Preoperative assessment of hilar cholangiocarcinoma: combination of cholangiography and CT angiography
2010-12-14ShiAnYuChengZhangJiaMinZhangGenJunMaoLongTangXuXiaoKangWuJinErShuGuangHongLvandZhangDongZheng
Shi-An Yu, Cheng Zhang, Jia-Min Zhang, Gen-Jun Mao, Long-Tang Xu,Xiao-Kang Wu, Jin-Er Shu, Guang-Hong Lv and Zhang-Dong Zheng
Jinhua, China
Preoperative assessment of hilar cholangiocarcinoma: combination of cholangiography and CT angiography
Shi-An Yu, Cheng Zhang, Jia-Min Zhang, Gen-Jun Mao, Long-Tang Xu,Xiao-Kang Wu, Jin-Er Shu, Guang-Hong Lv and Zhang-Dong Zheng
Jinhua, China
(Hepatobiliary Pancreat Dis Int 2010; 9: 186-191)
hilar cholangiocarcinoma;cholangiography;angiography;spiral-computed tomography
Introduction
With recent surgical techniques and devices, the results of surgery for hilar cholangiocarcinoma have improved during the past few years.[1-5]Aggressive surgery for hilar cholangiocarcinoma has been the only means of prolonging survival.[6-9]Precise information on regional anatomy is essential to the improvement of the curative resection rate. The extent of tumor invasion in the bile duct and vessels is one of the most important local factors for determining the resectablity of hilar cholangiocarcinoma. Direct cholangiography remains valuable in the diagnosis of longitudinal ductal in fi ltration of the tumor.[10,11]Multi-detector computed tomography (MDCT) with three-dimensional (3D) angiography has been applied clinically to diagnose the tumor invasion of the hepatic artery and portal vein.[12,13]Cholangiography combined with multi-slice spiral 3D CT angiography can be apotential approach for delineating the local invasion of hilar cholangiocarcinoma more accurately. From March 2007 to August 2009, we evaluated preoperatively the resectablity of hilar cholangiocarcinoma in 13 patients based on cholangiography and multi-slice spiral 3D CT angiography.
MethodsPatients
Based on the clinical manifestations and B-ultrasonography, 16 patients who were diagnosed with hilar cholangiocarcinoma were subjected to cholangiography and multi-slice spiral 3D CT angiography. Three patients were excluded from the study. One patient was con fi rmed with a hilar bile duct stone by cholangiography, and one was diagnosed with cholecyst carcinoma in fi ltration of the hilar duct. Tumor invasion of the hepatic artery and portal vein was shown by CT angiography in another patient but exploration was refused by the patient. The remaining 13 patients who had been operated upon and con fi rmed with cholangiocarcinoma pathologically (6 men and 7 women; mean age 65 years, range 54-79 years) were enrolled in this study. These patients demonstrated jaundice to varying degrees on admission, with a total bilirubin level of 82-428 mol/L and a disease course of one week to three months.
MDCT and 3D imaging
CT images were obtained with a 16-slice CT scanner(lightspeed 16; GE), and reconstructed images were obtained by an AW4.2 workstation. All patients underwent contrast-enhanced dual-phase scans from the diaphragm to the lower edge of the kidney after a general scan(scan parameters: 120 kV, 180-240 mA seconds; spiral pitch, 1.375∶1; rotation velocity, 0.5 s/r; reconstruction thickness, 0.625-1.25 mm). Subsequently, a total of 80 to 100 ml of non-ionic contrast material iohexol (omnipaque)was infused at a rate of 3 ml/s at a concentration of 350 mgI/ml. The delay scanning time of the arterial phase and portal venous phase after initiation of infusion was 25 seconds and 45 to 55 seconds, respectively. The MDCT data set was transferred to a workstation where the interim images were supplemented with different pseudocolors and saved. With 3D software, 3D CT arteriography and CT portography were achieved by multiplanar reformation (MPR), maximum intensity projection,volume rendering (VR), and curved planner reformation(CPR). Percutaneous transhepatic cholangiography (PTC)was performed in 9 patients, endoscopic retrograde cholangiopancreatography (ERCP) in 1, and magnetic resonance cholangiopancreatography (MRCP) in 3.
