茶树γ-氨基丁酸代谢途径对早期茶尺蠖取食为害的响应
2024-11-08孙娟陈慧刘关华张瀚黄福印王玉玺王诺保德孟施江戴伟东陈健付建玉



摘要:茶尺蠖(Ectropis obliqua Prout)为害会诱导茶树释放大量挥发性代谢物,这些代谢物作为重要信号物质在趋避害虫或吸引天敌方面的研究已被广泛报道,但茶尺蠖为害对茶树叶片中非挥发性代谢物质空间变化的影响及其作用尚不清楚。以茶树叶片为材料,限制茶尺蠖仅在叶尖部取食,再采集叶尖部、叶中部、叶基部3个位点组织,基于超高效液相色谱-四极杆轨道阱质谱(UHPLC-Q-Exactive/MS)的分析方法对这3个位点组织的非挥发性代谢物质进行鉴定和分析。结果表明,与空白对照和机械损伤相比,茶尺蠖为害可诱导6种二聚儿茶素类、3种氨基酸类(包括γ-氨基丁酸)、1种黄酮和黄酮苷类、1种酚酸类共11种差异代谢物。与空白对照相比,茶尺蠖为害后,茶树叶片3个位点的γ-氨基丁酸相对含量均明显增加,在叶中部和叶基部均增加了1.99倍,且γ-氨基丁酸生物合成途径中的关键基因在这3个位点均上调表达。茶尺蠖为害后,在叶片叶尖部和叶基部,γ-氨基丁酸的相对含量与其前体物质谷氨酸的相对含量呈显著正相关(P<0.05)。茶尺蠖取食添加了0.2、0.5、2.0 mg·g-1 γ-氨基丁酸的人工饲料后,其体质量和体长均显著小于对照组(P<0.05)。本研究表明,γ-氨基丁酸代谢途径在茶树抵御茶尺蠖为害的早期防御反应中发挥了重要作用,为进一步揭示茶树的生化抗性机制奠定基础。
关键词:茶树;茶尺蠖;γ-氨基丁酸;空间变化
中图分类号:S571.1;S435.711 文献标识码:A 文章编号:1000-369X(2024)05-816-15
Response of γ-Aminobutyric Acid Metabolic Pathway in Tea Plants to Early Infestation of Ectropis obliqua
SUN Juan1,2,3, CHEN Hui2,3, LIU Guanhua2,3, ZHANG Han2,3, HUANG Fuyin2,3, WANG Yuxi2,3, WANG Nuo2,3, BAO Demeng2,3, SHI Jiang2,4, DAI Weidong2,4, CHEN Jian1, FU Jianyu2,3*
1. College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou 310018, China;
2. Tea Research Institute, Chinese Academy of Agricultural Sciences, Hangzhou 310008, China;
3. Key Laboratory of Tea Quality and Safety Control, Ministry of Agriculture and Rural Affairs, Hangzhou 310008, China;
4. Key Laboratory of Tea Biology and Resources Utilization, Ministry of Agriculture and Rural Affairs, Hangzhou 310008, China
Abstract: Tea geometrid (Ectropis obliqua Prout) infestation induces tea plants to release massive amounts of volatile organic compounds (VOCs), which are widely reported as important chemical cues that either repel the pests or attract their enemies. However, the spatial variations and the roles of the non-volatile metabolites in tea leaves infested by the tea geometrids are confusing. Taking tea leaves as materials, the feeding of E. obliqua was limited at the leaf tip, and then the tissues at the leaf tip, middle and base were collected. The non-volatile metabolites of the tissues at the three sites were identified and analyzed by ultra-high performance liquid chromatography-quadrupole orbitrap mass spectrometry (UHPLC-Q-Exactive/MS). The results demonstrate that compared with the blank control and mechanical injury tea leaves, tea geometrids induced 11 differential metabolites, including six dimeric catechins, three amino acids (including γ-aminobutyric acid), one flavonoid and flavonoid glycoside, and one phenolic acid compound. After the infestation of the tea geometrids, the relative contents of γ-aminobutyric acid at the three sites in tea leaves were significantly increased compared to the blank control tea leaves, and increased by 1.99-fold in the middle and base of leaves. In addition, the key genes involved in the γ-aminobutyric acid biosynthetic pathway were upregulated at all three sites of tea leaves. There was a significant positive correlation between the relative content of γ-aminobutyric acid and the relative content of glutamic acid (P<0.05). When the tea geometrids were fed with artificial diet supplemented with 0.2 mg·g-1, 0.5 mg·g-1 and 2.0 mg·g-1 γ-aminobutyric acid, their body weight and length were both significantly decreased compared with the control (P<0.05). The present study indicates that the inhibitory neurotransmitter γ-aminobutyric acid plays a pivotal role in the early defense response against tea geometrids, which will shed light on the biochemical resistance mechanism of the tea plants.
