MRI shows clodronate-liposomes attenuating liver injury in rats with severe acute pancreatitis
2010-12-14JianXinZhangShengChunDangYongZhangXinShaLiRongZhangChuanSheWeiMinChenandDeLiJiang
Jian-Xin Zhang, Sheng-Chun Dang, Yong Zhang, Xin Sha, Li-Rong Zhang,Chuan-She Wei, Min Chen and De-Li Jiang
Zhenjiang, China
MRI shows clodronate-liposomes attenuating liver injury in rats with severe acute pancreatitis
Jian-Xin Zhang, Sheng-Chun Dang, Yong Zhang, Xin Sha, Li-Rong Zhang,Chuan-She Wei, Min Chen and De-Li Jiang
Zhenjiang, China
(Hepatobiliary Pancreat Dis Int 2010; 9: 192-200)
pancreatitis, acute;clodronate disodium;liver injury;MR imaging;macrophage
Introduction
Despite intensive research efforts, the systemic morbidity of severe acute pancreatitis (SAP) has increased over time.[1]SAP involves a complex array of mediators that initiate and amplify the systemic in fl ammatory response, which leads to the failure of distant organ systems. SAP-induced liver injury is dif fi cult to prevent and cure because of complicated pathogenesis.It frequently occurs in clinical practice, especially in theearly phase of SAP, and progresses rapidly. Though the mechanism underlying SAP-induced liver injury has not been fully elucidated, current studies suggest that it is potentially a complex pathophysiological process involving many in fl uential factors such as in fl ammatory mediators,oxidative stress, and microcirculatory disturbance.[2,3]
Recent studies have shown that Kupffer cells(KCs) not only act as phagocytes but also play a central role in various liver diseases as potent secretory cells.Accumulated evidence suggests that reduced numbers of KCs protect against liver injury, but no consensus on the protective mechanism has been reached.[4,5]Activated KCs can lead to a systemic in fl ammatory response, induce lipid peroxidation, impair membrane structure, result in injury to the pancreas and the other extrapancreatic organs, and eventually result in multiple organ dysfunction syndrome(MODS) by enhancing excessive secretion of cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin(IL)-6, or IL-1.[6,7]
Macrophage depletion can be achieved with the systemic injection of liposomes containing clodronate rather than free clodronate, which fails to permeate cellular membranes and has a short half-life in the systemic circulation.[8]Clodronate belongs to the family of bisphosphonates, bone-seeking agents that are potent osteoclast inhibitors. Like other bisphosphonates,clodronate has poor cell membrane permeability.[9,10]Liposomes are readily taken up by cells of the reticuloendothelial system, in particular macrophages. Liposomemediated delivery of clodronate inactivates and kills macrophages after effective phagocytosis but has no toxicity to nonphagocytic cells.[11]Superparamagnetic iron oxide nanoparticles (SPIO) have recently been shown to be an important tool for enhancing magnetic resonance contrast. Upon systemic application, SPIO particles are preferentially internalized by macrophages.[12]In this study, we employed a novel method to make SPIO-containing liposomes for macrophage labeling and MRI,which were able to bind clodronate for macrophage apoptosis and depletion.
In this study, we employed liposomes as carriers to deliver clodronate into macrophages (including KCs)to induce apoptosis, reduce the release of in fl ammatory mediators, and deliver SPIO for MRI. Our investigation of the effect of SPIO-clodronate-containing liposome on KCs in SAP provides a new basis for the treatment and MRI evaluation of liver injury with SAP.
MethodsAnimal models and experimental grouping
Forty-eight healthy Sprague-Dawley rats weighing 350-400 g were provided by the Laboratory Animal Center at Jiangsu University School of Medicine, China. The rats were housed in a controlled environment with an ambient temperature of 21-23 ℃ and a 12∶12-hour light-dark cycle. The rats were fed a standard laboratory diet, given water ad libitum, and fasted overnight before each experiment. All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee of Jiangsu University, China.Rats were handled according to regulations stipulated by the Institutional Animal Care and Use Committee.Maximal effort was made to minimize animal suffering,and the number of animals used was the minimum number required to obtain signi fi cant data. All rats were randomly divided into three groups: control (group C),SAP plus SPIO-liposomes (group P), and SAP plus SPIO-clodronate-containing liposomes (group T). Each group was further divided into two subgroups with periods of 2 or 6 hours. After anesthesia, the abdominal cavities of the rats were opened. SAP models were prepared by injecting 5% sodium taurocholate (2 ml/kg body weight)into the subcapsular space of the pancreas.[13]The rats were injected very slowly through the tail vein with either SAP plus SPIO-liposomes (2 ml/kg body weight) in the group P, SPIO-clodronate-containing liposomes (2 ml/kg body weight) in the group T, or normal saline (2 ml/kg body weight) in the group C. Dosing was performed as described above; all dosages had previously been proven to be effective. The suspension was shaken gently before administration. T2-weighted MRI scans (in the same plane) of the livers of rats in each group were performed.At 2 and 6 hours after SAP model creation, animals were sacri fi ced; the livers and pancreata were harvested.
