达格列净在胰岛素治疗效果不佳的2型糖尿病患者中的效果观察
2024-12-18周慧杨社珍蒋能美吴亮
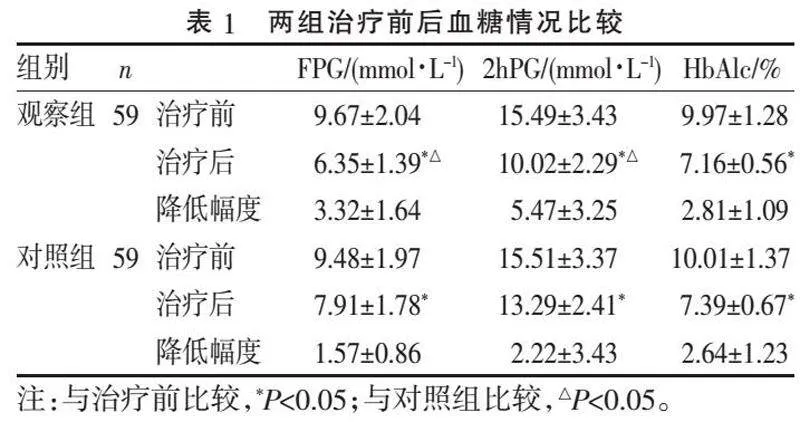
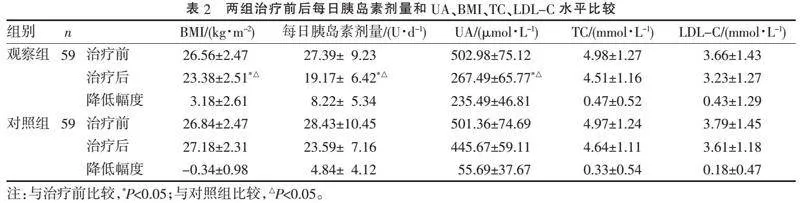
[摘" "要]" "目的:探讨达格列净在胰岛素治疗效果不佳的2型糖尿病(type 2 diabetes mellitus, T2DM)患者治疗中的效果和安全性。方法:筛查海门区人民医院内分泌科2020年6月—2021年12月期间就诊的胰岛素治疗效果不佳的T2DM患者,随机分为两组,各59例。对照组给予常规胰岛素或胰岛素联合1种及以上其他口服药降糖,观察组在此基础上加用达格列净片治疗。比较两组患者治疗前后BMI、每日胰岛素剂量、血糖、血脂、尿酸水平及不良反应情况。结果:治疗12周后,观察组空腹、餐后2 h血糖均低于对照组(P=0.046、0.039);两组糖化血红蛋白均明显下降(P=0.028、0.034),但组间比较差异无统计学意义(P=0.136)。两组治疗前后血清总胆固醇和低密度脂蛋白水平差异无统计学意义(P>0.05)。观察组BMI、每日胰岛素剂量、尿酸水平低于对照组(P=0.024、0.018、0.042)。观察组发生不良反应6例(10.17%),其中轻度低血糖4例,泌尿系感染2例;对照组发生不良反应8例(13.56%),其中轻度低血糖6例,中度低血糖1例,泌尿系感染1例,两组不良反应发生率比较差异无统计学意义(P=0.569)。结论:胰岛素治疗效果不佳的T2DM患者加用达格列净可有效降低患者血糖,减少每日胰岛素剂量,一定程度上降低因大剂量使用胰岛素带来的体质量增加、尿酸增高等风险,同时无明显不良反应,安全性可。
[关键词]" "2型糖尿病;达格列净;胰岛素
[中图分类号]" "R587.1" " " " " " " "[文献标志码]" "A" " " " " " " "[文章编号]" "1674-7887(2024)05-0435-04
Clinical efficacy observation of dapagliflozin in the treatment of
T2DM patients with poor insulin response*
ZHOU Hui YANG Shezhen, JIANG Nengmei, WU Liang " "(Department of Endocrinology, Nantong Haimen People's Hospital, Jiangsu 226100)
[Abstract]" "Objective: To explore the clinical efficacy and safety of dapagliflozin in the treatment of type 2 diabetes mellitus(T2DM) patients' with poor insulin response. Methods: T2DM patients with poor insulin response, who were treated in the Department of Endocrinology, Haimen People's Hospital from June 2020 to December 2021,were selected and randomly divided into two groups, with 59 patients in each group. The control group was treated with conventional insulin or insulin combined with one or more oral drugs to lower blood glucose level, and the observation group was additionally treated with dapagliflozin tablets on this basis. The BMI, insulin dose per day, blood glucose, lipids and uric acid levels, and incidence of adverse reactions were compared between two groups before and after treatment. Results: After treatment for 12 weeks, fasting blood glucose and 2-hour postprandial blood glucose in the observation group were lower than those in the control group(P=0.046, 