Stage and type of tumor
Based on the Bismuth-Corlette classi fi cation system,[14]the tumor was classi fi ed into four types: type Ⅰ, no obstruction of the con fl uence of the right and left hepatic ducts; type Ⅱ, limited obstruction to the right or left hepatic ducts; type Ⅲ, extensive obstruction of the right or left ductal rami fi cation; type Ⅳ, extensive obstruction of the ductal rami fi cation bilaterally.Tumor stages were as follows:[15]T1, tumor involving biliary con fl uence±unilateral extension to secondary biliary radicles, no liver atrophy or portal vein involvement; T2, tumor involving biliary con fl uence±unilateral extension to secondary biliary radicles with ipsilateral portal vein involvement±ipsilateral hepatic lobar atrophy, and no main portal vein involvement; T3,tumor involving biliary con fl uence+bilateral extension to secondary biliary radicles, or unilateral extension to secondary biliary radicles with contralateral portal vein involvement, or unilateral extension to secondary biliary radicles with contralateral hepatic lobar atrophy, main or bilateral portal venous involvement.
Judgment of blood vessel and bile duct invasion and resectability
All patients underwent operative explorations, and the operative results served as the gold standard to judge resectability. The judgment of blood vessel and bile duct invasion was based on postoperative pathologic fi ndings in patients whose tumors were removed. Tumor encirclement of the vessel, thickening or ankylosis of the vessel wall, stricture or obstruction of the vessel lumen, and granulation or node of the inner wall were considered to show blood vessel and bile duct invasion in patients whose tumors were not removed. Stage and type judgments were made by two radiologists and one surgeon, and resectability was evaluated preoperatively. The reference criteria for a unresectable tumor[16]were contralateral hepatic artery invasion,main or contralateral portal vein invasion longer than 2 cm, biliary extension to the contralateral secondary con fl uence farther than 2 cm from the hepatic hilum,enlarged lymph nodes at the celiac, portacaval, and paraaortic area as well as other ancillary fi ndings.
ResultsSurgical procedures and fi ndings
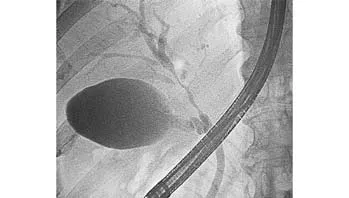
Fig. 1. ERCP showing tumor invasion of the left hepatic duct and level two branch of the right hepatic duct, Bismuth type Ⅳ.

Fig. 2. CT angiography showing the hepatic artery was not invaded.
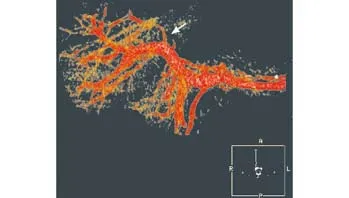
Fig. 3. CT angiography showing the left branch of the portal vein was encased and occluded (arrow).
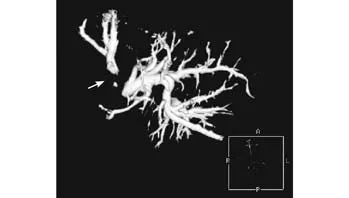
Fig. 4. Positive 3D CT biliary reconstruction: the right posterior hepatic duct was not shown (arrow).
Curative resection of the tumor was made in 7 patients,resection of the common hepatic duct and bifurcation,anastomosis of the left and right hepatic ducts to the jejunum in fi ve patients (Patients 1, 6, 11, 12 and 13) (Table). Quadrate lobectomy, resection of the extrahepatic bile duct and level one right hepatic duct,plasty of the right anterior and right posterior ducts and caudate lobe duct, and anastomosis of the left hepatic duct and shaped right duct to the jejunum were performed in Patient 3. Extensive left hemihepatectomy,plasty of the right anterior and right posterior ducts and caudate lobe hepatic duct, and anastomosis of shaped duct to the jejunum were performed in Patient 9.Palliative resection was made in one patient (Patient 4),and internal or external drainage was performed in fi ve patients. Stages and types were determined according to surgical fi ndings: type Ⅰ in 3 patients, type Ⅱ in 5, type Ⅲa in 2, type Ⅲb in 1, and type Ⅳ in 2. With regard to T-staging, T1 in 5 patients, T2 in 2, and T3 in 6 were also determined.