Materials
Clodronate was from Shanghai Weijing Technology Enterprise Co., Ltd. (China), sodium taurocholate from Sigma (USA), and ferric trichloride hexahydrate (analytical reagent) and ferrous sulfate heptahydrate (analytical reagent) from Sinopharm Chemical Reagent (China)and Shanghai Second Chemical Reagent Factory (China),respectively. The JJ-1 electric stirrer, electronic balance,and Tecnai-12 transmission electron microscope were from Jintan Medical Instrument Factory (Jiangsu, China),Sartorius (Beijing, China), and Philips (The Netherlands),respectively. We also used an automatic biochemistry analyzer CL-7300 (Shimadzu, Japan), rotary evaporators R-200 (BUCHI Labortechnik AG), and MR (Siemens Magnetom Trio Tim 3.0T).
SPIO preparation
SPIO nanoparticles with a diameter of 20-50 nm were prepared using the coprecipitation-hydrothermal method.Brie fl y, concentrated hydrochloric acid (0.85 ml) was added to 25 ml of distilled water. Nitrogen gas was then added to the solution for 30 minutes to expel the oxygen.FeCl3• 6H2O (5.2 g) and FeSO4• 7H2O (2.68 g) were added successively while stirring. The mixture was then dropped into a four-neck fl ask containing 250 ml of 1.5 mol/L NaOH and 2 g of PEG-2000 while the solution was vigorously stirred and fi lled with nitrogen gas. The fl ask was placed in a water bath at 60 ℃. The mixture was then stirred for another 60 minutes.
The pH value of the solution was maintained between 11 and 12 throughout the entire precipitation process. The fi nal black solution was transferred to a 100 ml hydrothermal kettle and maintained at 180 ℃ for 4 hours. After magnetic separation of the resulting solution,black precipitates were washed with deionized water until neutral pH was achieved. Samples were then vacuumdried at 50 ℃ for 8 hours to yield a black powder.
Preparation and use of SPIO-containing liposomes and SPIO-clodronate-containing liposomes
SPIO-containing liposomes and SPIO-clodronatecontaining liposomes were prepared by the reverse phase evaporation method as described previously by van Rooijen.[14]Brie fl y, phosphatidylcholine chloroform solution (100 mg/ml) was prepared and kept away from light at -20 ℃ for further use. In a 500 ml round-bottom fl ask, 8 mg of cholesterin was dissolved in 10 ml of chloroform. After that, 0.86 ml of the phosphatidylcholine chloroform solution containing 86 mg of phosphatidylcholine was added. Chloroform was then removed by rotary distillation (150 rpm) at 37 ℃under low-vacuum conditions (gradually decreased from 200 mbar to 150 mbar). Finally, a thin, uniform milky white phospholipid fi lm formed on the interior of the fl ask. The remaining fi lm was then dispersed by adding 10 ml PBS containing 1.0 mg of Fe3O4nanocrystals.Subsequently, 0.6 mol/L of clodronate phosphate buffer with 1.0 mg of Fe3O4nanocrystals and 2.5 g of clodronate dissolved in 10 ml of PBS was used for elution and dispersion of the phospholipid fi lm to result in a milky suspension. Thus, the fi nal SPIO-containing liposomes and SPIO-clodronate-containing liposomes were obtained.The preparation was kept at room temperature for 2 hours,sonicated at 50 Hz for 3 minutes in a cold-water bath,and then kept under nitrogen for an additional 2 hours at room temperature to swell the liposomes. In order to remove the non-encapsulated clodronate and SPIO, the preparations were washed three times using sterilized PBS(centrifugation at 10 000 g for 30 minutes). Finally, the pellet was re-suspended by the addition of 4 ml sterilized PBS. The suspensions were stored in nitrogen until use(within 2 weeks) and shaken gently before administration to animals. This concentration represents an estimate,thus doses of liposomes are described as volumes rather than as concentrations.[7]
MRI scan of liver
A Magnetom Trio Tim (3.0 T, Siemens) superconducting MRI scanner was used. A breast coil, self-made fi xation tool for rats, water model, and image postprocessing workstation (Leonardo) were utilized. The rats were put through the center of the coil along the vertical direction (that is, the long axis of the rat and the coil were mutually perpendicular). Physiological saline solution was placed below the coil for MRI. All rats were placed in the supine position. After rats were fi xed by using the self-made fi xation tool, they were wrapped through the coil and put on the scanning bed.All rats received coronal SE-T2WI (T2-weighted image)scans. Scan parameters were as follows: repetition time,3000 ms; echo time, 107 ms; scan fi eld, 180×100; slice number, 12; slice thickness, 3.0 mm; and slice spacing,0.3 mm. Circular regions of interest (ROI) were used to determine the signal intensity of the liver after injection of contrast agent. To reduce error, the signal values of ROI in the right lower lobe of the liver were determined to calculate average values. The scanning sites before and after injection of contrast agent were consistently kept as distant as possible. The pancreata in rats were too thin to undergo MRI examination.
