aMAP评分联合RAR及PIV构建慢性肝病患者肝细胞癌发生风险的预测模型
2025-02-15蒋晓涵曹杰刘丹丹薛丹郭志国



摘要:目的基于aMAP评分联合RAR及PIV构建并验证慢性肝病患者肝细胞癌(HCC)发生风险的预测模型。方法143例慢性肝病患者按照是否发生HCC分为HCC组32例及非HCC组111例,比较2组一般临床资料、aMAP评分及外周血指标水平。采用多因素Logistic回归分析住院慢性肝病患者发生HCC的影响因素,构建并验证列线图风险预测模型。结果与非HCC组比较,HCC组年龄大、男性比例高,总胆红素(TBIL)、红细胞分布宽度(RDW)、中性粒细胞计数(NEU)、单核细胞计数(MON)、aMAP评分、RDW与ALB比值(RAR)、泛免疫炎症值(PIV)水平高,白蛋白(ALB)、淋巴细胞计数(LYM)水平低(P<0.05)。多因素Logistic回归分析显示,较高aMAP评分、RAR、PIV是住院慢性肝病患者HCC风险的独立危险因素(P<0.05);据此构建的列线图风险预测模型受试者工作特征(ROC)曲线的曲线下面积(AUC)为0.823(95%CI:0.747~0.899),校准曲线显示预测值与实际观测值基本一致,Brier得分为0.125,决策曲线显示该模型具有明显的正向效益,Bootstrap法对预测模型进行内部验证的AUC为0.823(95%CI:0.820~0.825),提示模型具有良好的区分度。结论aMAP评分联合RAR及PIV构建的慢性肝病患者发生HCC的列线图风险预测模型预测性能良好,有助于指导个体化治疗及随访。
关键词:癌,肝细胞;列线图;ROC曲线;aMAP评分;慢性肝病;红细胞分布宽度与白蛋白比值;泛免疫炎症值中图分类号:R735.7文献标志码:A DOI:10.11958/20241112
Constructing a risk prediction model for hepatocellular carcinoma in patients with chronic liverdisease based on aMAP score combined with RAR and PIV
JIANG Xiaohan,CAO Jie△,LIU Dandan,XUE Dan,GUO Zhiguo
Department of Gastroenterology,Suzhou Hospital of Anhui Medical University,Suzhou Municipal Hospital ofAnhui Province,Suzhou 234000,China
△Corresponding Author E-mail:caojiewen@126.com
Abstract:Objective To construt and validate a risk prediction model for hepatocellular carcinoma(HCC)inpatients with chronic liver disease based on age-male-ALBI-platelets(aMAP)score combined with RAR and PIV.Methods A total of 143 patients with chronic liver disease were divided into the HCC group(32 cases)and the non-HCC group(111 cases)according to whether HCC occurred.General clinical data,aMAP score and peripheral blood indicator level were compared between two groups.Multivariate Logistic regression was used to analyze influencing factors of HCC in inpatients with chronic liver disease.A nomogram risk prediction model was constructed and validated.Results Compared with the non-HCC group,there were higher age,higher proportion of males,higher levels of total bilirubin(TBIL),red blood cell distribution width(RDW),neutrophil count(NEU)and monocyte count(MON),lower levels of albumin(ALB)and lymphocyte count(LYM),higher levels of aMAP score,RDW to ALB(RAR)and pan-immune inflammation value(PIV)in the HCC group(P<0.05).Multivariate Logistic regression showed that higher levels of aMAP score,RAR and PIV were independent risk factors for HCC in inpatients with chronic liver disease(P<0.05).The area under receiver operator characteristic(ROC)curve(AUC)of the nomogram risk prediction model constructed based on above factors was 0.823(95%CI:0.747-0.899).The calibration curve showed that the predicted value was basically consistent with the actual observed value,and the Brier score was 0.125.The decision curve showed that the model had a clear positive net benefit.The AUC of internal validation of the prediction model by Bootstrap method was 0.823(95%CI:0.820-0.825),indicating that the model had a good degree of differentiation.Conclusion The nomogram risk prediction model based on aMAP score,RARand PIV showed a good predictive performance of HCC in patients with chronic liver disease,which could benefits the individualized treatment and follow-up.