Keywords: Camellia sinesis, Ectropis obliqua, γ-aminobutyric acid, spatial variation
植物在进化过程中形成了复杂而巧妙的防御系统来应对害虫的取食为害,包括组成型和诱导型[1]。组成型防御是植物自身固有的,能够阻碍侵染的物理或化学防御性状,如植物组织表面的蜡质、刺、表皮毛等[2]。诱导型防御是指在受到攻击或外界诱导的情况下,植物的形态或内部代谢物发生变化,从而对害虫的某些行为能力产生负面影响的一种特性[3]。植物的诱导型防御又可分为直接防御和间接防御。直接防御是指植物自身积累的物质能够直接影响害虫的生理状态及行为,如生成广谱防御相关代谢物[4]。害虫的取食可以诱导这些代谢物在植物体内的空间分布发生变化,如新产生毒素,或在同一器官或整株植物中增加毒素的积累[5]。例如,斜纹夜蛾的取食会诱导玉米叶片中倍半萜的空间分布变化,倍半萜仅在受伤部位至叶尖之间产生,并呈现从叶尖到受害部位的梯度增加趋势[6]。同样,东方黏虫的取食部位也会影响玉米叶片中抗虫苯并噁嗪类化合物的产生和空间分布[7]。
γ-氨基丁酸(Gamma-aminobutyric acid,GABA)是一种广泛分布的非蛋白质氨基酸[8],其作为一种神经递质能够抑制害虫的生长发育,在烟草和拟南芥中对害虫的防御反应起关键作用[9-10]。研究表明,害虫的爬行或取食会导致植物组织和细胞的损伤,快速诱导γ-氨基丁酸合成和积累[11-13]。植物中有两条γ-氨基丁酸合成和转化途径:第一条途径为γ-氨基丁酸支路,是γ-氨基丁酸合成与代谢的主要途径,由谷氨酸脱羧酶(Glutamic acid decarboxylase,GAD)催化谷氨酸合成γ-氨基丁酸,再经γ-氨基丁酸转氨酶(GABA transaminase,GABA-T)和琥珀酸半醛脱氢酶(Succinic