Analysis of serum amylase, alanine aminotransferase(ALT), aspartate aminotransferase (AST), TNF-α and IL-6 levels
To assess serum amylase, ALT, AST, TNF-α, and IL-6 levels, blood was obtained from the superior mesenteric vein, placed on ice for 15 minutes, and centrifuged at 3000 rpm. The supernatant was retained and preserved at -20 ℃. Serum amylase, ALT, and AST were measured with an automatic biochemical analyzer.Serum TNF-α and IL-6 were measured by enzymelinked immunosorbent assays (ELISA) according to the manufacturer's protocol.
Immunohistochemical identi fi cation of macrophages in liver tissue
We detected CD68+cells by means of a standard indirect three-step immunohistochemical method. A commercially acquired monoclonal anti-rat CD68(macrophage-associated antigen) antibody was used to detect macrophages in formalin- fi xed, paraf fi nembedded tissues from rats.
Pathological examination
Paraf fi n-embedded pancreata and livers were sectioned (5 μm), then stained with hematoxylin and eosin. Experienced histologists, unaware of which treatment the animal was subjected to, scored each specimen histologically. This pathological assessment of pancreatic tissue was performed according to the scoring criteria proposed by Kaiser.[15]Liver injury was scored on a scale of 0-3 for each criterion.[16]
Statistical analysis
All data were expressed as mean±SD using SPSS statistical software (PASW Statistics for Windows, version 18.0). If equal variances were assumed, one-way ANOVA was used to evaluate the differences in serum amylase,ALT, AST, and T2WI; otherwise, the Kruskal-Wallis test was used. P<0.05 indicates a signi fi cant difference.
Results Observation of Fe3O4 particles and liposomes with electron microscopy
As revealed by electron microscopy, bare Fe3O4nanoparticles were nearly spherical. The majority of these nanoparticles aggregated, with an average diameter of 20-50 nm. The nanoparticles precipitated easily. After Fe3O4particles were entrapped in the cores of liposomes,the resulting superparamagnetic Fe3O4nanoparticles were dark brown in appearance, had a pH value of 6.47,contained 5 μmol Fe/ml, and showed stable suspension.The available liposomes had an average diameter of 100-200 nm. Under transmission electron microscopy,these liposomes displayed good shape and uniform size(Fig. 1).
Changes in MRI T2WI
The signal intensity of the liver on T2WI in the groups P and T was signi fi cantly lower than that in the group C at 2 and 6 hours (Fig. 2).
Changes in the levels of serum amylase, ALT, and AST
Levels of serum amylase, ALT, and AST at 2 and 6 hours in rats in the group P were higher than those in the group C (P<0.01). In contrast, the levels of serum amylase, ALT, and AST at both time points in the group T were lower than those in the group P (P<0.01) (Fig. 3).
Comparison of serum TNF-α and IL-6 levels
At both time points, higher levels of TNF-α and IL-6 were obtained in the group P than in the group C (P<0.01).Compared with the group P, the levels of TNF-α and IL-6 decreased in the group T (P<0.01) (Fig. 4).
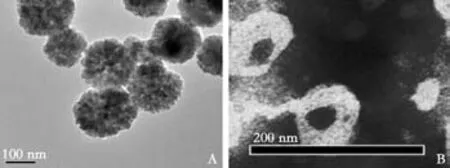
Fig. 1. A: Electron microscopy revealed that bare Fe3O4 nanoparticles were near-spherical and showed Fe3O4 electron diffraction patterns;B: SPIO-clodronate-containing liposomes were similar in size; iron particles were distributed uniformly.
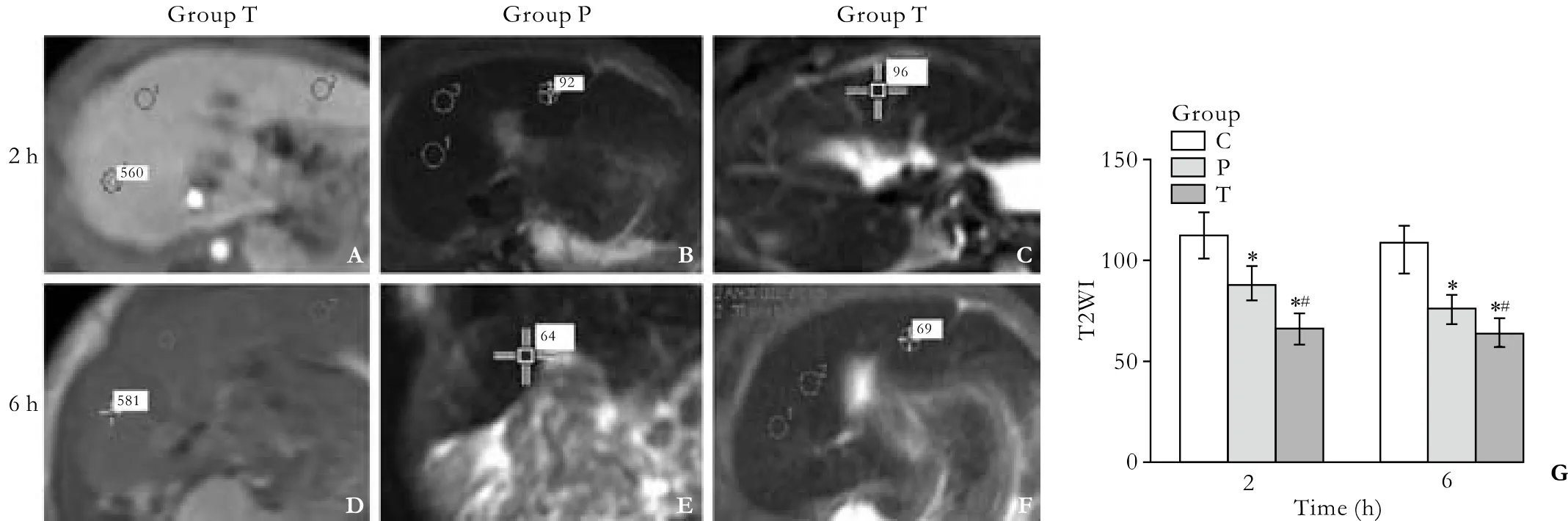
Fig. 2. Signal intensity of the liver on T2WI in the group C (A, D), group P (B, E), and group T (C, F). The signal intensity in the groups P and T was signi fi cantly lower than that in the group C (G). *: P<0.01, vs. the group C; #: P<0.01, vs. the group P. Data are mean±SD (n=8) as below.