Key words:carcinoma,hepatocellular;nomograms;ROC curve;aMAP score;chronic liver disease;RAR;PIV
肝癌居全球常见恶性肿瘤第6位,我国2022年原发性肝癌新增36.8万例,死亡31.7万例,分别占全球病例的42.4%与41.7%[1]。肝细胞癌(hepatocellular carcinoma,HCC)是主要的肝癌类型,HCC的5年生存率与癌症早期诊断率相关,然而目前我国HCC早期诊断率较低,总体中位生存期仅23个月,5年生存率不足14.1%[2-3]。另外,HCC治疗难度大、费用高。因此,如何提高HCC早期诊断率是目前亟待解决的重要问题。
慢性肝病人群是我国HCC的高危人群。对HCC风险人群进行风险评分,继而行分层管理可有效提升HCC早期诊断率和HCC风险人群获益率[4]。aMAP评分(age-male-ALBI-platelets score)由年龄、性别、外周血总胆红素(total bilirubin,TBIL)、白蛋白(albumin,ALB)及血小板计数(platelet count,PLT)5项指标构成,适用于HCC的风险预测[5],可作为基层医院及门诊慢性肝病患者HCC筛查的简便管理工具[6-7]。新型炎症标志物在肿瘤发生、发展及预后评估中发挥重要作用。研究表明,红细胞分布宽度(red blood cell distribution width,RDW)与ALB比值(RDW to ALB ratio,RAR)升高是癌症全因死亡率增加的独立预测因子[8]。泛免疫炎症值(pan-immune inflammation value,PIV)是一种由PLT、中性粒细胞计数(neutrophil count,NEU)、单核细胞计数(monocyte count,MON)、淋巴细胞计数(lymphocyte count,LYM)通过计算形成的复合指标,综合考虑了多种免疫和炎症相关因素。Liang等[9]研究显示,PIV可评估早期HCC患者接受根治性射频消融术后的无复发生存期和总生存期。然而,慢性肝病患者病情相对较重、合并症多,上述指标单独检测时对患者发生HCC风险的预测效能仍有限。本研究旨在建立并验证基于aMAP评分联合新型炎症指标的慢性肝病患者发生HCC风险的预测模型,以期提高对HCC高风险人群的识别率,辅助临床医师诊断和决策。
