Efficacy of middIe hepatic vein reconstruction in aduIt right-Iobe Iiving donor Iiver transpIantation
2010-06-29CiJunPengXiaoFeiWangBoLiYongGangWeiLuNanYanTianFuWenJiaYinYangWenTaoWangandJiChunZhao
Ci-Jun Peng, Xiao-Fei Wang, Bo Li, Yong-Gang Wei, Lu-Nan Yan, Tian-Fu Wen, Jia-Yin Yang, Wen-Tao Wang and Ji-Chun Zhao
Chengdu, China
OriginaI ArticIe / TranspIantation
Efficacy of middIe hepatic vein reconstruction in aduIt right-Iobe Iiving donor Iiver transpIantation
Ci-Jun Peng, Xiao-Fei Wang, Bo Li, Yong-Gang Wei, Lu-Nan Yan, Tian-Fu Wen, Jia-Yin Yang, Wen-Tao Wang and Ji-Chun Zhao
Chengdu, China
BACKGROUND:Congestion of the right anterior segment may lead to graft dysfunction in right-lobe living donor liver transplantation (LDLT) without a middle hepatic vein (MHV) trunk. Selective reconstruction of MHV tributaries with the interposition of vascular grafts has been introduced to overcome this problem. However, there is still no consensus on the definite criteria of MHV reconstruction.
METHODS:LDLT patients were reviewed to evaluate the effects of MHV reconstruction. From March 2005 to September 2008 in our transplantation center, 120 consecutive LDLTs were performed using a right-lobe graft without a MHV. Excluding 11 patients, among the remainder, 73 (67%) had reconstructed MHV tributaries, and the others 36 (33%) did not. The values of liver functional index and liver graft regeneration ratio were compared between the two groups.
RESULTS:There was a prolonged period of liver functional recovery in patients with small-for-size grafts and a graftrecipient weight ratio (GRWR) <1.0%, and without MHV reconstruction. The ratio of liver regeneration 1 month postoperatively in reconstruction cases was 81%, versus 78% in patients without reconstruction (P=0.352), but among smallfor-size grafts, there was a significant difference between the two groups (95% vs. 80%).
CONCLUSION:Our study shows that reconstruction of MHV tributaries is not necessary in all patients, but is beneficial for patients with GRWR <1.0%.
(Hepatobiliary Pancreat Dis Int 2010; 9: 135-138)
middle hepatic vein; reconstruction; living donor; liver transplantation; interposition vascular conduits
Introduction
Since 1989, when the first clinical living donor liver transplantation (LDLT) was reported,[1]it has become a valid therapeutic modality for end-stage liver disease. To overcome the matter of small-for-size graft, right-lobe grafts have been used in many centers. It is known that hepatic venous outflow of the right anterior sector (segments Ⅴ and Ⅷ) drains mainly into the middle hepatic vein (MHV). Thus it is important in right-lobe LDLT to maintain good MHV outflow drainage, including the MHV trunk or reconstructed MHV tributaries. Considering donor safety, most transplant centers have used right lobe grafts without a MHV trunk.[2-4]However, it is still controversial whether the reconstruction of MHV tributaries is necessary. In this study, we reviewed our LDLT cases to evaluate the effects of MHV reconstruction on right-lobe grafts with regard to liver functional recovery and graft regeneration in the early postoperative period.
Methods
From March 2005 to September 2008, a total of 120 patients underwent primary LDLT at West China Hospital of Sichuan University, Chengdu, China. We excluded 5 cases of dual graft of liver lobe transplantation, 1 case of combined liver and kidney transplantation, 2 cases of pediatric liver transplantation, and 3 deaths within one month postoperatively (one from disseminated intravascular coagulation on postoperative day 2, one from multiple organ dysfunction syndrome on postoperative day 6, and one from acute renal failure onpostoperative day 15). The remaining 109 patients received right lobe adult-to-adult LDLT (A-A LDLT). None of the grafts included a MHV. In our practice, if the diameter of segment Ⅴ or Ⅷ hepatic veins on the cut surface during donor hepatectomy was more than 5 mm, the MHV tributaries from segment Ⅴ or Ⅷ or both were reconstructed with cadaveric vascular conduits or the autologous great saphenous vein. In the recipient operation, the significant inferior right hepatic veins (IRHVs) were anastomosed to the inferior vena cava in an immediate end-to-side fashion and the interposition vascular conduits of reconstructed segment Ⅴ and/or segment Ⅷ were connected to the left hepatic vein stump or the inferior vena cava. The detailed surgical procedure is described as elsewhere.[5]The cases were divided into two groups according to whether or not segment Ⅴand/or segment Ⅷ were reconstructed. The cases of reconstruction of MHV tributaries from segment Ⅴ orⅧ or both comprised group A, and the others without reconstruction comprised group B.