Fig. 3. Levels of amylase (A), ALT (B), and AST (C) in serum at 2 and 6 hours in each group. *: P<0.01 vs. the group C; #: P<0.01 vs.the group P.
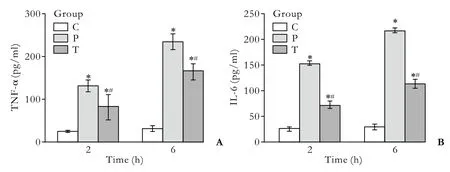
Fig. 4. Levels of TNF-α (A) and IL-6 (B) in serum at 2 and 6 hours in each group. *:P<0.01 vs. the group C; #: P<0.01 vs. the group P.
Morphological and pathological changes in the pancreas
Gross observation: In the group C, the pancreata showed no signi fi cant changes. In the group P, bloody ascites in the abdominal cavity as well as pancreatic congestion, edema, hemorrhage, and necrosis were noted. In the group T, pathological changes were milder than those in the group P. Light microscopy:In the group C, animals displayed normal pancreatic histology. In the group P, the pancreata were slightly edematous, with extensive in fi ltration by in fl ammatory cells (2 hours) and necrosis of the adjacent fat tissues,moderate hemorrhage, and more diffuse focal areas of nonviable pancreatic parenchyma; acinar cell necrosis was also observed (6 hours). The animals in the group T showed distinct signs of mild edematous pancreatitis characterized by interstitial edema, as well as in fi ltration of neutrophil and mononuclear cells, but without parenchyma necrosis and hemorrhage. These histological changes in the group T were less marked than those in the group P. According to Kaiser's criteria,the histological scores showed that signi fi cant differences existed in the groups P and T as compared to the group C.Less dramatic pathological changes were observed in the group T as compared to the group P (P<0.01) (Fig. 5).
Morphological and pathological changes in the liver
Gross observation: In the group C, the livers showed normal morphology. In the group P, varying degrees of congestion and swelling were seen at both time points;the liver became darker in color, and scattered necrotic foci were visible. In the group T, the above-mentioned changes were milder at 2 and 6 hours than those in the group P. Light microscopy: In the group C, the livers showed no morphological or structural abnormalities. In the group P, varying degrees of liver sinusoidal expansion,swelling and degeneration of liver cells, spotty or plaque hemorrhage, and necrosis in the parenchyma were seen at 2 and 6 hours. In the group T, pathological changes in the liver were signi fi cantly milder than those in the group P. The pathological severity score was higher in the groups P and T than in the group C (P<0.01), as well as lower in the group T than in the group P at 2 and 6 hours (P<0.01) (Fig. 6).
Immunohistochemistry for macrophage marker(CD68) in liver tissue
Immunostaining for the macrophage-speci fi c marker CD68 in rat liver tissue sections can be seen in Fig. 7.
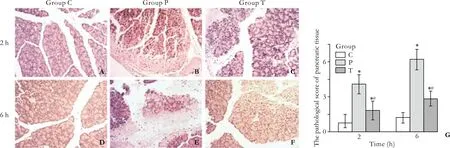
Fig. 5. Pathological changes in the pancreas. In the group C, the pancreata of rats showed no morphological or structural abnormalities (A, D); in the group P, there were varying degrees of focal interlobular edema, necrotic areas without structure,and red blood cells in the tissue space, as well as massive in fl ammatory cell in fi ltration (B, E); in the group T, pancreatic edema,hemorrhage, and necrosis, as well as inflammatory cell infiltration were milder than in the group P (C, F). Histological scores showed signi fi cant differences between the groups P and T as compared to the group C; pathological changes in the group T were less severe than those in the group P (G).