0.039). The glycosylated hemoglobin was decreased significantly in both groups(P=0.028, 0.034), but there was no significant diffe-rence in glycated hemoglobin between the two groups(P=0.136). There were no significant differences in serum total cholesterol and low-density lipoprotein levels between the two groups before and after treatment(Pgt;0.05). BMI," insulin dose per day and uric acid level in the observation group were lower than those in the control group(P=0.024, 0.018, 0.042). In the observation group, there were 6 cases(10.17%) of adverse reactions, including 4 cases of mild hypoglycemia and 2 cases of urinary tract infection. There were 8 cases(13.56%) of adverse reactions in the control group, including 6 cases of mild hypoglycemia, 1 case of moderate hyp-oglycemia and 1 case of urinary tract infection. There was no significant difference in the incidence of adverse reactions between the two groups(P=0.569). Conclusion: The additional use of dapagliflozin in T2DM patients with poor insulin response can effectively lower the blood glucose level, reduce the insulin dose per day, thereby reducing the risk of body weight gain and increased uric acid level induced by high doses of insulin to a certain extent, and it is safe, without obvious adverse reaction.
[Key words]" "type 2 diabetes mellitus; dapagliflozin; insulin
2型糖尿病(type 2 diabetes mellitus, T2DM)作为最常见的糖尿病类型,其发病率仍呈逐年升高趋势。T2DM患者长期使用胰岛素治疗会增加肥胖风险,进一步导致胰岛素抵抗,致使患者血糖控制不佳。达格列净作为首个我国批准上市的钠-葡萄糖协同转运蛋白2(sodium-glucose cotransporter 2, SGLT2)抑制剂,在糖尿病患者治疗中可与胰岛素及其他多种降糖药物联用,降糖过程中不依赖胰岛细胞功能,其安全性和有效性已被证实,已逐渐成为T2DM治疗的一线用药[1-2]。本研究旨为进一步观察达格列净在胰岛素治疗效果不佳的T2DM患者中的疗效,现报告如下。
1" "资料与方法
1.1" "一般资料" "选取2020年6月—2021年6月海门区人民医院内分泌科住院治疗的胰岛素治疗效果不佳的T2DM患者为研究对象,按随机数字表法随机分为两组,各59例。对照组男29例,女30例,年龄44~73岁,平均(56.24±5.32)岁;病程3~9年,平均(5.23±1.46)年;观察组男31例,女28例,年龄45~75岁,平均(56.37±5.28)岁;病程3~11年,平均(5.54±1.55)年;两组患者性别、年龄、糖尿病病程比较差异无统计学意义(Pgt;0.05)。本研究经过海门区人民医院伦理委员会审批通过(伦理批号:2021-KY10),所有研究对象均自愿参加研究,入组前均已签署知情同意书。