Information on demographic variables, and preoperative, intraoperative, and postoperative pathologic and clinical data were prospectively collected for all transplantations. Outcome analysis was made of the liver functional parameters (total bilirubin [TB], alanine aminotransferase [ALT], and international normalized ratio [INR]), and liver graft volume regeneration by computed tomography (CT) evaluation at 1 month postoperatively in recipients. These indices were compared in all cases and the cases with small-for-size grafts, namely, a graft-recipient weight ratio (GRWR) <1.0% between the two groups. The differences in each variable between groups A and B were analyzed by ANOVA or Student'sttest. All these statistical analyses were performed using the statistical software package SPSS.
Results
The indications for A-A LDLT of all the 109 patients were primary hepatocellular carcinoma (n=52, 48%), hepatic cirrhosis related to chronic hepatitis B (n=33, 30%), and others (n=24, 22%, including Budd-Chiari syndrome, fulminant hepatitis, hepatic echinococcosis, and autoimmune, alcoholic, or biliary cirrhosis). All the donors and recipients were ABO identical or compatible. Among the 109 patients, 73 (67%) had reconstruction ofⅤ and/or Ⅷ, including reconstructon of V in 34 (47%), reconstruction of Ⅷ in 17 (23%), and both in 22 (30%). There were 72 patients with GRWR <1.0%, of whom 51 had reconstructed MHV tributaries. All patients had a patent RHV and IRHV, and excepting 9 patients with Ⅴ/Ⅷ bridged conduit occlusion detected after 1 week postoperatively, the others had a patent bridged conduit confirmed by Doppler ultrasonography and/or CT within one month after transplantation. In group A, the interposition of vascular conduits consisted of the cadaveric iliac artery (37, 50%), cadaveric iliac vein (18, 25%), or autologous great saphenous vein (18, 25%). There was no significant difference in the recipients age, model for end-stage liver diseases (MELD) score, GRWR, anhepatic phase time, and intraoperative blood loss volume and urine volume between the two groups; the exception was cold ischemia time (Table 1).