1对象与方法
1.1研究对象随机抽取2019年10月—2023年12月在安徽省宿州市立医院住院治疗的慢性肝病患者143例,按照是否发生HCC分为HCC组32例和非HCC组111例。纳入标准:(1)年龄≥18岁;(2)HCC患者均存在慢性肝病史,同时符合《原发性肝癌诊疗指南(2022年版)》诊断标准(所有入组HCC患者重新按照2022年版标准诊断);(3)慢性肝病包括慢性乙型病毒性肝炎(chronic hepatitis B,CHB)、慢性丙型病毒性肝炎(chronic hepatitis C,CHC)、酒精性或非酒精性脂肪性肝病、自身免疫性肝病及上述或相关原因导致的肝硬化;(4)一般临床信息、血常规及肝功能等实验室资料完整。排除标准:(1)不配合正常诊疗;(2)精神异常;(3)合并其他系统原发肿瘤。本研究经安徽省宿州市立医院伦理委员会批准(批号A2023016)。
1.2研究方法
1.2.1一般临床资料通过住院病历系统收集患者的年龄、性别、吸烟史(≥20支/d,连续吸烟超过5年)、饮酒史(折合乙醇量男性≥40 g/d,女性≥20 g/d,连续饮酒>5年)、合并症(高血压、糖尿病史)等一般临床资料。
1.2.2实验室指标收集患者入院时的外周血TBIL、ALB、PLT、RDW、NEU、LYM、MON。计算aMAP评分、RAR、PIV,其中aMAP评分={[0.06×年龄+0.89×性别(男性:1;女性:0)+0.48×(log10 TBIL×0.66+ALB×-0.085)-0.01×PLT]+7.4}/14.77×100,RAR=RDW/ALB,PIV=(PLT×NEU×MON)/LYM。
1.3统计学方法采用SPSS 29.0及R软件分析数据。正态分布的计量资料以x±s表示,2组间比较采用独立样本t检验,偏态分布以M(P25,P75)表示,2组间比较采用秩和检验。计数资料以例或例(%)表示,组间比较采用χ2检验;影响因素分析采用Logistic回归分析,根据回归分析结果构建列线图预测模型。绘制受试者工作特征(ROC)曲线并计算曲线下面积(AUC)分析各指标及预测模型对住院慢性肝病患者发生HCC的预测价值,预测模型的内部验证应用Bootstrap法(抽样1 000次),校准曲线评价模型校准度,决策曲线评价模型临床效益。P<0.05为差异有统计学意义。
