席夫碱配体稀土配合物荧光感应识别
2024-12-31刘颖

摘 """""要: 稀土元素有着长的荧光寿命、大的斯托克斯位移和尖锐的特征发射等优势,但紫外吸光系数却很低。席夫碱配体的引入可以更好地吸收能量敏化稀土离子发射,其中天线效应为重要的传感机理。此外稀土配合物作为荧光探针检测各种小分子或离子时的机理主要有5种,介绍了这5种传感机理,用以探讨荧光猝灭或增强的原因。
关 "键 "词:稀土元素;稀土配合物;"传感机理;荧光探针
中图分类号:TQ133.3""""""文献标志码: A """"文章编号: 1004-0935(2024)07-1100-05
稀土元素包含镧系中的镧(La)、铈(Ce)、镨(Pr)、钕(Nd)、钪(Sc)、钇(Y)等共17种元素[1],是元素周期表中镧系元素系稀土类元素群的总称。正因为稀土离子多样,且存在丰富的发光能级,所以发光峰可以覆盖从紫外、可见到近红外区域,因此也被称为发光材料的宝库[2]。此外镧系稀土离子由于其4f 电子受到5S25P6 壳层屏蔽,其光谱性质受到电场、晶场、磁场、配位场影响较小,所以其发光峰位相对改变较少,且峰形尖锐,色纯度极好[3]。
但是由于镧系稀土离子Ln(Ⅲ)4f 电子中的 f-f 跃迁属于宇称禁阻跃迁,导致其光吸收能力较弱,发光效率低。而水杨醛类席夫碱配体不仅具有较高的吸光系数,而且制备简易且同时含有富电子N、O配位点,可采取螯合的方式与中心金属配位,已被广泛应用于金属-有机框架结构的搭建[4]。目前基于席夫碱配体和稀土金属构筑的多功能配合物在磁"学[5]、光学[6]以及医药学[7]等领域均有前沿性报道。
稀土配合物荧光发光方面有色谱纯度高、荧光寿命长等优点,在传感器[8]、生物成像[9]、有机电致发光二极管[10]、太阳能电池[11]、防伪[12]、光动力疗法[13]、化学疗法[14]、电致变色[15]及荧光探针[16]等众多领域有着广泛的研究。其中稀土离子Ln(Ⅲ)的发光原理为“天线效应”[17-20]。稀土离子在与席夫碱配体通过配位键相键合后,形成了稀土配合物,在紫外可见光的照射下配体吸收能量,从基态跃迁至激发态,最后向稀土离子的振动能级进行能量转移后,稀土离子从激发态回到基态,并发射出其特征荧光[21]。