When the mean values of liver functional index (TB, ALT, and INR) preoperatively and postoperatively on days 1, 3, 5, 7, and 14 were compared between groups A and B (Fig.), there was no significant difference in all patients between the two groups, but the serial changes were different in patients with GRWR <1.0%; the TB and ALT values of these patients were significantly higher in group B than in group A on postoperative days 3, 5 and 7, and remained higher (although not significantly) until postoperative day 14. The INR values showed no correlation with groups. Reconstruction of MHV tributaries was beneficial for liver functional recovery during the early postoperative period for patients with small-for-size grafts, but this effect was not seen in all patients.
Spiral enhancement computed tomography with graft volume evaluation was performed 30 days after transplantation. The graft regeneration ratio (GRR) was calculated using the formula: GRR=[(CT measured graft volume postoperatively on day 30−actual graft volume)/actual graft volume]×100%. The actual graft volume was determined after perfusion using University of Wisconsin solution during operation. The GRRin group A was 0.81±0.12 vs. 0.78±0.15 in group B at 30 days postoperatively (P=0.352; Table 2). In the patients with GRWR <1.0%, the GRR of the two groups was 0.95±0.11 and 0.80±0.16, respectively, and the difference was statistically significant. It is clear that reconstruction of MHV tributaries greatly promotes graft regeneration in patients with GRWR <1.0%. These data also demonstrate that small-for-size grafts have a higher regeneration activity than large grafts, which is consistent with another report.[6]
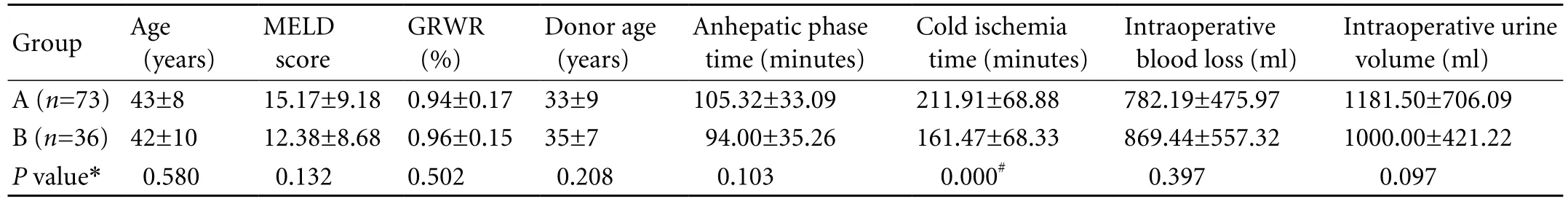
Table 1. Demographics and clinical data of groups A and B (mean±SD)
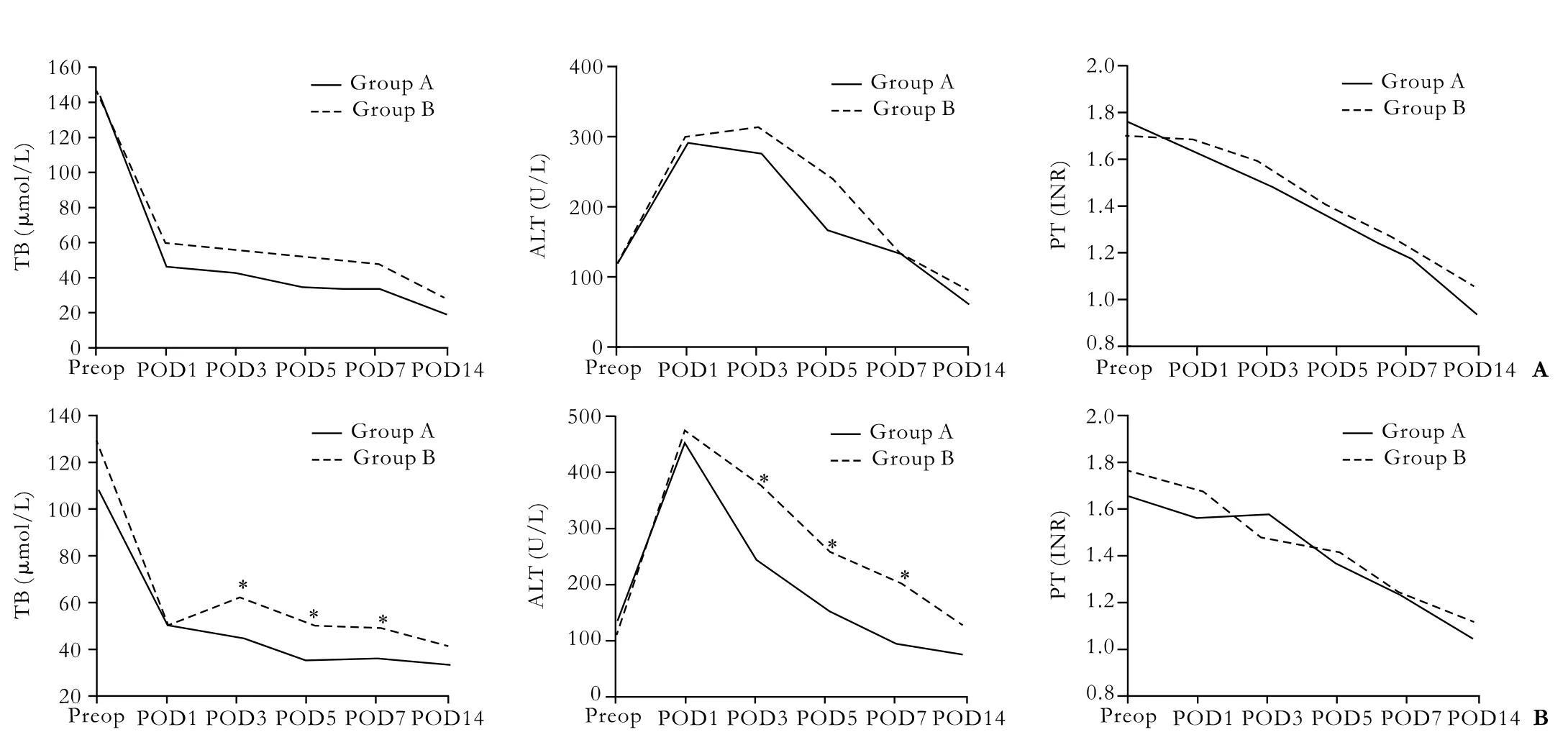
Fig. Liver functional index in early postoperative period. Solid and dotted lines represent mean values of liver functional index for groups A and B, respectively in all patients (A) and the patients with small-for-size grafts, GRWR <1.0% (B). *:P<0.05, the differences were analyzed by ANOVA. POD: postoperative day.