2结果
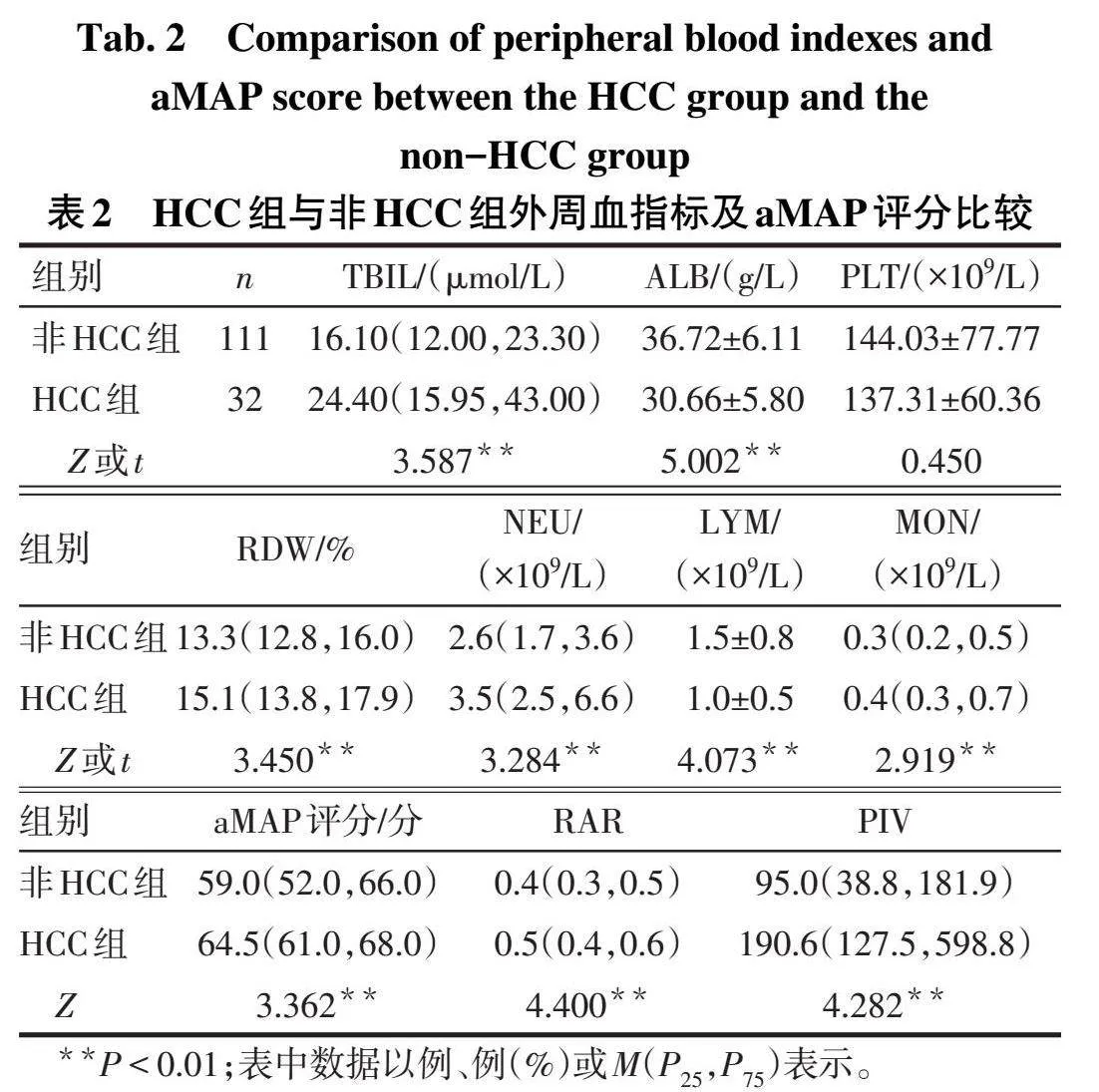
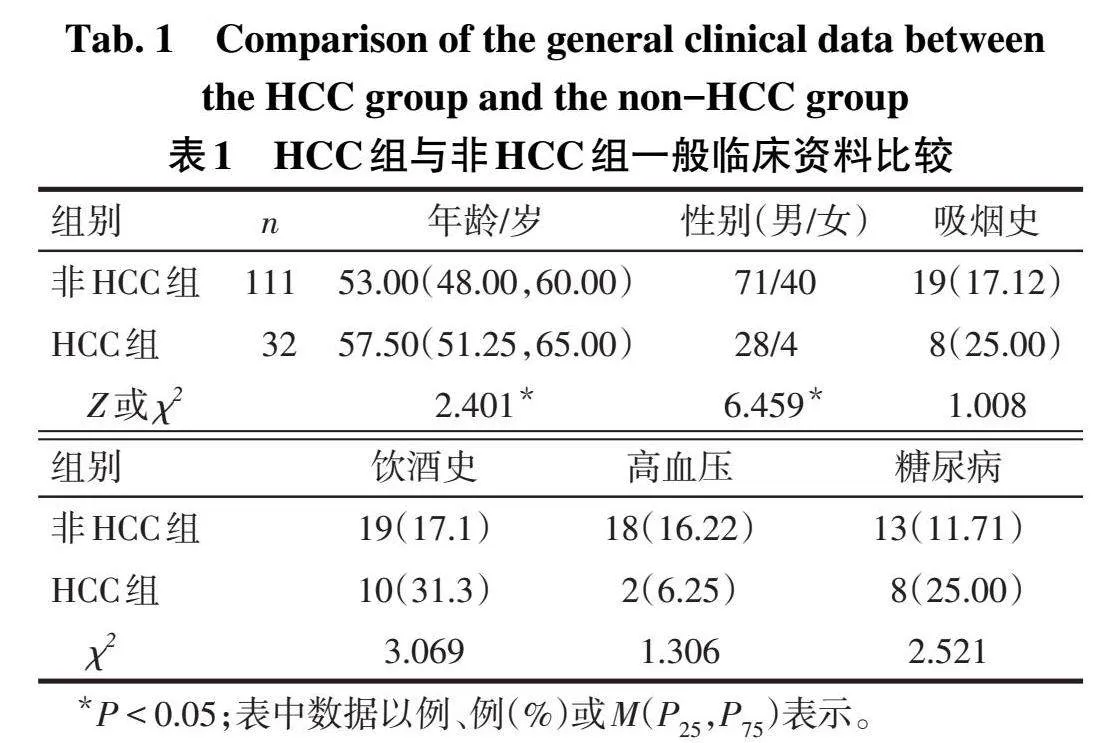
2.1 2组一般临床资料比较与非HCC组比较,HCC组年龄大、男性比例高(P<0.05),2组吸烟史、饮酒史、高血压、糖尿病等基础疾病差异无统计学意义(P>0.05),见表1。
2.2 2组外周血指标及aMAP评分比较与非HCC组比较,HCC组TBIL、RDW、NEU、MON、aMAP评分、RAR、PIV升高,而ALB、LYM水平降低(Plt;0.01),2组PLT差异无统计学意义,见表2。
2.3 HCC影响因素分析以aMAP评分、RAR、PIV为自变量,以是否发生HCC(否=0,是=1)为因变量,进行Logistic回归分析。结果显示,较高aMAP评分、RAR、PIV是住院慢性肝病患者发生HCC的独立危险因素(P<0.05),见表3。
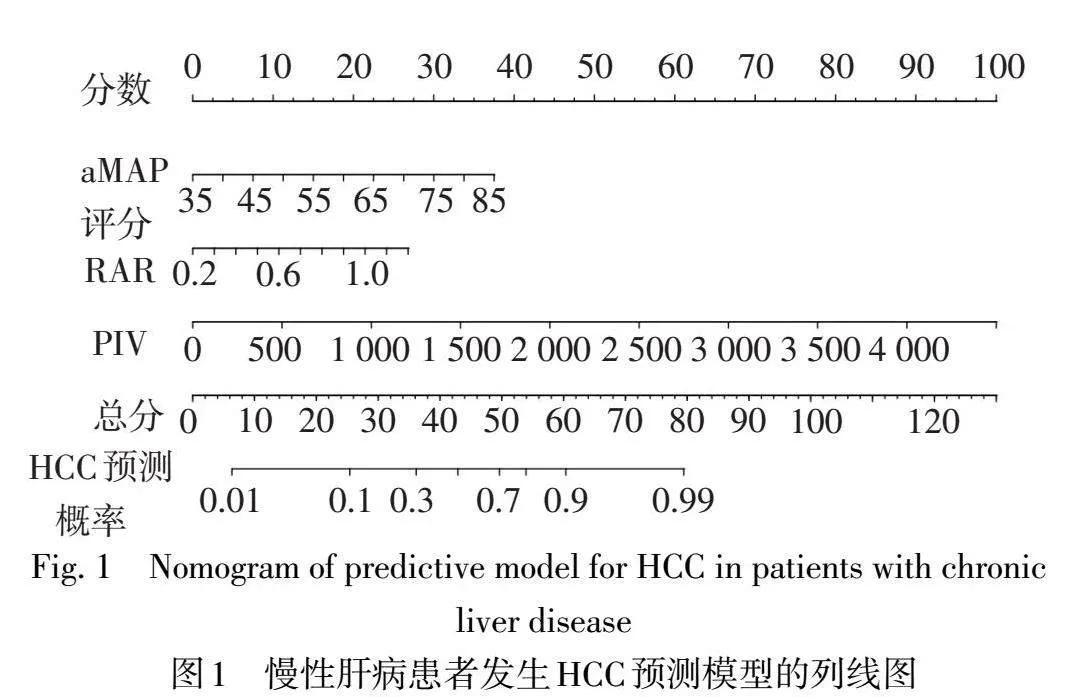
2.4慢性肝病患者发生HCC的列线图预测模型构建据Logistic回归分析结果构建预测模型的回归方程为Logit(P)=0.094×aMAP评分+3.419×RAR+0.003×PIV-9.363,绘制慢性肝病患者发生HCC的列线图,见图1。
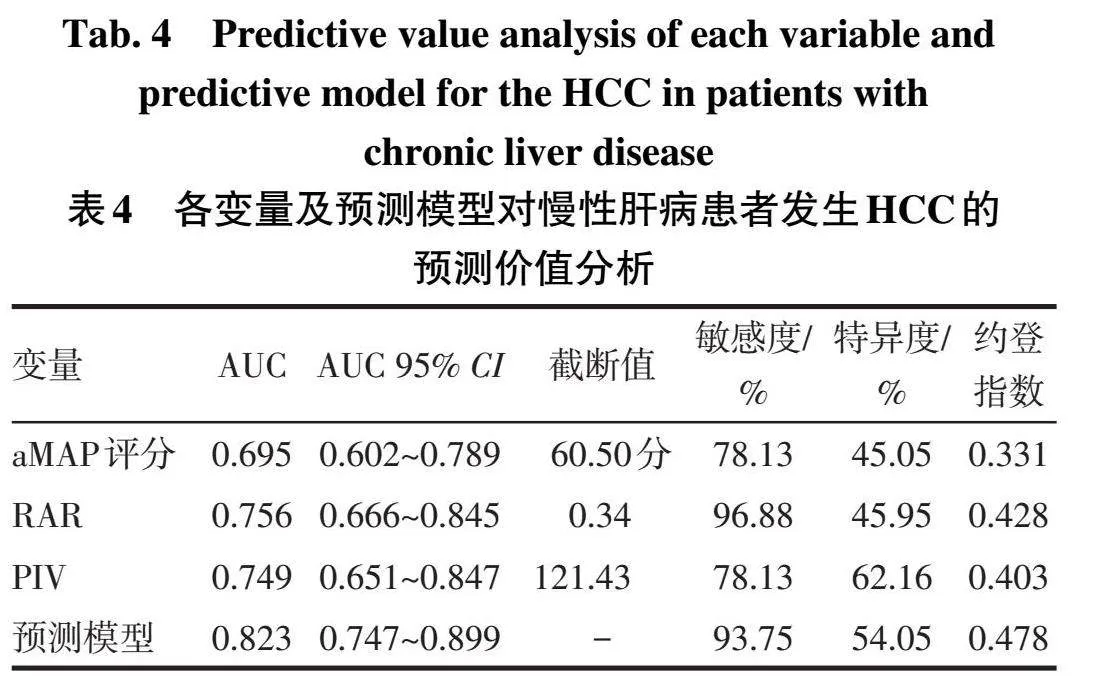
2.5列线图预测模型的评价与验证以aMAP评分、RAR、PIV及联合检测预测模型为检验变量,是否发生HCC(是=1,否=0)为状态变量,绘制ROC曲线。结果显示列线图预测模型具有较好的预测能力,其预测住院慢性肝病患者发生HCC的效能高于aMAP评分和RAR单独检测(Z分别为3.044和2.199,均P<0.05),与PIV比较差异无统计学意义(Z=1.446,P=0.148),见表4、图2。校准曲线示,预测值与实际观测值基本一致,Brier得分为0.125,说明模型预测的准确度较好,见图3A;决策曲线示,模型具有明显的正向净效益,有临床实用价值,见图3B。运用Bootstrap法对预测模型进行内部验证,内部验证的AUC为0.823(95%CI:0.820~0.825),提示模型具有良好的区分度。
3讨论