基于以上内容,经查阅文献,本文总结了5种稀土配合物发光的原理,用以探讨荧光猝灭或增强的原因。
1 "席夫碱稀土配合物的发光原理
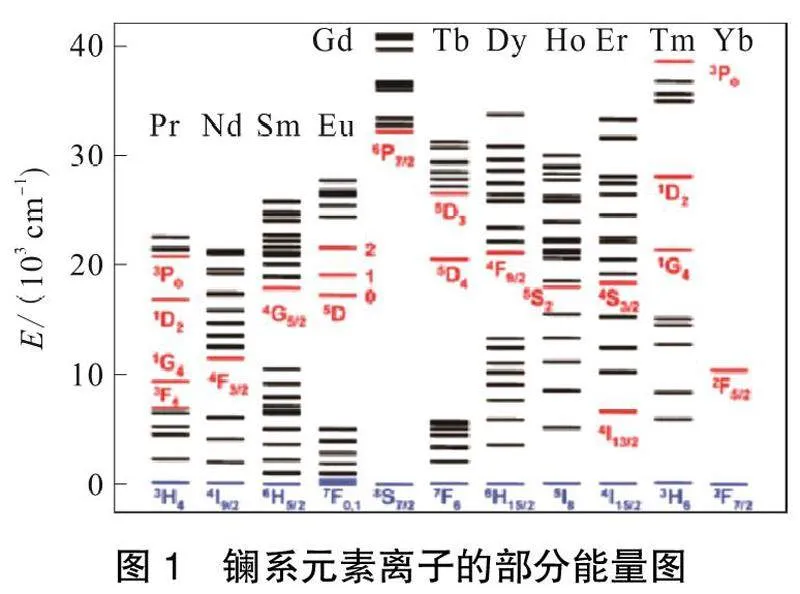
镧系离子独特的光物理性质(包括尖锐的吸收、长寿命的发光(微秒到毫秒)和狭窄的发射带)是由内部4f轨道内的电子跃迁造成的,由填充的5s25p6轨道屏蔽。镧系元素离子的部分能量图如图1所示。
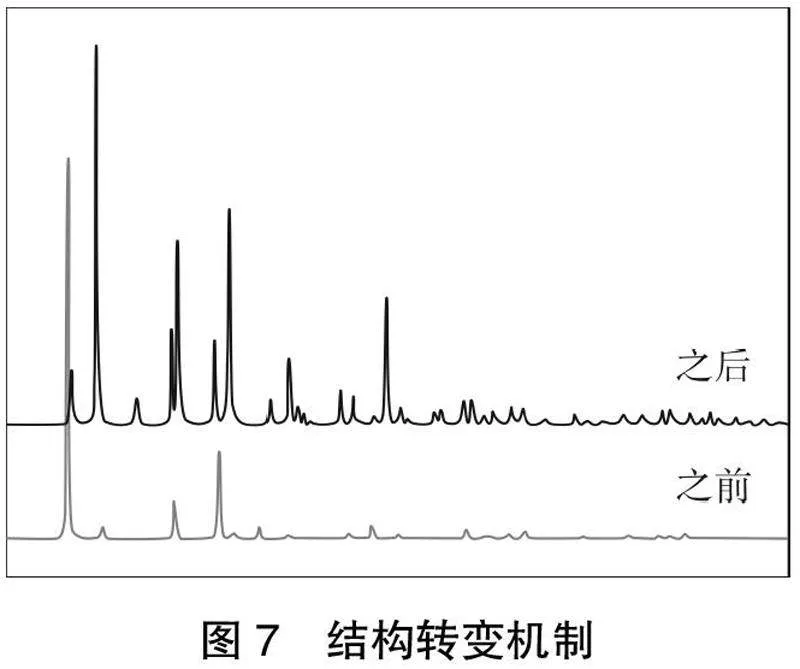
由图1可以看出,镧系离子具有丰富的电子激发态能级,除La3+和Lu3+外,所有镧系物都能通过f-f跃迁在电磁光谱可见区域产生紫外到NIR的发光。例如,蓝色、绿色、橙色和红色分别由Tm3+、Tb3+、Sm3+和Eu3+发出,而Yb3+、Nd3+和Er3+表现出NIR发射[22]。但是由于禁阻的f-f电子跃迁,三价镧系离子的摩尔吸收系数通常很小[23]。因此,这些离子的发光强度在直接激发时通常较低,尽管具有较高的本征量子产率。解决这个问题的方法是利用另一个发色团,它可以接受直接激发能,并通过一个称为敏化或天线效应的过程将其传递给这些镧系离子,如图2所示[24-25]。
镧系元素离子的激发是通过天线提供能量,如图3所示,将天线(即配体)从基态(S0)激发到单线态激发态(S1)。随后的系统间交叉(ISC)导致单线态激发态(S1)落回到三态激发态(T1),然后可以将能量转移到镧系离子上。最后,若镧系离子激发的单重态的零振动水平(S1)直接到基态(S0),它被称为荧光,而如果跃迁发生在三重态(T1)到基态(S0),则被称为磷光[26]。为了有效地敏化,天线的适当选择是很重要的,天线的三重态能级必须与镧系离子的能级匹配[24]。
2 "稀土配合物的荧光传感机理
经过查阅文献,总结了席夫碱配合物光致发光生物传感过程中5种可能存在的传感机制,包括光电子转移(PET)、共振能量转移(RET)、竞争吸收(CA)、结构转化(ST)[27]、寿命变化。
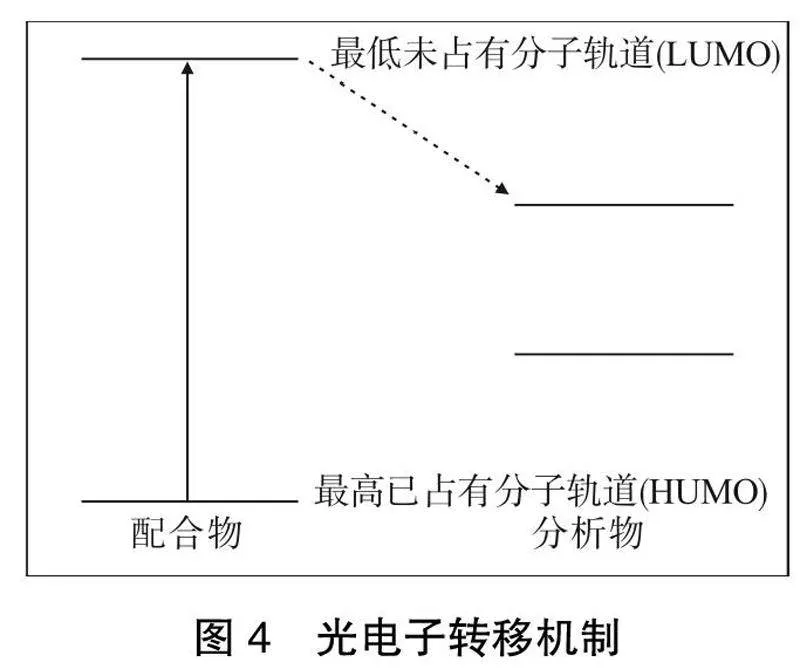
2.1 "光电子转移(PET)