semialdehyde dehydrogenase,SSADH)催化生成琥珀酸进入三羧酸(TCA)循环,最后由α-酮戊二酸催化谷氨酸脱氢酶(Glutamate dehydrogenase,GDH)生成谷氨酸;第二条途径为多胺降解途径,主要由精氨酸脱羧酶(Arginine decarboxylase,ADC)催化精氨酸生成胍丁胺再转化为腐胺,由铜胺氧化酶(Copper amine oxidase,CuAO)催化腐胺降解产物转化为γ-氨基丁酸[14-15]。
茶尺蠖(Ectropis obliqua Prout)是茶树上一类发生普遍且为害严重的咀嚼式口器害虫。近年来,茶园中茶尺蠖为害频繁发生,茶叶产量受到了极大的影响[16]。大量研究表明,茶尺蠖取食为害诱导茶树释放大量挥发物,以直接趋避昆虫或间接吸引天敌的方式来抵御虫害[17-18]。茶尺蠖取食可诱导茶树叶片中倍半萜合酶基因CsAFR和CsNES2表达水平的显著上调,增加(E,E)-α-法尼烯和(E)-橙花叔醇的释放量[19]。此外,茶尺蠖会诱导茶树释放高浓度的吲哚并促进茉莉酸和防御相关次生代谢物的产生[20]。茶尺蠖为害诱导的茶树苯乙腈的合成和释放量集中在白天,使茶树免受昼行性茶尺蠖幼虫的危害[21]。周围健康茶树植株也会感知受害植株释放的化学信号(如β-罗勒烯和萜类同系物DMNT等化学信号),提高自身抗性水平,进而实现群体防御[22]。
非挥发性代谢物在茶树对茶尺蠖的防御反应过程中也发挥着至关重要的作用,如拒食、驱避、毒害茶尺蠖等[23-24]。研究表明,茶尺蠖幼虫取食可显著诱导茶树叶片中槲皮素-