肝硬化和未抗病毒治疗的CHB等慢性肝病是导致肝癌的主要原因[4]。研究显示,85%~95%的肝癌患者曾患有肝硬化[10],而86%以上的肝硬化患者由CHB所致[11]。因此,患有慢性肝病者,尤其是CHB导致的肝硬化患者是临床需要重点关注的目标人群。
aMAP评分是近年新开发的预测HCC发生风险的评分系统,包含年龄、性别、TBIL、ALB、PLT五项指标。CHB由乙型肝炎病毒(hepatitis B virus,HBV)感染所致,研究显示,母婴传播是HBV传播的重要途径,大部分HBV感染发生在婴儿期,呈慢性感染状态,病毒复制持续活跃,因此CHB所致HCC发病风险随年龄增加呈上升趋势[12]。Liu等[13]研究亦证实,CHB发生HCC风险随年龄增加而增加。研究显示,我国男性HCC的发病率约是女性的3倍[14],原因可能为男性有饮酒等不良习惯者较多,女性X染色体富含免疫相关基因且性激素的免疫调节作用也产生了影响[15]。TBIL是结合胆红素与未结合胆红素的总和,为血红素分解代谢的最终产物,可反映肝脏的清除代谢功能。ALB由肝细胞合成,可反映肝脏的合成功能及营养状态。ALB、TBIL是aMAP评分的重要部分,可体现肝脏的储备功能,广泛用于HCC治疗后的预后价值评估[16-17]。PLT是骨髓成熟的巨核细胞胞质裂解脱落的小块胞质,参与肿瘤微环境的形成,可用于早期癌症检测、疾病进展监测及肿瘤预后评估[18-19]。因此,包含以上指标的aMAP评分可用于预测HCC的发生。Innes等[20]研究表明,aMAP评分对肝硬化和已治愈的丙型肝炎病毒(hepatitis C virus,HCV)感染患者发生HCC风险的预测性能较其他预测模型更好。Yamashita等[21]研究也表明,aMAP评分可用于预测达到持续病毒学反应的HCV患者发生HCC的风险程度。本研究结果亦证实,与非HCC组比较,HCC组年龄、男性占比及TBIL水平高,ALB水平低,但2组PLT水平无差异,考虑原因为本研究中纳入的均是住院患者,病情相对重所致。
RAR是RDW与ALB的比值,为一种新型炎症标志物。RDW反映了外周血中红细胞体积异质性,可反映机体的炎症程度,其升高通常与多种肝脏疾病相关。研究显示,RDW与CHB的严重程度呈正相关,可以独立预测CHB相关肝硬化的长期预后[22]。RDW也与HCC肿瘤大小有关,是预测HBV相关HCC患者生存和预后的潜在有价值的血液学标志物[23-25]。