PET是一个激发态电荷转移过程,其中一个光电子从激发态供体转移到基态受体。当供体的最低未占据分子轨道(LUMO)的能级高于受体时,光电子有可能从激发态供体转移到基态受体,而不是回基态,如果发生将导致供体的发射猝灭[28-29]。这种光致发光传感机制已在许多生物物种的检测中被确定,如抗生素[30-31]和杀虫剂[32]。密度泛函理论(DFT)计算通常用于分析供体和受体的能量图。在大多数情况下,分子轨道的计算与观察到的发光猝灭相一致。光电子转移机制如图4所示,当配体的LUMO能级位于分析物的LUMO能级之上时,光电子可以有效地从配体转移到分析物,通过这一过程,配合物中稀土的发光可以被猝灭,这可以被认为是被分析物的一个信号。一般来说,被分析物的LUMO能级越低,光电子转移越容易,因此猝灭效率越高。
2.2 "共振能量传递(RET)
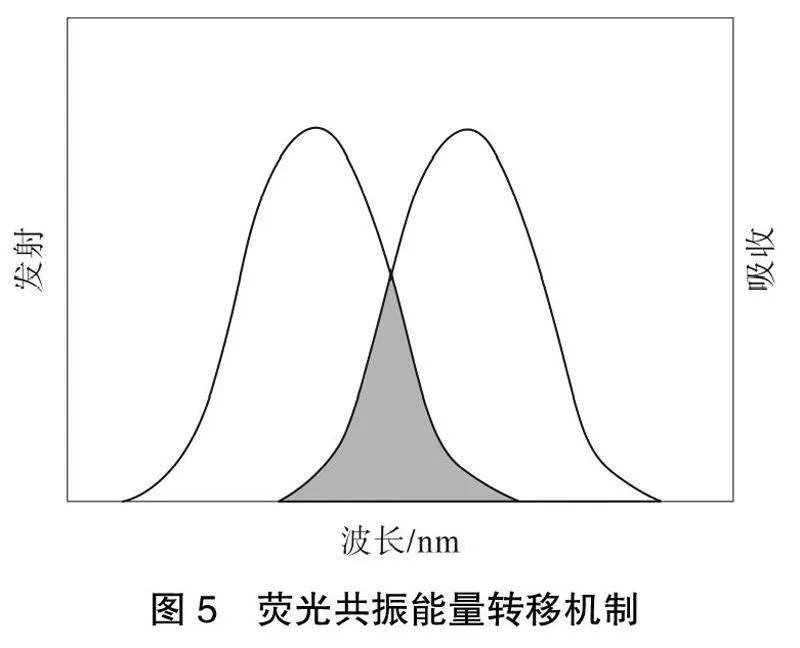
荧光共振能量转移(RET)是一种经常被使用的光致发光传感机制,其中一个激发态供体返回到基态同时,一个受体通过这2个过程之间的非辐射能量转移被提升到激发态,导致供体的发射猝灭和受体的发射增强[28,33]。能量传递是一个与距离相关的物理过程,能量传递的效率通常取决于光谱重叠程度、主客体的发射谱和吸收谱以及偶极-偶极相互作用、主客体的距离和相对取向[34-35]。光谱重叠可以通过实验来确定,并可能发生RET,导致稀土配合物的发光猝灭。荧光共振能量转移机制如图5""所示。
假设客体的紫外-可见吸收光谱与稀土配合物的发射光谱重叠,这一过程通常被称为荧光共振能量转移(FRET),除了光谱重叠的程度外,能量转移的效率也可以通过主客偶极-偶极相互作用来调制。然而,与光谱重叠不同,偶极-偶极相互作用在这一点上更多的是推测。
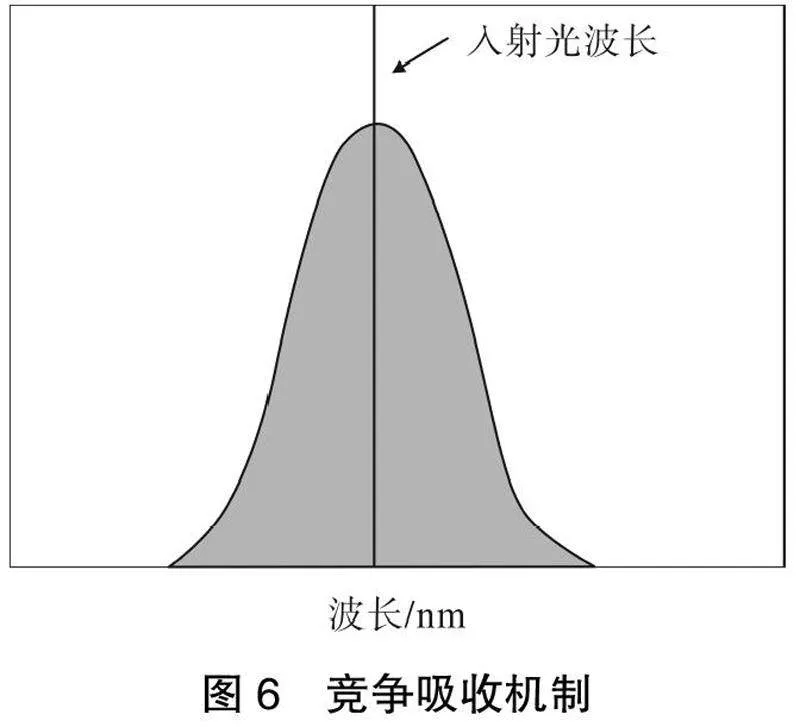
2.3 "竞争吸收(CA)
当被分析物的吸收光谱与配合物的激发光谱重叠时,在配合物和被分析物之间很可能会发生对激发光的吸收竞争。直观地说,分析物对激发光的吸收减少了配合物的总可用能量,导致激发态的能量减少,从而使配合物发光猝灭,如图6所示。这种机制通常被提出用于传感Fe3+[36]和一些挥发性有机化合物(挥发性有机物),如丙酮[37]。由于许多生物物种吸收近紫外光或可见光,具有适当激发波长的稀土配合物可以应用于这些生物物种的传感。