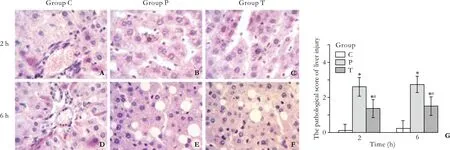
Fig. 6. In the group C, rat livers showed no morphological or structural abnormalities, and liver sinusoidal expansion was occasionally noted (A, D); in the group P, varying degrees of liver sinusoidal expansion, swelling, and hepatocyte cell degeneration were seen at 2 hours; in fl ammatory cell in fi ltration, predominantly distributed in the portal area and necrotic areas, was noted at 6 hours (B, E); in the group T, pathological changes in the liver were signi fi cantly milder than those in the group P (C, F). Liver injury scores (0-3) were determined as described in the Methods section (G).
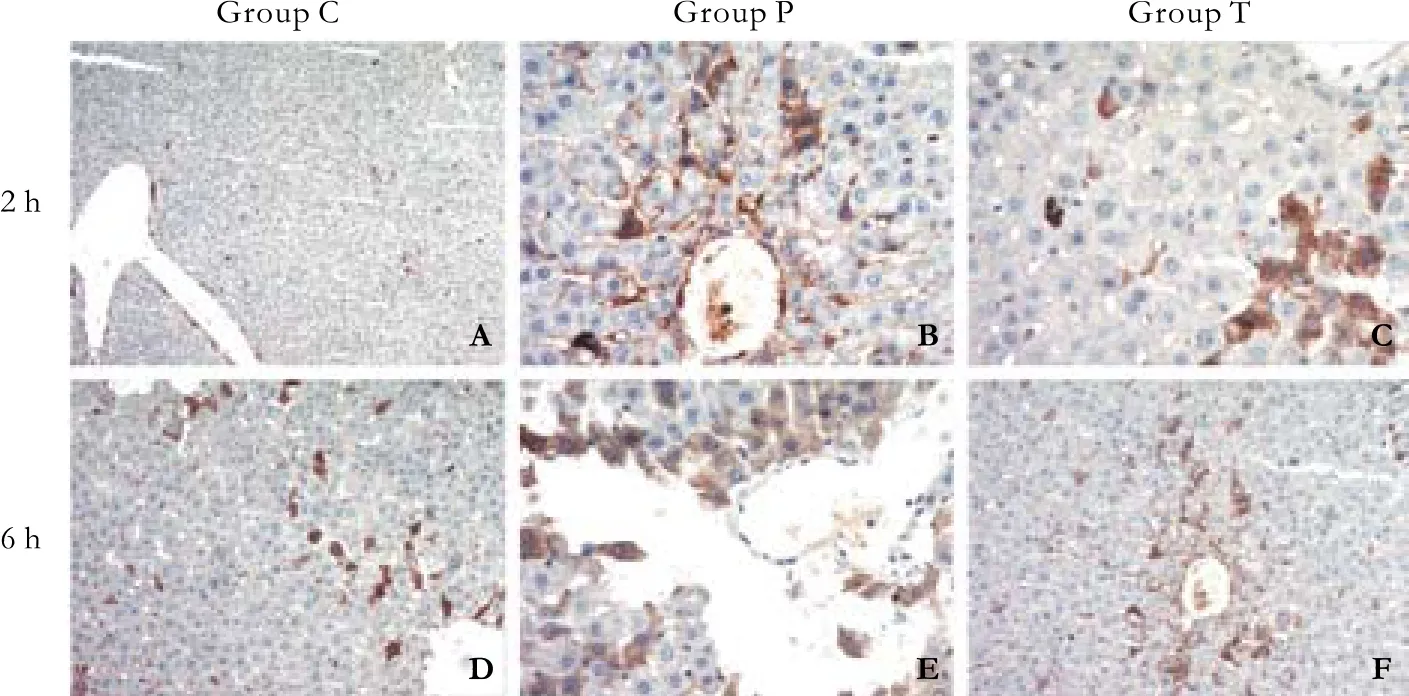
Fig. 7. Immunostaining for the macrophagespeci fi c marker CD68 in rat liver tissue sections.Under homeostatic conditions (A, D); numerous KC clusters were observed in the group P; the tissue architecture was massively distorted (B, E);fewer KCs were observed in liver tissue sections from the group T (C, F).