因此,RAR可同时反映机体的炎症和营养状况,能更全面地反映疾病的发展状态。PIV是以外周血NEU、MON、PLT和LYM为基础的新型标志物,可反映机体全身免疫炎症水平。NEU是全身及局部炎症过程的关键介质,参与了肝癌发生发展和转移的多个环节,通过产生和释放促血管生成的因子、蛋白水解酶及多种细胞因子来刺激肿瘤增殖和转移[26]。研究显示,NEU与采用手术或局部治疗的肝癌患者的高肿瘤负荷、血管侵犯、肝外转移和甲胎蛋白水平存在显著相关性[27]。LYM在肿瘤免疫循环中发挥核心作用,低LYM是肿瘤预后不良的预测因素[28]。MON可发挥吞噬作用和免疫调节作用。研究表明,高水平NEU、MON及低水平LYM的HCC患者总生存期降低、疾病进展较快[27]。本研究结果显示,较高aMAP评分、RAR、PIV是影响住院慢性肝病患者HCC风险的独立危险因素,三者联合检测预测HCC的效能较高,证实了上述指标联合检测对HCC的发生有一定的预测价值,且与aMAP评分、RAR、PIV任一指标单独检测的诊断效能相比,提高了AUC或敏感度。本研究进一步将多因素Logistic回归分析得到的影响因素构建慢性肝病患者发生HCC的风险预测模型,并以列线图的形式可视化呈现,该预测模型在预测慢性肝病患者发生HCC的AUC较高,校准曲线示预测值与实际观测值基本一致,模型具有良好的准确度,决策曲线示模型具有明显的正向净效益,预测模型内部验证提示模型具有良好的区分度。此外,模型中所纳入的指标检测成本低、易获得,便于临床评估;因此,笔者认为临床中可通过该列线图评估住院慢性肝病患者发生HCC的风险,简单、便捷,实用性强。
综上所述,aMAP评分联合RAR、PIV构建的慢性肝病患者发生HCC的风险预测模型预测性能良好,有助于识别HCC高风险人群以制定个体化治疗及随访策略,具有临床应用价值。然而,由于本研究为回顾性研究,所纳入病例数偏少、为单中心研究,存在选择偏倚问题,并且有待开展外部验证。
参考文献
[1]LI Q,DING C,CAO M,et al.Global epidemiology of liver cancer 2022:an emphasis on geographic disparities[J].Chin Med J(Engl),2024,137(19):2334-2342.doi:10.1097/CM9.0000000000003264.