3-O-葡萄糖苷、儿茶素、表儿茶素和表没食子儿茶素没食子酸酯等次级代谢产物含量显著积累,最终产生直接防御反应[25-26]。受害茶树叶片产生的茉莉酸信号能够传递到未受害的相邻叶片,并诱导Kunitz型蛋白酶抑制剂基因CsKPI1的表达,增强茶树对茶尺蠖的防御能力[27]。当前,对虫害诱导茶树抗性的研究主要以挥发性物质为主,非挥发性物质种类庞杂且难以准确定量,因此单片茶树叶片中非挥发性代谢物对虫害的响应及其机制研究鲜见报道。本研究以茶树叶片为研究材料,利用代谢组学技术解析茶尺蠖为害叶尖后非挥发性代谢物在该叶片中的时空变化,并分析关键物质γ-氨基丁酸合成途径的基因表达水平,结合生物测定方法,为揭示茶树非挥发性代谢物对虫害的化学防御机制提供重要基础。
1 材料与方法
1.1 供试茶苗
供试茶苗为2年生龙井43扦插苗,种植在中国农业科学院茶叶研究所嵊州综合实验基地,选择完好健康茶枝作为研究材料。
1.2 供试茶尺蠖
茶尺蠖幼虫由中国农业科学院茶叶研究所昆虫饲养室提供。室内饲养一代后的3龄幼虫,饥饿3 h后用于单片茶树叶片处理。室内饲养一代后的初孵幼虫,取食人工饲料至3龄后用于体质量和体长指标测定。
1.3 供试饲料
人工饲料参考杨子威等[28]的配方并适当改进。称取10 g茶叶干粉、5 g大豆粉、0.35 g抗坏血酸、1.5 g酵母粉混匀备用。称取1.2 g琼脂粉,加入100 mL蒸馏水,加热4 min至透明,立即加入1 g蔗糖、0.05 g山梨醇和0.085 g对羟基苯甲酸甲酯混匀,待温度降到60 ℃时,分别添加0.2、0.5、2.0 mg·g-1的γ-氨基丁酸,对照组不添加γ-氨基丁酸。最后分别倒入混匀的茶叶干粉中并搅拌均匀,待饲料凝固后放入﹣20 ℃冰箱保存待用。
1.4 仪器与试剂
主要仪器:超高效液相色谱-四极杆轨道阱质谱仪(Ultra-high performance liquid chromatography-quadrupole orbitrap mass spectrometry,UHPLC-Q-Exactive/MS),美国Thermo