Table 2. Graft regeneration ratio 1 month postoperatively (mean±SD)
Discussion
The necessity of reconstructing MHV tributaries in A-A LDLT has been controversial.[7-10]Marcos et al[11]performed 25 right lobe LDLTs without the MHV, with an excellent recipient and graft survival rate of 88%. Ito et al[2,12]found no significant difference in the extent of congestion of the anterior segment between right-lobe grafts with and without segments Ⅴ and/or Ⅷ hepatic vein reconstruction one month after transplantation. These trials revealed that it is unnecessary to reconstruct MHV tributaries in right-lobe grafts without a MHV trunk. Some hypotheses support this opinion. Kaneko et al[13]thought that intrahepatic collaterals can develop after ligation of the major hepatic veins. This kind of venous collateral can produce venous flow into the right hepatic vein after the ligation of tributaries of the MHV and develops within 10 days of transplantation. However, other results have shown severe congestion of the right anterior segment (AS) in some right-lobe grafts after transplantation, which may influence graft regeneration and lead to sustained graft injury, and even graft loss.[3,14-16]
In right-lobe A-A LDLT, the MHV is usually left to the donor to assure donor safety, which may result in potential right anterior sector (segments Ⅴ andⅧ) congestion in grafts since some AS veins depend partially on MHV for outflow drainage.[17]Selective reconstruction of MHV tributaries may prevent AS congestion; however, it may lead to a prolonged operation time and additional complexity. On the other hand, we obtained satisfactory results after transplantation without MHV reconstruction as Ito et al[12]described. Therefore, the reconstruction of Ⅴ/Ⅷis under debate. Fan et al[7]summarized the views of five Asian liver transplantation centers on the necessity of providing venous drainage for the AS in a right lobe graft. They all recognized the need for adequate drainage of the AS, but the strategy varies from absolute inclusion of the MHV in the graft in every case[18]to selectiveinclusion on the basis of criteria such as the appearance of a dusky area, the results of hepatic artery or hepatic vein occlusion tests,[19]donor-recipient ratio and hepatic vein anatomy, or the presence of a dominant segmentⅤ and Ⅷ hepatic vein. Kim et al[6]advocated selective reconstruction of MHV tributaries in patients with smallfor-size grafts of GRWR <0.8%, but not for all grafts.
Our study showed that the patients with small-forsize grafts of GRWR <1.0%, without a reconstructed MHV, had a prolonged period of liver function recovery, and reconstruction of the MHV can greatly promote graft regeneration in small-for-size grafts. However, reconstruction of the MHV tributaries can prolong the cold ischemia time, which may impair graft quality. In conclusion, we think that it is unnecessary to reconstruct MHV tributaries in all patients, but it can significantly accelerate liver functional recovery and promote liver regeneration in patients with small-for-size grafts. The results are in line with the reports of Kim et al[6]and De Carlis et al.[20]Taking full account of this study and the safety of the patient after transplantation, we recommend that the MHV tributaries should be reconstructed when GRWR<1.0% as far as possible.
Funding:None.
Ethical approval:Not needed.
Contributors:PCJ wrote the first draft of this paper. All authors contributed to the intellectual context and approved the final version. LB is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Raia S, Nery JR, Mies S. Liver transplantation from live donors. Lancet 1989;2:497.
2 Yamamoto H, Maetani Y, Kiuchi T, Ito T, Kaihara S, Egawa H, et al. Background and clinical impact of tissue congestion in right-lobe living-donor liver grafts: a magnetic resonance imaging study. Transplantation 2003;76:164-169.
3 Gyu Lee S, Min Park K, Hwang S, Hun Kim K, Nak Choi D, Hyung Joo S, et al. Modified right liver graft from a living donor to prevent congestion. Transplantation 2002;74:54-59.
4 Yu PF, Wu J, Zheng SS. Management of the middle hepatic vein and its tributaries in right lobe living donor liver transplantation. Hepatobiliary Pancreat Dis Int 2007;6:358-363.
5 Yan L, Wu H, Chen Z, Luo Y, Lu Q, Zhang Z, et al. Intrahepatic venous collaterals formation following outflow block in adult-to-adult living donor liver transplantation. J Surg Res 2008;146:172-176.
6 Kim DG, Moon IS, Kim SJ, Lee YJ, Lee MD. Effect of middle hepatic vein reconstruction in living donor liver transplantation using right lobe. Transplant Proc 2006;38: 2099-2101.