2.4 "结构变化(ST)
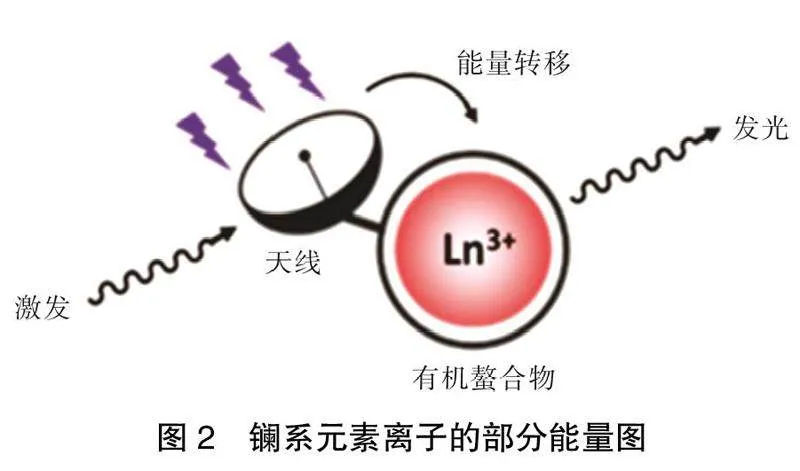
分析物的加入可能会导致配合物结构的改变,这可以通过实验得到的配合物的XRD粉末衍射数据对比由晶体CIF文件模拟得到的数据的比较来进行判断,如图 7所示。
如果得到的谱图发生了变化,说明稀土荧光性能的变化可能是由于配合物结构的坍塌造成,而结构坍塌基本不能复原,所以稀土发射峰消失不见。产生这种现象部分原因是席夫碱配体稀土配合物会出现未配位的原子,分析物的加入可能导致未配位原子与分析物进行配位,影响整个配合物的发光表现。常见的氧化还原反应、缩合反应等都可能导致整个配合物的结构转变,导致荧光性能的改变。
2.5""寿命变化
荧光变化从配合物自身的寿命变化来看,过程主要通过动态和静态增强模式进行。前者导致荧光寿命的变化,而后者的寿命没有变化[38-39]。前者与荧光分子与被分析物之间的碰撞有关,并伴随着荧光寿命的变化,另一方面,后者涉及基态分子缔合的形成,而没有寿命的变化。
对发光强度进行分析时,一般选取浓度与荧光强度之间的变化,通常在发光猝灭过程使用S-V[40]方程拟合,荧光增强过程可以使用浓度与强度直接进行拟合[41]。
3""结 论
讨论了配合物的发光原理主要为天线效应,此外总结了席夫碱稀土配合物光致发光生物传感平台的5种传感机制,光电子转移(PET)、共振能量转移(RET)、竞争吸收(CA)、结构转化(ST)、寿命变化,这在研究配合物感应传感机理方面有重要意义。
参考文献:
[1] 张倩,张亚如.稀土材料的应用及发展[J].科技风,2019(12):135.
[2] BUNZLI"J"C"G,PIGUET"C. Taking advantage of luminescent lanthanide ions[J]. Chem. Soc. Rev., 2005, 34(12): 1048-1077.
[3] 王浩,王红宇. 新型光功能稀土配合物研究及应用进展[J]. 发光学报2022,43(10):1509-1523.