1.2" "纳入及排除标准" "纳入标准:符合《中国2型糖尿病防治指南(2020年版)》[3]中T2DM相关诊断标准;常规胰岛素(或联合非SGLT2抑制剂类降糖药)治疗≥3个月,糖化血红蛋白(glycosylated hemoglobin A1c, HbAlc)仍≥7.0%。排除标准:严重肝、肾功能不全[肾小球滤过率估计值(estimated glomerular filtration rate, eGFR)<45 L/min,ALT、AST大于正常上限的2倍],合并严重感染性疾病、恶性肿瘤、免疫性疾病者;合并高血糖高渗状态、糖尿病酮症以及其他重症疾病危及生命者;对本研究药物过敏者。
1.3" "治疗方法" "对照组以门冬胰岛素30注射液[诺和诺德(中国)制药有限公司,国药准字S20133006]滴定治疗为基础,必要时联合二甲双胍(天方药业有限公司,国药准字H20031225,0.5 g/片)、磷酸西格列汀(杭州默沙东制药有限公司,国药准字H20237004,
100 mg/片)等非SGLT2抑制剂类口服降糖药等常规降糖治疗。观察组在上述治疗基础上加用达格列净(阿斯利康制药有限公司,国药准字HJ20170119,
20170120,10 mg/片)口服治疗。治疗期间,监测患者三餐前后指尖血糖,根据患者血糖水平2~3 d调整1次胰岛素剂量,治疗12周后随访。
1.4" "监测指标" "(1)血糖水平。检测各患者基线与观察12周后(治疗前后)静脉血空腹血糖(fast plasma glucose, FPG)、餐后2 h血糖(2-hour postprandial blood glucose, 2hPG)、HbAlc水平。(2)治疗前后BMI、每日胰岛素剂量、尿酸(uric acid, UA)、血清总胆固醇(total cholesterol, TC)、低密度脂蛋白胆固醇(low density lipoprotein-cholesterol, LDL-C)水平。(3)不良反应情况。主要包括低血糖[4](轻度低血糖:血糖3.0~3.9 mmol/L;中度低血糖:血糖1.5~3.0 mmol/L;重度低血糖:血糖<1.5 mmol/L或伴有明显神经精神症状等情况),肝肾功能异常及泌尿系感染等。
1.5" "统计学方法" "相关数据统计分析采用SPSS 20.0统计软件进行,其中组间正态连续变量采用独立样本t检验,数据以x±s表示。非正态连续变量资料采用非参数检验,分类变量资料采用χ2检验。Plt;0.05为差异有统计学意义。
2" "结" " " 果
2.1" "血糖水平比较" "治疗前两组各血糖指标差异无统计学意义(Pgt;0.05);治疗12周后,观察组FPG、2hPG水平均低于对照组(P=0.046、0.039);两组HbAlc均较治疗前明显下降(P=0.028、0.034),但组间比较差异无统计学意义(P=0.136),见表1。
2.2" "每日胰岛素剂量、UA、BMI、TC、LDL-C水平比较" "两组患者治疗前后TC和LDL-C水平均有所下降,但组间比较差异无统计学意义(Pgt;0.05)。治疗前两组每日胰岛素剂量、UA水平差异无统计学意义(Pgt;0.05);治疗后,观察组BMI、每日胰岛素剂量、UA水平显著低于对照组(P=0.024、0.018、0.042),见表2。
2.3" "不良反应" "两组均无重度低血糖和肝肾功能异常发生。观察组发生低血糖4例(6.78%),均为轻度低血糖;泌尿系感染2例(3.39%),不良反应发生率为10.17%。对照组发生低血糖7例(11.86%),其中轻度低血糖6例,中度低血糖1例;泌尿系感染1例,不良反应发生率为13.56%。两组不良反应发生率比较差异无统计学意义( χ2=0.324, P=0.569)。
3" "讨" " " 论
T2DM作为内分泌科最常见的疾病,其发病与遗传、环境、生活方式、年龄等密切相关。随着病程的延长,T2DM患者胰岛β细胞功能进一步耗竭,机体糖脂等代谢进一步紊乱,导致患者单用口服药物降糖疗效欠佳,需要外源性补充胰岛素治疗。而长期胰岛素治疗引起胰岛素抵抗,导致胰岛β细胞负担加重、胰岛素使用剂量增加,进而增加肥胖、低血糖等并发症发生风险,甚至出现大血管病变等[3]。因此,对于使用胰岛素降糖治疗的T2DM患者,无禁忌证时应尽早联合其他口服降糖药物治疗,以期改善胰岛功能、延缓疾病进展。
达格列净是最早在国内上市的SGLT2抑制剂,通过抑制肾小管重吸收、增加尿糖排泄发挥降糖作用。达格列净发挥降糖作用的同时不依赖胰岛素分泌,单用或与其他药物联用时,可有效降低空腹及餐后血糖,明显降低HbA1c[4-5]。本研究中,两组患者治疗前后FPG及2hPG均明显下降,且组间比较差异有统计学意义。治疗后两组HbA1c均明显下降,但可能由于病例数有限,且观察周期较短等原因,差异无统计学意义(Pgt;0.05)。