Fisher公司;低温冷冻离心机,德国Eppendorf公司;数控超声波清洗器,昆山市超声仪器有限公司;LightCycler®480 Ⅱ实时荧光定量聚合酶链式反应仪,上海罗氏制药有限公司;万分之一电子天平,奥豪斯仪器(常州)有限公司;超景深显微镜(VHX-6000),基恩士有限公司。
试剂:甲醇(色谱纯)、抗坏血酸购自上海阿拉丁生化科技股份有限公司,乙腈(色谱纯)购自西格玛奥德里奇(上海)贸易有限公司,RNA提取试剂盒购自天根生化科技(北京)有限公司,逆转录试剂盒和荧光定量PCR试剂盒均购自南京诺唯赞生物科技股份有限公司,γ-氨基丁酸购自北京中农思辰生物科技有限公司,大豆粉购自山东艾科特农业发展有限公司,酵母粉购自赛默飞世尔科技公司,琼脂粉和蔗糖均购自国药集团化学试剂有限公司,山梨醇购自北京科奥科技有限公司,对羟基苯甲酸甲酯购自合肥博美生物科技有限责任公司。
1.5 茶树叶片处理
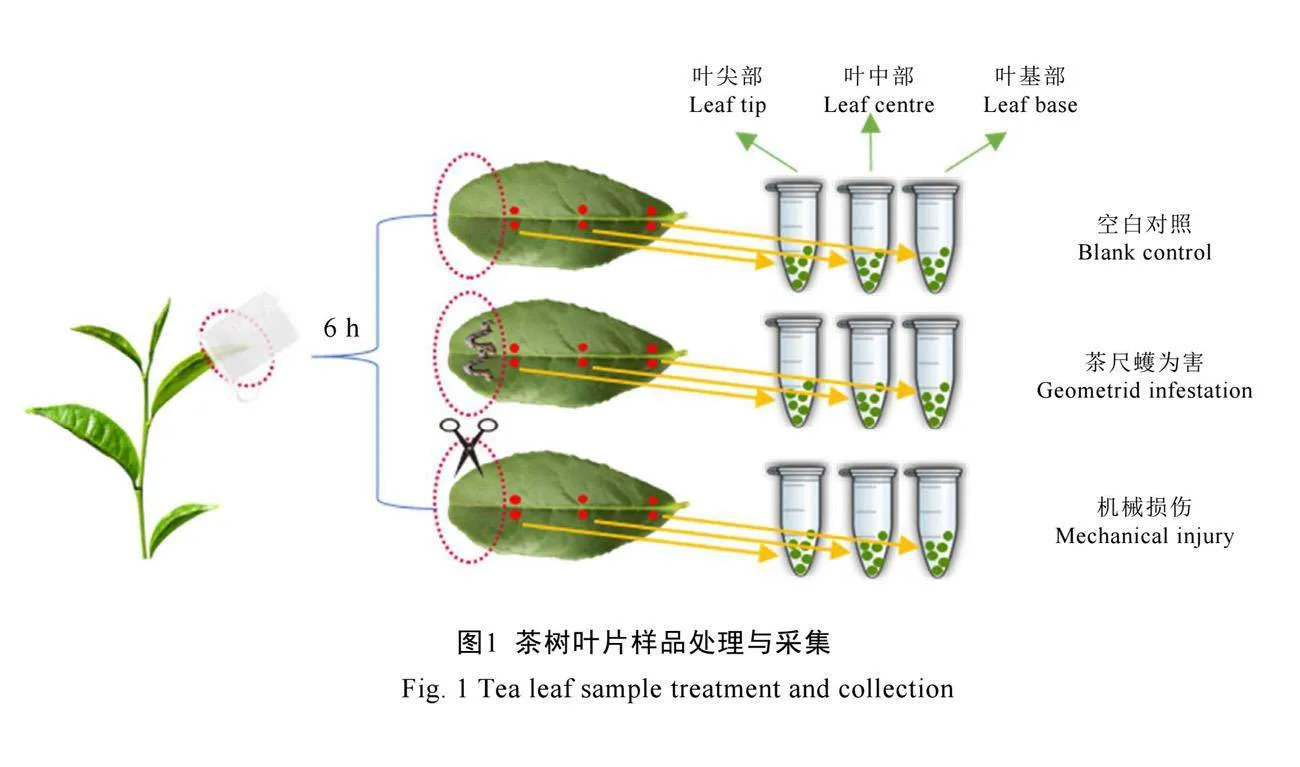
茶尺蠖为害组将2头3龄茶尺蠖幼虫接种于茶树新梢芽下第3叶的叶尖部,套以透气网袋防止逃逸。机械损伤组处理用消毒剪刀模拟茶尺蠖的取食方式,在茶树新梢芽下第3叶的叶尖部形成创伤后,套以透气网袋。空白对照组仅在茶树新梢芽下第3叶套透气网袋(图1)。
1.6 样品采集
处理6 h后取芽下第3叶的叶尖部、叶中部、叶基部(2 mm×2 mm圆形叶片,约0.83 mg)用于非挥发性代谢物及基因表达量分析。6个同一位点叶片组织作为1个混样,每组处理3个混样重复。
1.7 非挥发性代谢物质的测定
1.7.1 样品前处理
样品中加入100 μL 70%的甲醇溶液(V甲醇∶
V水=7∶3),溶解后20 ℃水浴超声30 min,以10 000 r·min-1离心10 min,取上清液待测。