7 Fan ST, De Villa VH, Kiuchi T, Lee SG, Makuuchi M. Right anterior sector drainage in right-lobe live-donor liver transplantation. Transplantation 2003;75:S25-27.
8 de Villa VH, Chen CL, Chen YS, Wang CC, Lin CC, Cheng YF, et al. Right lobe living donor liver transplantationaddressing the middle hepatic vein controversy. Ann Surg 2003;238:275-282.
9 Morioka D, Sekido H, Matsuo K, Takeda K, Sugita M, Kubota T, et al. Middle hepatic vein tributary reconstruction could not act as a complete substitute for an entirely preserved middle hepatic vein. Hepatogastroenterology 2005;52:208-211.
10 Sugawara Y, Makuuchi M, Sano K, Imamura H, Kaneko J, Ohkubo T, et al. Vein reconstruction in modified right liver graft for living donor liver transplantation. Ann Surg 2003; 237:180-185.
11 Marcos A, Fisher RA, Ham JM, Shiffman ML, Sanyal AJ, Luketic VA, et al. Right lobe living donor liver transplantation. Transplantation 1999;68:798-803.
12 Ito T, Kiuchi T, Yamamoto H, Maetani Y, Oike F, Kaihara S, et al. Efficacy of anterior segment drainage reconstruction in right-lobe liver grafts from living donors. Transplantation 2004;77:865-868.
13 Kaneko T, Kaneko K, Sugimoto H, Inoue S, Hatsuno T, Sawada K, et al. Intrahepatic anastomosis formation between the hepatic veins in the graft liver of the living related liver transplantation: observation by Doppler ultrasonography. Transplantation 2000;70:982-985.
14 Lee S, Park K, Hwang S, Kim K, Ahn C, Moon D, et al. Anterior segment congestion of a right liver lobe graft in living-donor liver transplantation and strategy to prevent congestion. J Hepatobiliary Pancreat Surg 2003;10:16-25.
15 Fan ST, Lo CM, Liu CL, Wang WX, Wong J. Safety and necessity of including the middle hepatic vein in the right lobe graft in adult-to-adult live donor liver transplantation. Ann Surg 2003;238:137-148.
16 Maetani Y, Itoh K, Egawa H, Shibata T, Ametani F, Kubo T, et al. Factors influencing liver regeneration following living-donor liver transplantation of the right hepatic lobe. Transplantation 2003;75:97-102.
17 Nakamura S, Sakaguchi S, Hachiya T, Suzuki S, Nishiyama R, Konno H, et al. Significance of hepatic vein reconstruction in hepatectomy. Surgery 1993;114:59-64.
18 Liu CL, Zhao Y, Lo CM, Fan ST. Hepatic venoplasty in right lobe live donor liver transplantation. Liver Transpl 2003;9: 1265-1272.
19 Sano K, Makuuchi M, Takayama T, Sugawara Y, Imamura H, Kawarasaki H. Technical dilemma in living-donor or splitliver transplant. Hepatogastroenterology 2000;47:1208-1209.
20 De Carlis L, Lauterio A, Giacomoni A, Slim AO, Pirotta V, Mangoni J, et al. Adult living donor liver transplantation with right lobe graft: the venous outflow management in the Milan-Niguarda experience. Transplant Proc 2008;40:1944-1946.
April 14, 2009
Accepted after revision January 28, 2010
Author Affiliations: Department of Liver and Vascular Surgery and Liver Transplantation Center, West China Hospital, Sichuan University, Chengdu 610041, China (Peng CJ, Wang XF, Li B, Wei YG, Yan LN, Wen TF, Yang JY, Wang WT and Zhao JC)
Bo Li, MD, Department of Liver and Vascular Surgery and Liver Transplantation Center, West China Hospital, Sichuan University, Chengdu 610041, China (Tel: 86-28-81812470; Email: doctorlibo@ 163.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Protective effects of MCP-1 inhibitor on a rat model of severe acute pancreatitis
- Extrahepatic right hepatic duct diverticulum:a rare entity
- MRI shows clodronate-liposomes attenuating liver injury in rats with severe acute pancreatitis
- Preoperative assessment of hilar cholangiocarcinoma: combination of cholangiography and CT angiography
- Surgical therapy and prognosis of sarcomatoid carcinoma of the gallbladder
- Small-duct primary sclerosing cholangitis with hepatocellular carcinoma requiring liver transplantation