[4] 杨慧,刘珊珊. 中性席夫碱单核稀土配合物的合成、晶体结构及荧光性质[J]. 广州化工,2022,50(12):24-27.
[5] ZHANG"P, GUO"Y"N,TANG"J. Recent advances in dysprosium-based single molecule magnets: structural overview and synthetic strategies[J]. Coord. Chem. Rev., 2013, 257(11-12): 1728-1763.
[6] CHEN"L,FU"P"Y,WANG"H"P,et al. Excited-state intramolecular proton transfer (ESIPT) for optical sensing in solid state[J]. Adv. Opt. Mater., 2021, 9: 2001952.
[7] CHUNDAWAT"N"S,JADOUN"S, ZARRINTAJ"P,et al. Lanthanide complexes as anticancer agents: a"review[J]. Polyhedron, 2021, 207: 115387.
[8] LIU"J,JI"G,XIAO"J,et al. Ultrastable 1D europium complex for simultaneous and quantitative sensing of Cr(Ⅲ) and Cr(Ⅵ) ions in aqueous solution with high selectivity and sensitivity[J]. Inorg. Chem.,"2017, 56(7): 4197-4205.
[9] MARTINIC"I, ELISEEVA"S"V,NGUYEN"T"N,et al. Near-infrared optical imaging of"necrotic cells by photostable lanthanide-based metallacrowns[J]. J. Am. Chem. Soc.,"2017, 139(25): 8388-8391.
