 2003; 278: 5750-9.
[29]Nakashima T,Huang CL,Liu D,et al.Expression of vascular endothelial growth factor-A and vascular endotheial growth factor-C as prognostic factos for non-small cell lung cancer[J].Med Sci Monit 2004;10: BR157-65.
[30]Zhang D,Li B,Shi J,et al.Suppression of tumor growth and metastasis by simultaneously blocking vascular endotheial growth factor (VEGF)-A and VEGF-C with a receptor-immunoglobulin fusion protein[J].Cancer Res 2010; 70: 2495-503.
[31]Janic B,Arbab AS.The role and therapeutic potential of endotheial progenitor cells in tumor neovascularization[J].Scientific World J 2010; 10: 1088-99.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Decrease of Peripheral Blood CD8+/CD28- Suppressor T Cell Followed by Dentritic Cells Immunomodulation among Metastatic Breast Cancer Patients
- Chinese Journal of Cancer Research Guidelines for Authors
- Inhibition of Proliferation Induced by Cyclin D1 Gene Silence in Human Renal Carcinoma ACHN Cells
- High Expression of ERCC1 Is a Poor Prognostic Factor in Chinese Patients with Non-small Cell Lung Cancer Receiving Cisplatin-based Therapy
- Association of Lysosome Associated Protein Transmembrane 4 Beta Gene Polymorphism with the Risk of Pancreatic Cancer
- Recurrent Patterns and Factors Involved in Node-negative Advanced Gastric Cancer