1.7.2 UHPLC-Q-Exactive/MS分析条件
试验样品的代谢组学分析采用UHPLC-Q-
Exactive/MS进行数据采集。
UHPLC条件:T3色谱柱(100 mm×2.1 mm,1.8 μm),柱温40 ℃,流速0.4 mL·min-1,进样量为3 μL。流动相A为0.1%甲酸-水溶液,流动相B为0.1%甲酸-乙腈溶液。洗脱程序:0 min,98% A;0~0.5 min,98% A;0.5~8.0 min,85% A;8.0~13.0 min,65% A;13.0~15.0 min,30% A;15.0~16.0 min,15% A;16.0~16.5 min,98% A;16.5~20.0 min,98% A。
MS条件:采用电喷雾电离(Electrospray ionization,ESI)离子源,正离子全扫描模式,质量扫描范围为质荷比(m/z)80~1 200,毛细管电压3.5 kV,毛细管温度300 ℃,辅助气温度350 ℃,辅助气流速10 L·min-1。
代谢物结构鉴定参考前期研究结果和建立的数据库[29-30]。
1.8 基因表达量分析
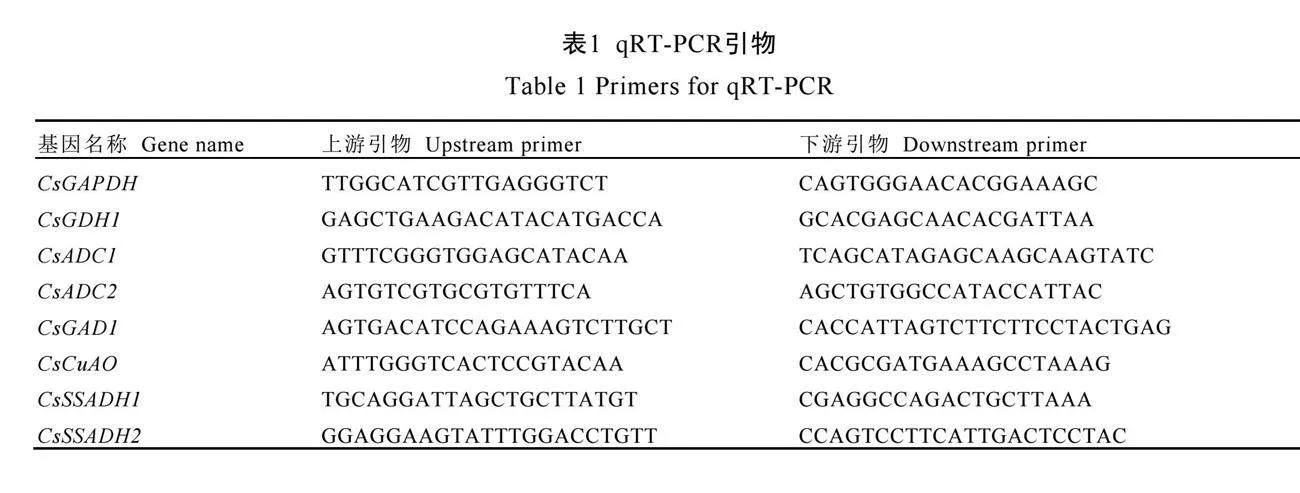
使用多糖多酚植物总RNA提取试剂盒(离心柱型)提取样本RNA,将质量合格的RNA采用逆转录试剂盒反转录为cDNA。使用LightCycler®480 Ⅱ实时荧光定量聚合酶链式反应仪及荧光定量PCR试剂盒进行实时荧光定量PCR(qRT-PCR)。PCR反应条件:95 ℃预变性30 s;95 ℃变性10 s,60 ℃退火30 s,40个循环。每个样品3次生物学重复。采用 法分析基因的相对表达水平。CsGAPDH作为内参基因[31],引物序列见表1。
1.9 体质量和体长测定