Imaging fi ndings
Nine patients underwent PTC: type Ⅰ in 2 patients,type Ⅱ in 3, type Ⅲa in 2, type Ⅲb in 1, and type Ⅳin 1. One patient underwent ERCP and was diagnosed with type Ⅳ, and 3 underwent MRCP, which con fi rmed a diagnosis of type Ⅰ in 2 patients and type Ⅱ in 1. In these patients MDCT revealed that reconstructions of the hepatic artery and portal vein were successful.Hepatic artery invasion was observed in 4 patients and portal vein invasion in 5. T1 was in 6 patients, T2 in 1,and T3 in 6.
Combined imaging evaluation
Imaging fi ndings were consistent with surgical fi ndings in 4 patients with hepatic artery invasion, and in 5 of the 6 patients with portal vein invasion. In 12 of the 13 patients, the preoperative Bismuth type and T-staging based on combined imaging were consistent with operative exploration fi ndings. Seven of 8 patients who had been estimated to be suitable for an operation by imaging were curatively treated, and one (Patient 5) underwent internal drainage because of peritoneal dissemination of the tumor. Five patients who had been found unsuitable for curative surgery by cholangiographyand CT angiography underwent palliative procedures.In patient 4, ERCP showed tumor in fi ltration of the left hepatic duct and level two right hepatic duct (Fig. 1),and CT angiography showed tumor in fi ltration of the left branch of the portal vein but the hepatic artery was normal (Figs. 2 and 3). This patient underwent extensive left hemihepatectomy, plasty of the right anterior and right posterior ducts and caudate lobe duct, and anastomosis of shaped duct to the jejunum. However,pathologic examination revealed a positive margin of the right anterior hepatic duct, and palliative resection was considered for the patient.
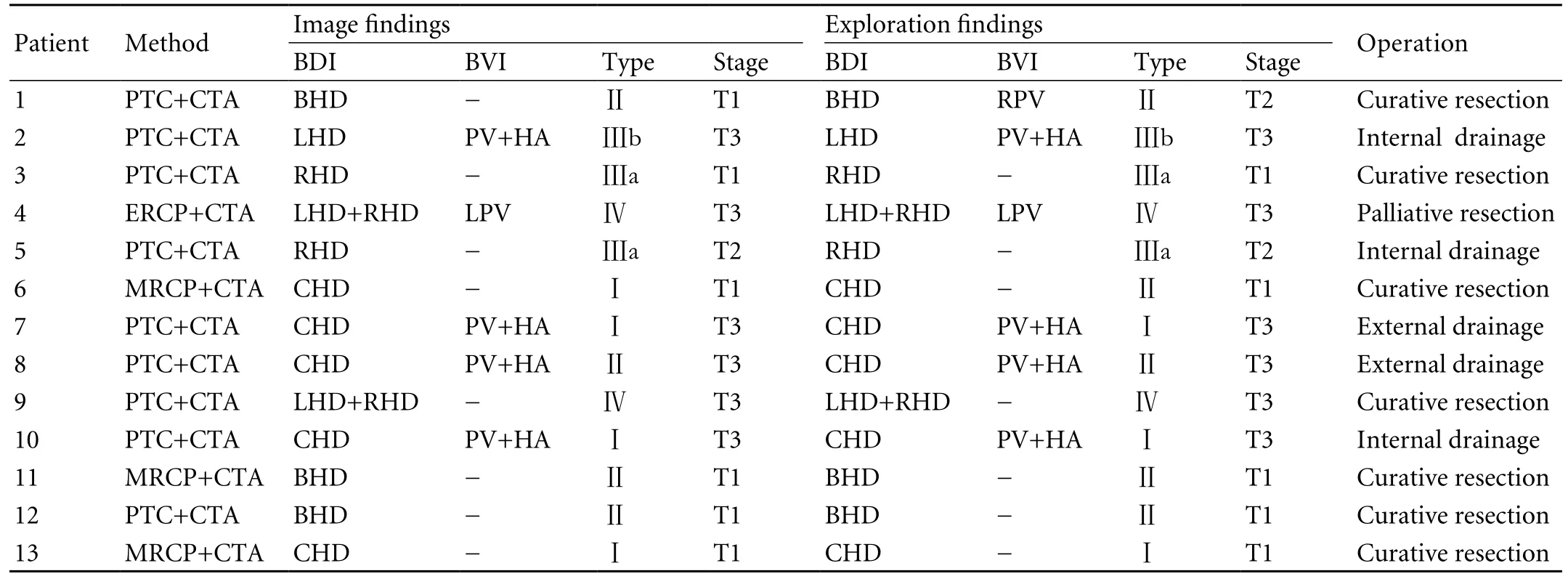
Table. Evaluation of combined imaging for hilar cholangiocarcinoma
Discussion
Curative resection is one of the most important predictive factors in the treatment of cholangiocarcinoma.[1,17,18]Yet the curative resection rate in recent reports remains unsatisfactory.[2,19]The improvement of the treatment of hilar cholangiocarcinoma requires accurate preoperative evaluation, appropriate planning of surgical procedures,and performance of the procedures. Compared with traditional imaging methods, combined cholangiography and CT angiography will provide surgeons with accurate information about complex cases.