[2]PARK J W,CHEN M,COLOMBO M,et al.Global patterns of hepatocellular carcinoma management from diagnosis to death:the BRIDGE Study[J].Liver Int,2015,35(9):2155-2166.doi:10.1111/liv.12818.
[3]ALLEMANI C,MATSUDA T,DI CARLO V,et al.Global surveillance of trends in cancer survival 2000-14(CONCORD-3):analysis of individual records for 37513025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries[J].Lancet,2018,391(10125):1023-1075.doi:10.1016/S0140-6736(17)33326-3.
[4]杨永平,卢实春.原发性肝癌的分层筛查与监测指南(2020版)[J].中华肝脏病杂志,2021,29(1):25-40.YANG YP,LU S C.Guideline for stratified screening and surveillance of primary liver cancer(2020 Edition)[J].Chinese Journal of Hepatology,2021,29(1):25-40.doi:10.3760/cma.j.cn112152-20201109-00970.
[5]FAN R,PAPATHEODORIDIS G,SUN J,et al.aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis[J].J Hepatol,2020,73(6):1368-1378.doi:10.1016/j.jhep.2020.07.025.
[6]李秀华,郝新,邓永红,等.应用aMAP评分评估基层医院慢性肝病人群的肝癌发生风险[J].中华肝脏病杂志,2021,29(4):332-337.LI X H,HAO X,DENG Y H,et al.Application of aMAP score to assess the risk of hepatocarciongenesis in population of chronic liver disease in primary hospitals[J].Chin J Hepatol,2021,29(4):332-337.doi:10.3760/cma.j.cn501113-20210329-00144.
[7]王丽旻,张鸿飞,甘雨,等.aMAP评分评估门诊慢性HBV感染者肝细胞癌风险的价值分析[J].临床肝胆病杂志,2022,38(10):2242-2246.WANG L M,ZHANG H F,GAN Y,et al.Value of aMAP score in prediction of hepatocellular carcinoma risk in outpatients with chronic hepatitis B virus infection[J].J Clin Hepatol,2022,38(10):2242-2246.doi:10.3969/j.issn.1001-5256.2022.10.009.
[8]LU C,LONG J,LIU H,et al.Red blood cell distribution width-to-albumin ratio is associated with all-cause mortality in cancer patients[J].J Clin Lab Anal,2022,36(5):e24423.doi:10.1002/jcla.24423.
[9]LIANG X,BU J,JIANG Y,et al.Prognostic significance of pan-immune-inflammation value in hepatocellular carcinoma treated by curative radiofrequency ablation:potential role for individualized adjuvant systemic treatment[J].Int J Hyperthermia,2024,41(1):2355279.doi:10.1080/02656736.2024.2355279.
[10]FORNER A,REIG M,BRUIX J.Hepatocellular carcinoma[J].Lancet,2018,391(10127):1301-1314.doi:10.1016/S0140-6736(18)30010-2.
[11]WANG M,WANG Y,FENG X,et al.Contribution of hepatitis B virus and hepatitis C virus to liver cancer in China north areas:experience of the Chinese National Cancer Center[J].Int J Infect Dis,2017,65:15-21.doi:10.1016/j.ijid.2017.09.003.
[12]CHEN L Z,ZHOU W Q,ZHAO S S,et al.A nested case-control study of maternal-neonatal transmission of hepatitis B virus in a Chinese population[J].World J Gastroenterol,2011,17(31):3640-3644.doi:10.3748/wjg.v17.i31.3640.