研究[6-7]表明,单用胰岛素治疗血糖控制欠佳的T2DM患者加用达格列净治疗,可有效降低三酰甘油、LDL-C水平,减少胰岛素用量。本研究结果发现,两组患者治疗前后TC、LDL-C水平均有所下降,但组间比较差异无统计学意义(P>0.05)。这可能与样本量偏少,随访时间较短有关。根据治疗前后血脂水平推测,随着达格列净治疗时间的推进,患者血脂水平可能会进一步降低,达格列净可有效改善肥胖患者血脂代谢紊乱。在有效降脂的同时,T2DM患者发生动脉粥样硬化的风险降低,心血管事件发生率进一步下降[8]。随着达格列净的广泛应用,其不良反应也逐渐被人们重视。研究[9]发现,达格列净在T2DM的治疗过程中,不增加低血糖发生风险,在与沙格列汀等药物合用时,一定程度上可减少低血糖事件发生[6],具有较高的安全性。由于达格列净发挥降糖作用机制的特殊性,大量葡萄糖经泌尿系统排出,T2DM在选择该药治疗时更应关注是否导致泌尿生殖系感染。一项包含4种SGLT2抑制剂的安全性研究的Meta分析[10]显示,T2DM患者使用达格列净治疗会导致泌尿生殖系感染发生风险增加,但多均为轻中度感染,及时治疗后可有效控制。因此在选择该药降糖的同时,医护人员应提前评估患者泌尿生殖系感染风险,做好健康宣教,避免药物因素导致感染事件的发生或加重。
综上所述,联合使用达格列净治疗胰岛素治疗效果不佳的T2DM患者,可有效降低患者血糖,减少胰岛素注射剂量,降低患者体质量,同时其低血糖等不良反应发生率无明显升高,安全性较好。
[参考文献]
[1]" "杜婉笛, 杨晓蕾, 刘玉萍, 等. 达格列净联合用药治疗糖尿病的临床研究进展[J]. 中国医院药学杂志, 2021, 41(19):2026-2030.
[2]" "ELSAYED N A, ALEPPO G, ARODA V R, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023[J]. Diabetes Care, 2023, 46(Suppl 1):S140-S157.
[3]" "中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2020年版)[J]. 中华糖尿病杂志, 2021, 13(4):315-409.
[4]" "SAHAY R K, GIRI R, SHEMBALKAR J V, et al. Fixed-dose combination of Dapagliflozin+Sitagliptin+Metformin in patients with type 2 diabetes poorly controlled with metformin: phase 3, randomized comparison with dual combinations[J]. Adv Ther, 2023, 40(7):3227-3246.
[5]" "赵香芳, 常连庆, 吕春雷, 等. 达格列净治疗2型糖尿病的研究进展[J]. 医学综述, 2021, 27(4):783-787.
[6]" "严佳栋, 钱震宇, 钱佳瑜. 达格列净片联合胰岛素治疗单用胰岛素血糖控制不佳2型糖尿病患者的临床疗效[J]. 中国药物经济学, 2020, 15(12):51-53, 61.
[7]" "GONZÁLEZ-CLEMENTE J M, GARCÍA-CASTILLO M, GORGOJO-MARTÍNEZ J J, et al. Beyond the glycaemic control of dapagliflozin: impact on arterial stiffness and macroangiopathy[J]. Diabetes Ther, 2022, 13(7):1281-1298.
[8]" "ZELNIKER T A, RAZ I, MOSENZON O, et al. Effect of dapagliflozin on cardiovascular outcomes according to baseline kidney function and albuminuria status in patients with type 2 diabetes: a prespecified secondary analysis of a randomized clinical trial[J]. JAMA Cardiol, 2021, 6(7):801-810.
[9]" "赵惟超, 项荣武, 杜闪闪, 等. 达格列净治疗2型糖尿病有效性及安全性的Meta分析[J]. 沈阳药科大学学报, 2017, 34(10):917-928.
[10]" "QIU M, DING L L, ZHANG M, et al. Safety of four SGLT2 inhibitors in three chronic diseases: a meta-analysis of large randomized trials of SGLT2 inhibitors[J]. Diab Vasc Dis Res, 2021, 18(2):14791641211011016.
[收稿日期] 2023-10-18