Discussion
SAP is commonly known as "acute abdomen"; its underlying pathogenesis has not been fully elucidated. It is currently believed that, during the early phase of SAP,pancreatic acinar cells produce and release excessive in fl ammatory cytokines, which causes the in fl ammatory cascade to run out of control, induces or exacerbates the systemic in fl ammatory response syndromes, and results in MODS.[17]Excessive release of systemic proin fl ammatory cytokines is a dominating factor for both local and systemic aggravation of SAP. In fi ltration and activation of macrophages not only triggers the initial events of SAP, but also functions as an important pathophysiological step in multiple organ failure.[18]
As the largest reservoir of macrophages in the body, the human liver plays a unique role in the systemic response to SAP. KCs in the liver account for approximately 50% of all macrophages, or 80%-90%of fi xed macrophages in the body. KCs are the main effector cells in the production of in fl ammatory cytokines. Since liver injury is closely related to the development and prognosis of SAP, exploration of the mechanism underlying the development of liver injury is of great signi fi cance for preventing injury to extrapancreatic organs and reducing the occurrence of MODS. Experiments showed that cytokines generated by macrophages, such as TNF-α and IL-1β, directly cause liver injury and liver dysfunction. Therefore suppressing TNF-α and IL-1β produced by macrophages could reduce liver injury.[19-23]Gloor et al[24]investigated the roles of cytokines secreted by KCs in SAP and found that,in untreated pancreatitis patients, the levels of cytokines were lowest in the portal vein, higher in the liver vein,and highest in peripheral blood. After blocking KCs with gadolinium chloride, the levels of cytokines in the liver vein and peripheral blood declined signi fi cantly, and the extent of liver injury was signi fi cantly lessened. This result suggests that cytokines that originate from KCs elevate cytokine levels in peripheral blood and play a role in related liver injury.
Bisphosphonate clodronate, clinically used in the treatment of osteoporosis, is known to deplete monocytic lineage cells. van Rooijen et al[25]found that intravenous injection of clodronate-containing liposomes selectively eliminates macrophages in vivo. Clodronate is a bisphosphonate drug that inhibits the viability of macrophages via the induction of apoptosis, possibly mediated by competing with ATP as a substrate for intracellular ATPase. After clodronate is incorporated within liposomes, its uptake by phagocytic cells such as macrophages is greatly enhanced, resulting in selective lethality that targets macrophage populations.[26,27]Liposomal clodronate inhibits the growth of cultured macrophages but has no effect on endothelial or smooth muscle cells.[28]
Since SPIO nanoparticles are engulfed by KCs in vivo,they can be used for MR tracking and speci fi c imaging in tissues containing high numbers of macrophages such as the liver, spleen, and lymph nodes. SPIO nanoparticles signi fi cantly reduce the signal values of T1WI and T2WI. The main effect of the SPIO particle in the MR image is a shortening of the T2 relaxation time. The signal intensity of tissues on T2WI decreases with an increase in SPIO content. As nanoscale particles, SPIO particles have uniform size, are water-soluble, and show no mutual aggregation. After being introduced into the liver, SPIO particles are recognized and engulfed by KCs that form part of the reticuloendothelial system. As a result, the signal intensity of the corresponding region is reduced. KCs in normal liver account for 80% of all reticuloendothelial cells. The vast majority of SPIO particles are engulfed by KCs after they are introduced into the body. The extent to which SPIO particles are engulfed in the liver depends on KC distribution. SPIO-enhanced T2WI MRI scans permit evaluation of the extent of liver injury in SAP. The signal intensity of the liver on T2WI was signi fi cantly lower in the group P than in the group C.
In the present study, we investigated the timecourse of changes in KC expression of the macrophagespeci fi c marker CD68 with SAP in rat liver using immunohistochemistry. Serum amylase, TNF-α, and IL-6 levels were notably increased after induction of SAP.Levels in the group T were lower than those in the group P. Pancreas and liver injuries in the group T were milder than those in the group P.
In our model, the degree of liver injury, which was assessed according to the standard scale of pathological examination, was closely paralleled by CD68 expression.In conclusion, KCs are involved in the pathogenesis of liver injury in SAP. The data in the present report indicate that macrophages play a crucial role in the development of SAP, and that activated macrophages contribute to the progression of liver injury. Moreover,clodronate-containing liposomes induce the apoptosis of macrophages in SAP rats. Thus, we can reduce the release of in fl ammatory mediators and lessen the in fl ammatory response by inducing the apoptosis of macrophages,and further improve the prognosis of SAP. This study shows that inactivation of macrophages by systemic administration of liposome-encapsulated clodronate inhibits intestinal macrophages in rats with SAP. These results validate our hypothesis that macrophages play a pivotal role in the pathogenesis of aggravated liver injury. In the study, liposomal clodronate was safely administered to macrophages. The doses were suf fi cient to relieve the in fl ammatory reaction by inhibiting macrophage function. These fi ndings suggest that liposomal delivery of clodronate offers an effective novel approach to treat SAP in rats. This study also provides a new approach for clinical treatment of SAP and SAP-induced liver injury. Modulation of macrophage function may be of great value in the evolution of SAP.SPIO can be used as a tracer for MRI examination of liver injury.
Funding: This study was supported by grants from the National Natural Science Foundation of China (No. 30772117) and the Natural Science Foundation of Jiangsu Province (No. BK2007096).
Ethical approval: Animal care and experimental procedures were performed in accordance with the Guidelines for Animal Experimentation of Jiangsu University with the approval of the Institutional Animal Care and Use Committee. Efforts were made to minimize animal suffering, and the number of animals used was minimal to obtain signi fi cant data.
Contributors: ZJX proposed the study. DSC wrote the fi rst draft and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. ZJX is the guarantor.
Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Glisic T, Sijacki A, Vukovic V, Subotic A. Bernard Organ Failure Score in estimation of most severe forms of acute pancreatitis. Srp Arh Celok Lek 2009;137:166-170.
2 Zhang XP, Zhang J, Ren Z, Feng GH, Zhu W, Cai Y, et al.Study on protecting effects of baicalin and octreotide on hepatic injury in rats with severe acute pancreatitis. World J Gastroenterol 2008;14:6551-6559.
3 Zhang XP, Wang L, Zhang J. Study progress on mechanism of severe acute pancreatitis complicated with hepatic injury. J Zhejiang Univ Sci B 2007;8:228-236.
4 Li JY, Gu X, Zhang WH, Jia S, Zhou Y. GdCl3abates hepatic ischemia-reperfusion injury by inhibiting apoptosis in rats.Hepatobiliary Pancreat Dis Int 2009;8:518-523.
5 Kincius M, Liang R, Nickkholgh A, Hoffmann K,Flechtenmacher C, Ryschich E, et al. Taurine protects from liver injury after warm ischemia in rats: the role of kupffer cells. Eur Surg Res 2007;39:275-283.
6 Oikawa K, Ohkohchi N, Sato M, Masamume A, Satomi S.Kupffer cells play an important role in the cytokine production and activation of nuclear factors of liver grafts from non-heartbeating donors. Transpl Int 2002;15:397-405.
7 Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Yang K, et al.Intestinal damage mediated by Kupffer cells in rats with endotoxemia. World J Gastroenterol 2002;8:923-927.
8 van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 1994;174:83-93.
9 Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status.Clin Cancer Res 2006;12:6222s-6230s.
10 Hoshino H, Yamazaki K. Mechanisms of action in bisphosphonates. Clin Calcium 2005;15:88-92.
11 Selander KS, Monkkonen J, Karhukorpi EK, Harkonen P,Hannuniemi R, Vaananen HK. Characteristics of clodronateinduced apoptosis in osteoclasts and macrophages. Mol Pharmacol 1996;50:1127-1138.
12 Siglienti I, Bendszus M, Kleinschnitz C, Stoll G. Cytokine pro fi le of iron-laden macrophages: implications for cellular magnetic resonance imaging. J Neuroimmunol 2006;173:166-173.
13 Dang SC, Zhang JX, Qu JG, Wang XQ, Fan X. Ligustrazine alleviates gastric mucosal injury in a rat model of acute necrotizing pancreatitis. Hepatobiliary Pancreat Dis Int 2007;6:213-218.
14 van Rooijen N, van Kesteren-Hendrikx E. "In vivo" depletion of macrophages by liposome-mediated "suicide". Methods Enzymol 2003;373:3-16.
15 Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML.Relationship between severity, necrosis, and apoptosis in fi ve models of experimental acute pancreatitis. Am J Physiol 1995;269:C1295-1304.
16 Zhang XP, Zhang L, Wang Y, Cheng QH, Wang JM, Cai W,et al. Study of the protective effects of dexamethasone on multiple organ injury in rats with severe acute pancreatitis.JOP 2007;8:400-412.
17 Raraty MG, Connor S, Criddle DN, Sutton R, Neoptolemos JP.Acute pancreatitis and organ failure: pathophysiology, natural history, and management strategies. Curr Gastroenterol Rep 2004;6:99-103.
18 Shifrin AL, Chirmule N, Zhang Y, Raper SE. Macrophage ablation attenuates adenoviral vector-induced pancreatitis.Surgery 2005;137:545-551.
19 Yang J, Denham W, Carter G, Tracey KJ, Norman J.Macrophage paci fi cation reduces rodent pancreatitisinduced hepatocellular injury through down-regulation of hepatic tumor necrosis factor alpha and interleukin-1beta.Hepatology 1998;28:1282-1288.
20 Wang X, Zhao X, Shi C, Borjesson A, Chen Z, Axelsson J, et al. Potential mechanisms and signi fi cance of acute pancreatitis-associated liver injury. Scand J Gastroenterol 2006;41:604-613.
21 Peng Y, Gallagher SF, Haines K, Baksh K, Murr MM. Nuclear factor-kappaB mediates Kupffer cell apoptosis through transcriptional activation of Fas/FasL. J Surg Res 2006;130:58-65.
22 Peng Y, Sigua CA, Gallagher SF, Murr MM. Protein kinase C-zeta is critical in pancreatitis-induced apoptosis of Kupffer cells. J Gastrointest Surg 2007;11:1253-1261.
23 Yang J, Gallagher SF, Haines K, Epling-Burnette PK, Bai F,Gower WR Jr, et al. Kupffer cell-derived Fas ligand plays a role in liver injury and hepatocyte death. J Gastrointest Surg 2004;8:166-174.
24 Gloor B, Blinman TA, Rigberg DA, Todd KE, Lane JS, Hines OJ, et al. Kupffer cell blockade reduces hepatic and systemic cytokine levels and lung injury in hemorrhagic pancreatitis in rats. Pancreas 2000;21:414-420.
25 van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods 1996;193:93-99.