[13]LIU M,TSENG T C,JUN D W,et al.Transition rates to cirrhosis and liver cancer by age,gender,disease and treatment status in Asian chronic hepatitis B patients[J].Hepatol Int,2021,15(1):71-81.doi:10.1007/s12072-020-10113-2.
[14]CHEN W Q,ZHENG R S,BAADE P D,et al.Cancer statistics in China,2015[J].CA Cancer J Clin,2016,66(2):115-132.doi:10.3322/caac.21338.
[15]BUETTNER N,THIMME R.Sexual dimorphism in hepatitis B and C and hepatocellular carcinoma[J].Semin Immunopathol,2019,41(2):203-211.doi:10.1007/s00281-018-0727-4.
[16]ZHAO SJ,WANG M M,YANG Z Y,et al.Comparison between Child-Pugh score and Albumin-Bilirubin grade in the prognosis of patients with HCC after liver resection using time-dependent ROC[J].Ann Transl Med,2020,8(8):539.doi:10.21037/atm.2020.02.85.
[17]HO S Y,HSU C Y,LIU P H,et al.Albumin-bilirubin(ALBI)grade-based nomogram for patients with hepatocellular carcinoma undergoing transarterial chemoembolization[J].Dig Dis Sci,2021,66(5):1730-1738.doi:10.1007/s10620-020-06384-2.
[18]BALLERINI P,CONTURSI A,BRUNO A,et al.Inflammation and cancer:from the development of personalized indicators to novel therapeutic strategies[J].Front Pharmacol,2022,13:838079.doi:10.3389/fphar.2022.838079.
[19]BIHARI C,RASTOGI A,SHASTHRY S M,et al.Platelets contribute to growth and metastasis in hepatocellular carcinoma[J].APMIS,2016,124(9):776-786.doi:10.1111/apm.12574.
[20]INNES H,JEPSEN P,MCDONALD S,et al.Performance of models to predict hepatocellular carcinoma risk among UK patients with cirrhosis and cured HCV infection[J].JHEP Rep,2021,3(6):100384.doi:10.1016/j.jhepr.2021.100384.
[21]YAMASHITA Y,JOSHITA S,SUGIURA A,et al.aMAP score prediction of hepatocellular carcinoma occurrence and incidence-free rate after a sustained virologic response in chronic hepatitis C[J].Hepatol Res,2021,51(9):933-942.doi:10.1111/hepr.13689.
[22]WANG J,HUANG R,YAN X M,et al.Red blood cell distribution width:a promising index for evaluating the severity and long-term prognosis of hepatitis B virus-related diseases[J].Dig Liver Dis,2020,52(4):440-446.doi:10.1016/j.dld.2019.12.144.
[23]JING J S,FU X L,ZHAO W,et al.Red cell distribution width as a prognostic factor inpatients with hepatocellular carcinoma[J].Clin Lab,2020,66(7):1237-1248.doi:10.7754/Clin.Lab.2019.191027.
[24]TAN M Q,LIU B,YOU R L,et al.Red blood cell distribution width as a potential valuable survival predictor in hepatitis B virus-related hepatocellular carcinoma[J].Int J Med Sci,2023,20(7):976-984.doi:10.7150/ijms.79619.
[25]VIDILI G,ZINELLU A,MANGONI A A,et al.Red cell distribution width as a predictor of survival in patients with hepatocellular carcinoma[J].Medicina(Kaunas),2024,60(3):391.doi:10.3390/medicina60030391.
[26]XIONG S M,DONG L L,CHENG L.Neutrophils in cancer carcinogenesis and metastasis[J].J Hematol Oncol,2021,14(1):173.doi:10.1186/s13045-021-01187-y.
[27]HONG Y M,YOON K T,HWANG T H,et al.Pretreatment peripheral neutrophils,lymphocytes and monocytes predict long-term survival in hepatocellular carcinoma[J].BMC Cancer,2020,20(1):937.doi:10.1186/s12885-020-07105-8.
[28]UEDA K,SUEKANE S,KUROSE H,et al.Absolute lymphocyte count is an independent predictor of survival in patients with metastatic renal cell carcinoma treated with nivolumab[J].Jpn J Clin Oncol,2022,52(2):179-186.doi:10.1093/jjco/hyab157.
(2024-08-19收稿2024-10-29修回)
(本文编辑陆荣展)