[10] METLIN"M"T, GORYACHII"D"O, AMINEV"D"F,et al. Bright Yb3+"complexes for efficient pure near-infrared OLEDs[J]. Dyes Pigm.,"2021, 195: 109701.
[11] GAVRILUTA"A,FIX"T,NONAT"A,et al. Tuning the chemical properties of europium complexes as downshifting agents for copper indium gallium selenide solar cells[J]. J. Mater. Chem. A.,"2017, 5(27): 14031-14040.
[12] MOUDAM"O,LAKBITA"O. Potential end-use of a europium binary photoluminescent ink for anti-counterfeiting security documents[J]. Acs Omega,"2021, 6(44): 29659-29663.
[13] RODRIGUES"C"V,JOHNSON"K"R,LOMBARDI"V"C,et al. Photo- cytotoxicity of thiophene- and bithiophene-dipicolinato luminescent lanthanide complexes[J]. J. Med. Chem.,"2021, 64(11): 7724-7734.
[14] PATRA"D,KUMAR"P,DASH"T"K,et al. Gadolinium(Ⅲ) coordinated theranostic polymer for proficient sequential targeting-combinational chemotherapy and T-1 weighted magnetic resonance imaging[J]. Acs Appl. Polym. Mater.,"2022, 4(3): 1752-1763.
[15] KANAZAWA"K, KOMIYA"Y,NAKAMURA"K,et al. Red luminescence control of Eu(III) complexes by utilizing the multi-"colored electrochromism of viologen derivatives[J]. PCCP,"2017, 19(26): 16979-16988.
[16] PARKER"D,FRADGLEY"J"D,WONG"K"L. The design of responsive luminescent lanthanide probes and sensors[J]. Chem. Soc. Rev.,"2021, 50(14): 8193-8213.
[17] BINNEMANS"K. Lanthanide-based luminescent hybrid materials[J]. Chem. Rev.,"2009, 109(9): 4283-4374.
[18] FENG"J, ZHANG"H. Hybrid materials based on lanthanide organic complexes: a review[J]. Chem. Soc. Rev.,"2013, 42(1): 387-410.
[19] CARLOS"L"D, FERREIRA"R"A"S,"BERMUDEZ"V"d"Z,"et al. Lanthanide-containing light-emitting organic-inorganic hybrids: a bet on the future[J]. Adv. Mater.,"2009, 21(5): 509-534.
[20] LI"H,SHAO"H, WANG"Y,et al. Soft material with intense photoluminescence obtained by dissolving Eu2O3"and organic ligand into a task-specific ionic liquid[J]. Chem. Commun,"2008(41): 5209-5211.
[21] 李焕荣,王天任. 基于稀土配合物和离子液体的新型稀土发光材料研究进展[J]. 发光学报,2018,39(4):425-439.
[22] ELISEEVA"S"V, BUENZLI"J"C"G. Lanthanide luminescence for functional materials and bio-sciences[J]. Chem. Soc. Rev., 2010, 39(1): 189-227.
[23] RUNOWSKI"M, STOPIKOWSKA"N, LIS, S. UV-Vis-NIR absorption spectra of lanthanide oxides and fluorides[J]. Dalton Trans., 2020, 49(7): 2129-2137.
[24] JOAQUINA B"L"M, SOLIS-CESPEDES"E,PAEZ-HERNANDEZ"D. The role of the excited state dynamic of the antenna ligand in the lanthanide sensitization mechanism[J]. Dalton Trans., 2020, 49(22): 7444-7450.
[25] AULSEBROOK"M"L,GRAHAM"B, GRACE"M"R,et al. Lanthanide complexes for "luminescence-based sensing of low molecular weight analytes[J]. Coord. Chem. Rev., 2018, 375: 191-220.
[26] ALBRECHT C."Joseph R. Lakowicz: Principles of fluorescence spectroscopy, 3rd Edition [J]. Anal. Bioanal. Chem., 2008, 390(5): 1223-1224.
[27] ZHAO Y, ZENG H, ZHU"X W, et al. Metal-organic frameworks as photoluminescent biosensing platforms: mechanisms and applications [J]. Chem Soc Rev, 2021, 50(7): 4484-4513.
[28] SUN"X,WANG"Y,LEI"Y. Fluorescence based explosive detection: from mechanisms to sensory materials[J]. Chem. Soc. Rev.,2015, 44(22):"8019-8061.