The factors concerning the radical resection of hilar cholangiocarcinoma include 1) systemic factors such as surgery not being tolerated because of organ dysfunction or coagulation dysfunction; 2) distant metastasis or peritoneal dissemination of the tumor; and 3) extensive local invasion of the tumor. The Bismuth type and T-stage classi fi cations are widely used in China and abroad to determine the resectability of cholangiocarcinoma.Invasion of liver parenchyma and liver atrophy can be detected more easily by 2D CT. However, to determine the invasion of blood vessels and bile ducts, further diagnostic imaging techniques are often needed. Direct cholangiography including ERCP and PTC has been used to determine tumor in fi ltration along the bile duct and is considered an important index for the diagnosis of vertical invasion of the tumor. MRCP has also been widely used in clinical cases. Despite the advantages and disadvantages of each of the three techniques,their values are similar in diagnosis of the Bismuth type.[11,20]Patients with hilar cholangiocarcinoma often demonstrate jaundice to varying degrees when they seek medical intervention, and some of them need preoperative biliary drainage. Endoscopic nasobiliary drainage (ENBD) can be performed after ERCP when needed or percutaneous transhepatic cholangial drainage(PTCD) after PTC. Therefore, direct cholangiography is adopted more commonly. Kloek et al[21]compared the outcomes of ENBD and PTCD in patients with resectable hilar cholangiocarcinomas and found that infectious complications were more common in the ENBD patients.In addition, when there is complete obstruction in the hilar bile duct, the biliary tree above the obstruction cannot be shown by ERCP, PTC or PTCD. These indicate why PTC was used more commonly in this study.
Vertical assessment of tumor invasion is another crucial aspect for staging hilar cholangiocarcinomas because vascular involvement is the most frequent criterion for the unresectability of the tumor. 2D MDCT images including an axial image and MPR can reveal the density of the tumor, the extent of invasion and peritoneal metastasis, but 3D images can visualize the involvement of the hepatic artery and portal vein as well as the extent and length of the involved vasculature,thus providing more accurate information about the relationship between the hilar cholangiocarcinoma and adjacent vessels.[22]
At present, Bismuth type for hilar cholangiocarcinoma is widely used, while the T-stage is more conducive to the judgment of the resectability of hilar cholangiocarcinoma[23]because the invasion of the portal vein and liver lobe is taken into account. Of the 13 patients in our study (PTC was performed in 9 patients, ERCP in 1, and MRCP in 3), 12 (92.3%)had Bismuth classi fi cation consistent with the results of surgical exploration. Patient 6 underwent MRCP and had the preoperative Bismuth classi fi cation for type Ⅰ. Biopsy revealed a tumor in fi ltrating the hepatic duct con fl uence (Bismuth classi fi cation, type Ⅱ). This underestimation may be due to tumor in fi ltration along the bile duct wall, but the duct lumen is not narrow or obstructed. All of the 13 patients underwent a successful 3D reconstruction of the hepatic artery and portal vein. Four patients were diagnosed with hepatic artery involvement, and 5 patients with portal vein involvement. The fi ndings of CT angiography were consistent with those of surgical exploration in 4 patients with hepatic artery invasion; in 6 patients with portal vein invasion, only 5 patients (83.3%) had the similar fi ndings. In Patient 1, a wedge resection was performed for the vascular wall invasion of the right branch of the portal vein, and no vascular abnormalities were shown by 3D CT imaging. Preoperative T-stages de fi ned by joint imaging were consistent with those de fi ned by surgical exploration in 12 (92.3%) of the 13 patients. Furthermore, the exception was patient 1 who had the invasion of the right branch of the portal vein wall and whose preoperative stage was T1 and postoperative stage was T2 (Table).