将20头初孵幼虫接在放置有人工饲料的塑料培养皿中饲养,每2 d更换1次人工饲料。饲喂至3龄后,用万分之一电子天平和超景深显微镜(VHX-6000)分别测量幼虫的体质量和长度。每组处理重复3次。
1.10 数据分析
UHPLC-Q-Exactive/MS分析得到的原始图谱采用Compound Discoverer 3.2软件进行峰匹配与峰面积提取,采用SIMCA-P 14.1软件进行有监督的偏最小二乘回归分析(Partial least squares discriminant analysis,PLS-DA),采用IBM SPSS Statistics 26软件进行单因素方差分析,GraphPad Prism 9绘制柱状图,热图分析由Tbtools-IIv 2.061软件绘制,在基迪奥生物信息云平台(https://www.omicshare.com)采用皮尔逊相关系数法完成相关性分析。
2 结果与分析
2.1 茶尺蠖为害诱导茶树叶片中代谢物的变化
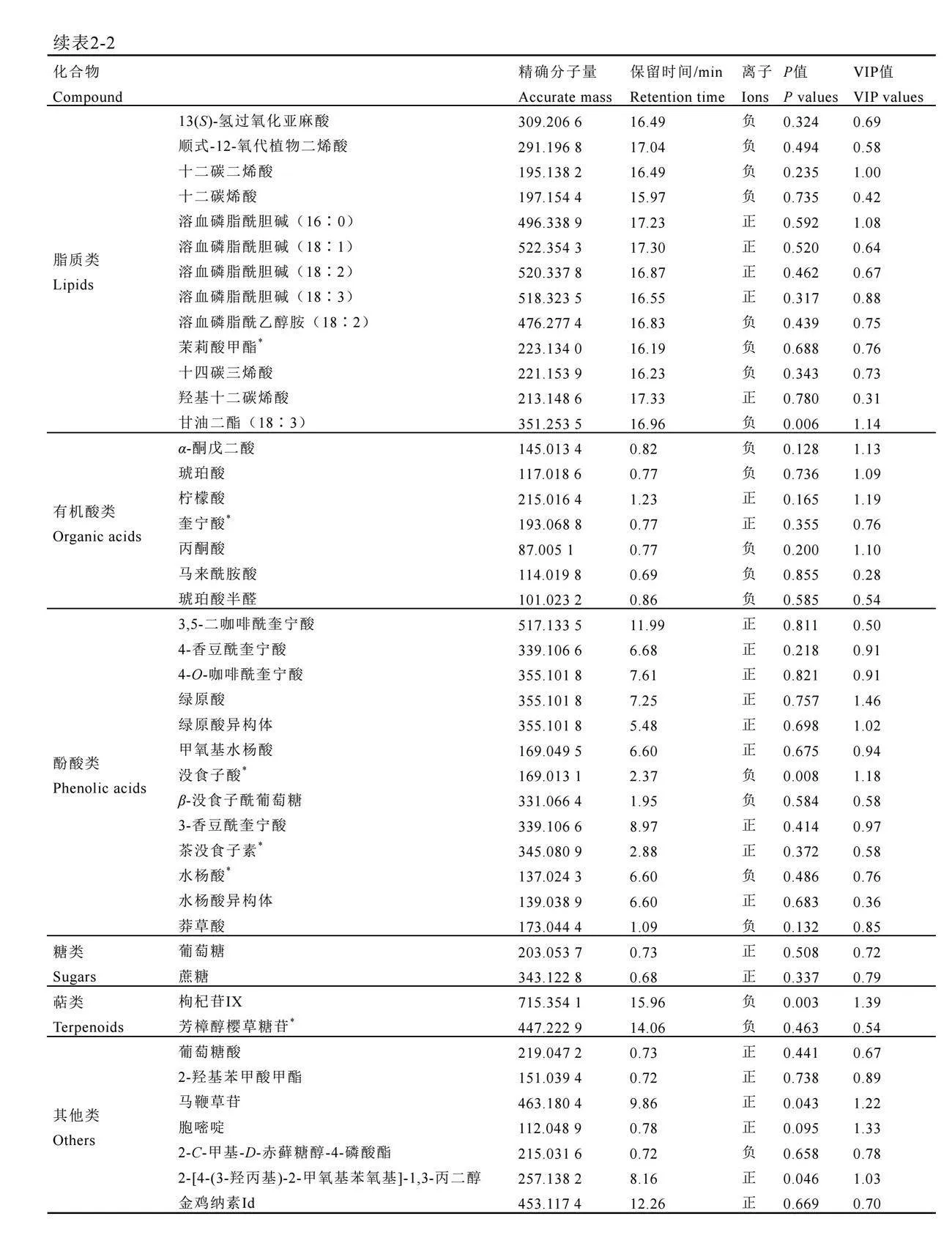
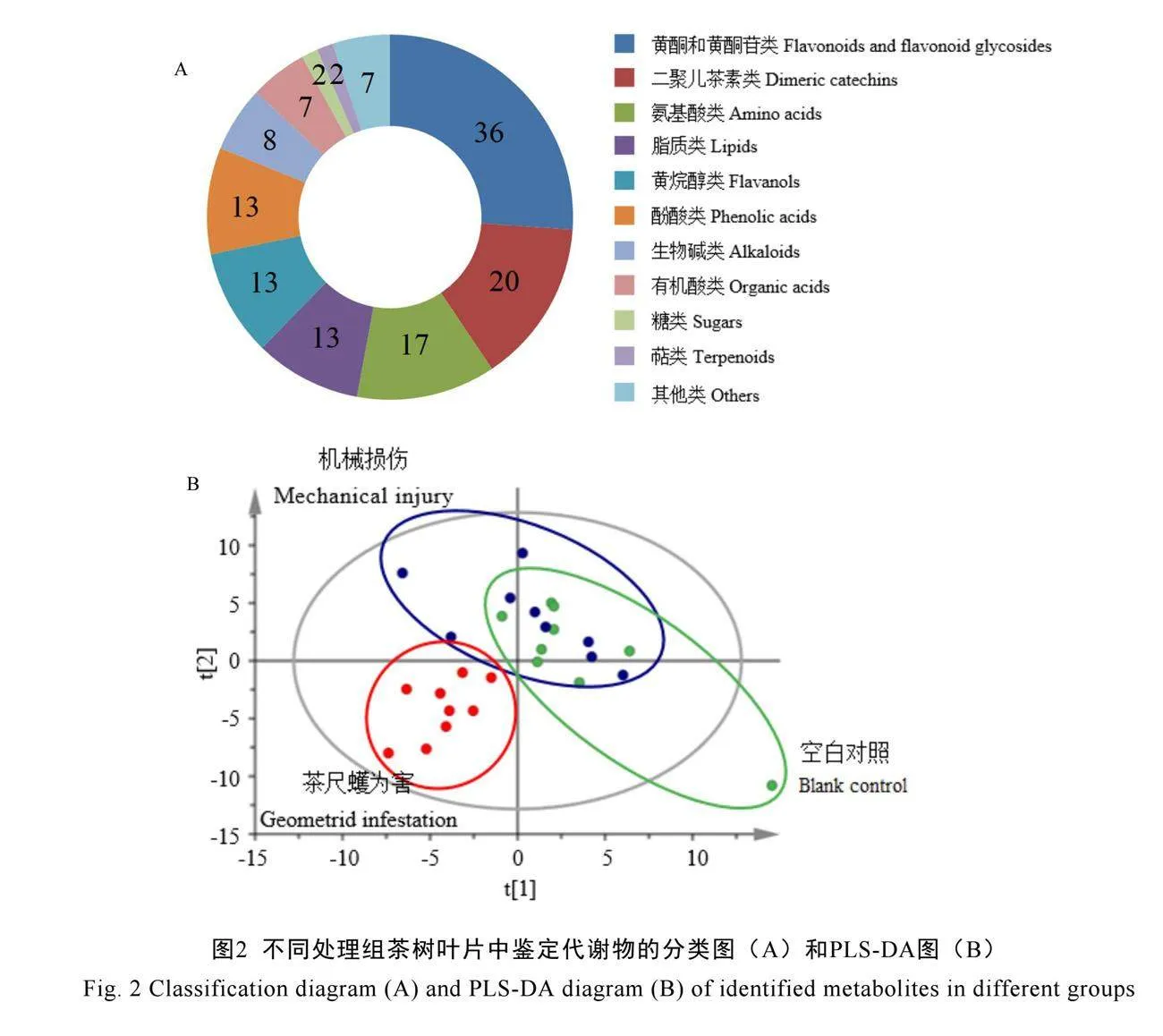
通过一级质谱、二级质谱与标准品比较分析,从茶尺蠖为害后的茶树叶片中共鉴定出138种化合物(表2),包括36种黄酮和黄酮苷类(26%)、20种二聚儿茶素类(14%)、17种氨基酸类(12%)、13种脂质类(9%)、13种黄烷醇类(9%)、13种酚酸类(9%)、8种生物碱类(6%)、7种有机酸类(5%)、2种糖类(1%)、2种萜类(1%)、7种其他类(5%)(图2A)。
PLS-DA显示,茶尺蠖为害后的叶片与空白对照组的叶片在第1主成分上(R2X[1]=0.282)有明显的分离,与机械损伤后的叶片在第2主成分上(R2X[2]=0.199)有较明显的分离(图2B)。该结果表明,茶尺蠖为害能够诱导单片茶树叶片的非挥发性代谢物积累量发生明显改变。