[29] JUNG"H"S, VERWILST"P,KIM"W"Y,et al. Fluorescent and colorimetric sensors for the detection of humidity or water content[J]. Chem. Soc. Rev., 2016, 45(5): 1242-1256.
[30] WANG"B,LV"X"L,FENG"D,et al. Highly stable Zr(IV)-based metal-"organic frameworks for the detection and removal of antibiotics and organic explosives in water[J]. J. Am. Chem. Soc., 2016, 138(19): 6204-6216.
[31] ZHU"K,FAN"R,ZHENG"X,et al. Dual-emitting dye-CDs@MOFs for selective and sensitive identification of antibiotics and MnO4-"in water[J]. J. Mater. Chem. C., 2019, 7(47): 15057-15065.
[32] TAO"C"L,CHEN"B,LIU"X"G,et al. A highly luminescent entangled metal-organic framework based on pyridine-substituted tetraphenylethene for efficient pesticide detection[J]. Chem. Commun., 2017, 53(72): 9975-9978.
[33] ZHANG"Y"Z, XIANG"X, MEI"P,et al. Spectroscopic studies on the interaction of Congo Red with bovine serum albumin[J]."Acta A Mol. Biomol, 2009, 72(4): 907-914.
[34] CAI"Y, HUA"Y, YIN"M,et al. Fabrication of test strips with gold-silver nanospheres and metal-organic frameworks: a fluorimetric method for sensing trace cysteine in hela cells[J]. Sens. Actuators B Chem, 2020, 302: 127198.
[35] LIU"X,QIU"J. Recent advances in energy transfer in bulk and nanoscale luminescent materials: from spectroscopy to applications[J]. Chem. Soc. Rev.,2015, 44(23): 8714-8746.
[36] XU"H,GAO"J,QIAN"X,et al. Metal-organic framework nanosheets for fast-response and highly sensitive luminescent sensing of Fe3+[J]. J. Mater. Chem. A., 2016, 4(28): 10900-10905.
[37] HUANG"Y"L,QIU"P"L,BAI"J"P,et al. Exclusive recognition of acetone in a luminescent BioMOF through multiple hydrogen-bonding interactions[J]. Inorg. Chem., 2019, 58(12): 7667-7671.
[38] REN"K,WU"S"H,GUO"X"F,et al. Lanthanide organic framework as a reversible luminescent sensor for sulfamethazine antibiotics[J]. Inorg. Chem., 2019, 58(7): 4223-4229.
[39] WANG"B,WANG"P, XIE"L"H,"et al. A stable zirconium based metal-organic framework for specific recognition of representative polychlorinated dibenzo-p-dioxin molecules[J]. Nat. Commun., 2019, 10: 3861.
[40] LIU J, ZHONG Y, LU"P, et al. A superamplification effect in the detection of explosives by a fluorescent hyperbranched poly- (silylenephenylene) with aggregation-enhanced emission characteristics [J]. Polym. Chem., 2010, 1(4): 426-429.
[41] LENG X, SHI D, YANG"X, et al. Construction of a luminescent square-like Cd6Eu2"nanocluster for the quantitative detection of 2,6-dipicolinic acid as an anthrax biomarker [J]. J. Mater. Chem. C., 2022, 10(9): 3510-3516.
Fluorescence Sensing Recognition of Rare Earth
Complexes with Schiff Base Ligand
LIU Ying
(College of Chemistry and Material Engineering, Wenzhou University, Wenzhou Zhejiang 325035, China)
Abstract:"Rare earth elements have the advantages of long fluorescence lifetime, large Stokes shift and sharp characteristic emission, but the ultraviolet absorption coefficient is very low. The introduction of Schiff base ligand can better absorb energy-sensitized rare earth ion emission, in which antenna effect is an important sensing mechanism. In addition, there are four main mechanisms for the detection of various small molecules or ions by rare earth complexes as fluorescent probes. In"this paper, these five sensing mechanisms were introduced to explore the reasons for fluorescence quenching or enhancement.
Key words:""Rare earth element ; Rare earth complexes; Sensing mechanism; Fluorescent probes