Cholangiography combined with multi-slice spiral CT and 3D angiography can be a more accurate method for assessing the resectability of hilar cholangiocarcinoma.Lee et al[16]reported the criterion for unresectable tumor,in their series of 5 patients who could not be given radical resection. During surgical exploration, patients 2 and 10 underwent internal drainage, and patients 7 and 8,external drainage. Despite radical resection, the incision margin of the right hepatic duct was pathologically positive in patient 4, but the patient received palliative resection. Joint imaging predicted successfully the 5 patients who could not sustain a radical resection(speci fi city 100%). The tumor was estimated to be removable by imaging in another 8 patients, and 7 of them underwent a radical resection (sensitivity 87.5%).Patient 5 showed peritoneal metastasis during operation and underwent internal drainage.
Multi-slice spiral CT is limited in diagnosis of peritoneal metastasis. It has been reported that, after contrast agent fi lls the biliary tract, the positive image of 3D CT can show the biliary tree more perfectly. We found that the advantages of this method vary from non-overlapping of two-dimensional images, a dynamic rotation of 360°, clear demonstration of the biliary tree to localization of the relationship between branches. The positive 3D biliary reconstruction relies on the fi lling of a contrast agent in the bile duct. When the fi lling is not complete under PTC/PTCD or ERCP/ENBD, the 3D imaging of the biliary tree is also incomplete (Fig. 4).
In conclusion, combined cholangiography and 3D CT angiography can show the local invasion of hilar cholangiocarcinoma, improve the assessment of the resectability of the tumor, and make treatment planning and surgical options perfect. However, this study has limitations due to the small number of cases.
Funding: None.
Ethical approval: Not needed.
Contributors: ZJM proposed the study. YSA wrote the fi rst draft. ZC analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. ZJM is the guarantor.
Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC,Bodniewicz BS J, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001;234:507-519.
2 Lee SG, Lee YJ, Park KM, Hwang S, Min PC. One hundred and eleven liver resections for hilar bile duct cancer. J Hepatobiliary Pancreat Surg 2000;7:135-141.
3 Lygidakis NJ, Sgourakis GJ, Dedemadi GV, Vlachos L,Sa fi oleas M. Long-term results following resectional surgery for Klatskin tumors. A twenty-year personal experience.Hepatogastroenterology 2001;48:95-101.
4 Munoz L, Roayaie S, Maman D, Fishbein T, Sheiner P, Emre S, et al. Hilar cholangiocarcinoma involving the portal vein bifurcation: long-term results after resection. J Hepatobiliary Pancreat Surg 2002;9:237-241.
5 Lygidakis NJ, Singh G, Bardaxoglou E, Dedemadi G, Sgourakis G, Nestoriois J, et al. Changing trends in the management of Klatskin tumor. Hepatogastroenterology 2004;51:689-696.
6 Lai EC, Lau WY. Aggressive surgical resection for hilar cholangiocarcinoma. ANZ J Surg 2005;75:981-985.
7 Otto G, Romaneehsen B, Hoppe-Lotichius M, Bittinger F.Hilar cholangiocarcinoma: resectability and radicality after routine diagnostic imaging. J Hepatobiliary Pancreat Surg 2004;11:310-318.
8 Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg 2000;7:155-162.
9 Kondo S, Hirano S, Ambo Y, Tanaka E, Okushiba S,Morikawa T, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg 2004;240:95-101.
10 Vogl TJ, Schwarz WO, Heller M, Herzog C, Zangos S, Hintze RE, et al. Staging of Klatskin tumours (hilar cholangiocarcinomas): comparison of MR cholangiography,MR imaging, and endoscopic retrograde cholangiography.Eur Radiol 2006;16:2317-2325.
11 Manfredi R, Brizi MG, Masselli G, Vecchioli A, Marano P. Malignant biliary hilar stenosis: MR cholangiography compared with direct cholangiography. Radiol Med 2001;102:48-54.
12 Sugiura T, Nishio H, Nagino M, Senda Y, Ebata T, Yokoyama Y, et al. Value of Multidetector-row Computed Tomography in Diagnosis of Portal Vein Invasion by Perihilar Cholangiocarcinoma. World J Surg 2008;32:1478-1484.
13 Park HS, Lee JM, Choi JY, Lee MW, Kim HJ, Han JK, et al.Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am J Roentgenol 2008;190:396-405.
14 Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992;215:31-38.
15 Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg 1998;228:385-394.
16 Lee HY, Kim SH, Lee JM, Kim SW, Jang JY, Han JK, et al.Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology 2006;239:113-121.