26 Monkkonen J, Taskinen M, Auriola SO, Urtti A. Growth inhibition of macrophage-like and other cell types by liposomeencapsulated, calcium-bound, and free bisphosphonates in vitro. J Drug Target 1994;2:299-308.
27 Frith JC, Monkkonen J, Blackburn GM, Russell RG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5'-(beta,gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J Bone Miner Res 1997;12:1358-1367.
28 Danenberg HD, Fishbein I, Gao J, Monkkonen J, Reich R,Gati I, et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation 2002;106:599-605.
BACKGROUND: Studies have revealed that macrophages play an important role in the development of severe acute pancreatitis (SAP). Activated macrophages can lead to a systemic in fl ammatory response, induce lipid peroxidation,impair membrane structure, result in injury to the liver and the other extrahepatic organs, and eventually result in multiple organ dysfunction syndrome by promoting excessive secretion of cytokines. Liver injury can further aggravate the systemic in fl ammatory response and increase mortality by affecting the metabolism of toxins and the release of excessive in fl ammatory mediators. Clodronate is a synthetic bisphosphonate, which is often used for treating bone changes caused by osteoporosis and other factors. In the current study,we created liposomes containing superparamagnetic iron oxide particles (SPIOs) for macrophage labeling and magnetic resonance imaging, using a novel method that can bind the clodronate to induce apoptosis and deplete macrophages.
METHODS: Superparamagnetic Fe3O4nanoparticles were prepared by chemical coprecipitation. SPIO-containing liposomes and SPIO-clodronate-containing liposomes were prepared by the thin fi lm method. SAP models were prepared by injection of sodium taurocholate (2 ml/kg body weight)into the subcapsular space of the pancreas. Sprague-Dawley rats were randomly divided into a control group, a SAP plus SPIO-liposome group, and a SAP plus SPIO-clodronatecontaining group. Two and six hours after SAP models were available, T2-weighted MRI scans (in the same plane) of the livers of rats in each group were performed. At the end of the scans, 2 ml of blood was taken from the superior mesenteric vein to measure the levels of serum amylase, ALT, AST, TNF-α,and IL-6. Pathological changes in the liver and pancreas were assessed.
RESULTS: Transmission electron microscopy showed that the liposomes had a uniform size. No pathological changes in the pancreata of rats in the control group were noted. The pathological changes in the pancreata and livers of rats in the SAP plus SPIO-clodronate-containing liposome group were milder than those in the SAP plus SPIO-liposome group.The MRI signal intensity of the livers in the SAP plus SPIO-liposome and SAP plus SPIO-clodronate-containing groups was signi fi cantly lower than that in the control group. There were signi fi cant changes in the two experimental groups(P<0.01). In addition, the levels of serum amylase, ALT, AST,TNF-α, and IL-6 in rats in the SAP plus SPIO-liposome group were higher than those in the control group (P<0.01), while the corresponding levels in the SPIO-clodronate-containing liposome group were signi fi cantly lower than those in the SAP plus SPIO-liposome group (P<0.01).
CONCLUSION: Clodronate-containing liposomes protect against liver injury in SAP rats, and SPIO can be used as a tracer for MRI examination following liver injury in SAP rats.
Author Af fi liations: Department of General Surgery (Zhang JX, Dang SC,Zhang Y and Sha X), Department of MRI (Zhang LR and Wei CS), Af fi liated Hospital of Jiangsu University, Zhenjiang 212001, China; School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, China(Chen M and Jiang DL)
Jian-Xin Zhang, Professor, Department of General Surgery, Af fi liated Hospital of Jiangsu University, Zhenjiang 212001, China(Tel: 86-511-85082208; Fax: 86-511-85038661; Email: zhangjx@ujs.edu.cn)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
September 28, 2009
Accepted after revision March 4, 2010
Correction
In the article entitled Clinical management of hepatitis B virus infection correlated with liver transplantation by Zhang et al (Hepatobiliary Pancreat Dis Int 2010;9:15-21.), the Unit of the dose in the Table should be μg.
In the article entitled Contrast-free air cholangiography-assisted unilateral plastic stenting in malignant hilar biliary obstruction by Singh et al (Hepatobiliary Pancreat Dis Int 2010;9:88-92.), the authors of reference 12 should be Singh V, Singh G, Verma GR, Gupta V, Gupta R, Kapoor R, et al for Contrast-free balloon-assisted unilateral plastic stenting in malignant hilar biliary obstruction: a new method. Dig Endosc 2008;20:190-193. The authors of reference 13 should be Bismuth H, Corlette MB for Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 1975;140:170-178.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Delayed hepatocarcinogenesis through antiangiogenic intervention in the nuclear factor-kappa B activation pathway in rats
- Effect of blueberry on hepatic and immunological functions in mice
- Impact of human leukocyte antigen matching on hepatitis B virus recurrence after liver transplantation
- Prognostic models for acute liver failure
- Proteomic analysis of differentially expressed proteins involving in liver metastasis of human colorectal carcinoma
- Protective effects of MCP-1 inhibitor on a rat model of severe acute pancreatitis