17 Yubin L, Chihua F, Zhixiang J, Jinrui O, Zixian L, Jianghua Z, et al. Surgical management and prognostic factors of hilar cholangiocarcinoma: experience with 115 cases in China.Ann Surg Oncol 2008;15:2113-2119.
18 Forsmo HM, Horn A, Viste A, Hoem D, Ovrebo K. Survival and an overview of decision-making in patients with cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2008;7:412-417.
19 Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S,Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg 2003;238:84-92.
20 Choi JY, Kim MJ, Lee JM, Kim KW, Lee JY, Han JK, et al.Hilar cholangiocarcinoma: role of preoperative imaging with sonography, MDCT, MRI, and direct cholangiography. AJR Am J Roentgenol 2008;191:1448-1457.
21 Kloek JJ, van der Gaag NA, Aziz Y, Rauws EA, van Delden OM, Lameris JS, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg 2010;14:119-125.
22 Endo I, Shimada H, Sugita M, Fujii Y, Morioka D, Takeda K, et al. Role of three-dimensional imaging in operative planning for hilar cholangiocarcinoma. Surgery 2007;142:666-675.
23 Chen RF, Li ZH, Zhou JJ, Wang J, Chen JS, Lin Q, et al.Preoperative evaluation with T-staging system for hilar cholangiocarcinoma. World J Gastroenterol 2007;13:5754-5759.
BACKGROUND: Hilar cholangiocarcinoma is one of the most dif fi cult carcinomas to manage because of the location of the main tumor at the hepatic hilus and the complex anatomy of the biliary, arterial, and portal systems. To plan an operation,it is important to acquire accurate information about the relationship between hilar cholangiocarcinoma and adjacent vessels. This study aimed to evaluate the clinical value of cholangiography combined with spiral CT three-dimensional(3D) angiography for a preoperative assessment of hilar cholangiocarcinoma.
METHODS: From March 2007 to August 2009, cholangiography was performed in 13 patients with hilar cholangiocarcinoma.Meanwhile, contrast-enhanced abdominal scanning was performed using 16-slice spiral CT, and the 3D images of the hepatic artery and portal vein were acquired. The level and range of invasion of the hepatic artery, the portal vein, and the bile duct, the preoperative Bismuth classi fi cation, and T-staging were recorded and compared with those after surgical exploration.
RESULTS: The hepatic artery and portal vein were reconstructed successfully in all these patients. Percutaneous transhepatic cholangiography was performed in 9 patients,endoscopic retrograde cholangiopancreatography in 1, and magnetic resonance cholangiopancreatography in 3. The CT angiography records of invasion of the hepatic artery were consistent with the results of explorations in these patients.The data from 5 of the 13 patients were consistent with those on invasion of the portal vein. The results of the Bismuth classi fi cation and the T-staging system were consistent with those of surgical exploration in 12 of the 13 patients. Seven of 8 patients who were estimated to be suitable for operation based on images were curatively treated and 5 who were judged to be unsuitable for curative operation by cholangiography and CT angiography were con fi rmed intraoperatively and underwent palliative procedures.
CONCLUSIONS: Cholangiography combined with multi-slice spiral 3D CT angiography can satisfactorily delineate the local invasion of hilar cholangiocarcinoma and accurately evaluate the resectability. This approach, therefore, contributes to the planning of safe operation.
Author Af fi liations: Department of General Surgery, Jinhua Central Hospital,Jinhua 321000, China (Yu SA, Zhang JM, Mao GJ, Xu LT, Wu XK, Shu JE, Lv GH and Zheng ZD); Wenzhou Medical College, Wenzhou 325035, China(Zhang C)
Jia-Min Zhang, MD, Department of General Surgery,Jinhua Central Hospital, Jinhua 321000, China (Tel: 86-579-82552710; Fax:86-579-2318024; Email: zhangjiamin600323@126.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
October 15, 2009
Accepted after revision March 13, 2010
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Protective effects of MCP-1 inhibitor on a rat model of severe acute pancreatitis
- Extrahepatic right hepatic duct diverticulum:a rare entity
- MRI shows clodronate-liposomes attenuating liver injury in rats with severe acute pancreatitis
- Surgical therapy and prognosis of sarcomatoid carcinoma of the gallbladder
- Small-duct primary sclerosing cholangitis with hepatocellular carcinoma requiring liver transplantation
- Impact of human leukocyte antigen matching on hepatitis B virus recurrence after liver transplantation