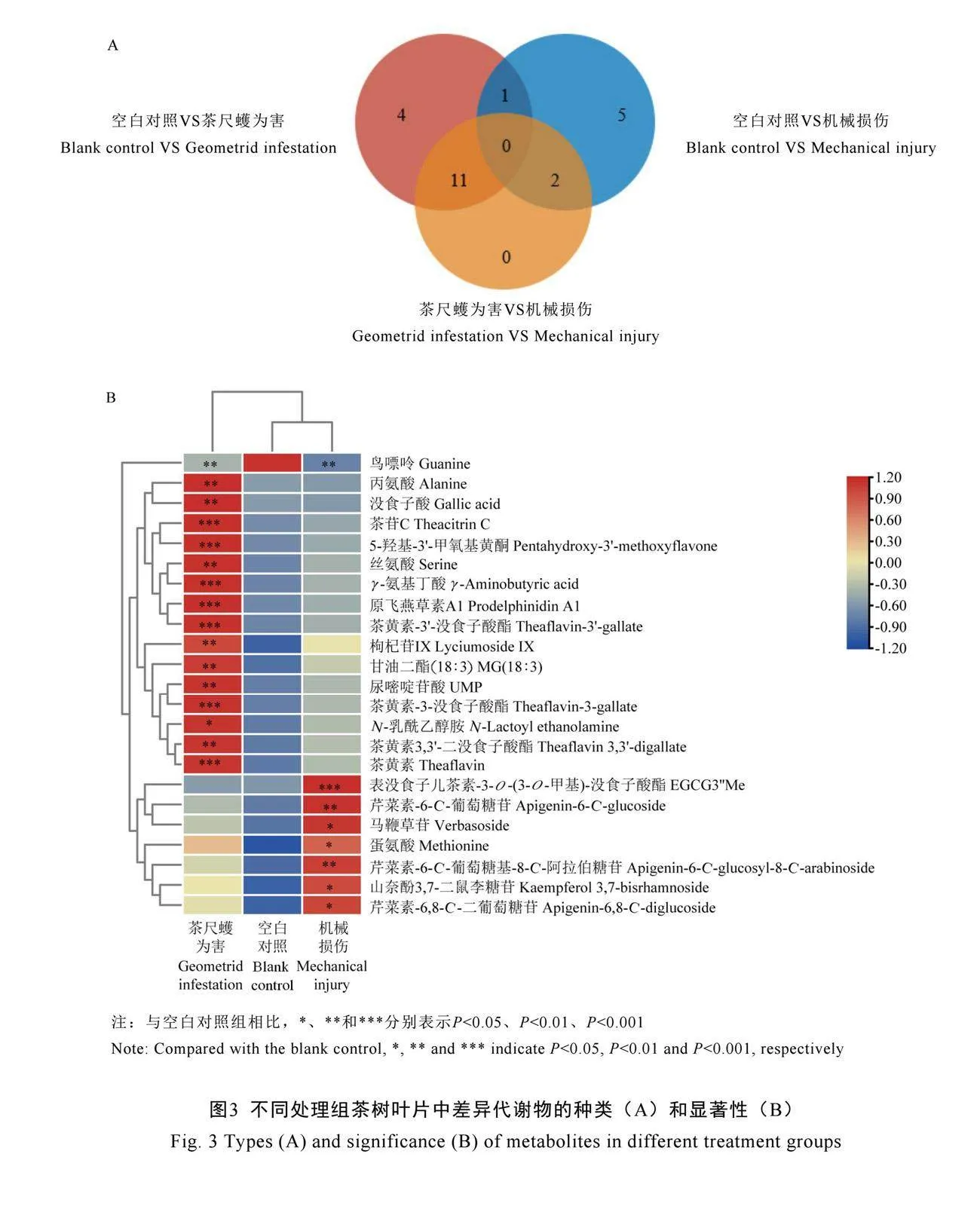
基于VIP>1,P<0.05的筛选标准,在不同处理组的茶树叶片中共筛选出23种差异代谢物。与空白对照相比,茶尺蠖为害诱导产生16种差异代谢物,包括6种二聚儿茶素类(茶苷C、原飞燕草素A1、茶黄素、茶黄素-3-没食子酸酯、茶黄素-3′-没食子酸酯、茶黄素3,3′-二没食子酸酯),3种氨基酸类(γ-氨基丁酸、丙氨酸、丝氨酸),3种生物碱类(鸟嘌呤、尿嘧啶苷酸、N-乳酰乙醇胺),1种黄酮和黄酮苷类(5-羟基-3′-甲氧基黄酮),1种酚酸类(没食子酸),1种萜类(枸杞苷IX),1种脂质类[甘油二酯(18∶3)]。其中,原飞燕草素A1、茶黄素-3-没食子酸酯、茶黄素3,3′-二没食子酸酯、5-羟基-3′-甲氧基黄酮、茶苷C、茶黄素、茶黄素-3′-没食子酸酯、枸杞苷IX相对含量变化显著,分别增加5.90倍、4.38倍、4.21倍、3.89倍、3.53倍、3.06倍、3.06倍、2.03倍(图3)。
与机械损伤相比,茶尺蠖为害诱导产生13种差异代谢物,包括7种二聚儿茶素类[茶苷C、原飞燕草素A1、茶黄素-3′-没食子酸酯、茶黄素-3-没食子酸酯、茶黄素3,3′-二没食子酸
酯、茶黄素、表没食子儿茶素-3-O-(3-O-甲基)-没食子酸酯],3种氨基酸类(γ-氨基丁酸、丙氨酸、丝氨酸),2种黄酮和黄酮苷类(5-羟基-3′-甲氧基黄酮、芹菜素-6-C-葡萄糖苷),1种酚酸类(没食子酸)。其中,原飞燕草素A1、5-羟基-3′-甲氧基黄酮、茶苷C、茶黄素-3-没食子酸酯、茶黄素3,3′-二没食子酸酯、茶黄素-3′-没食子酸酯、茶黄素相对含量变化显著,分别增加3.39倍、2.88倍、2.74倍、2.50倍、2.30倍、2.29倍、2.01倍(图3)。
茶尺蠖为害显著增加了茶树叶片中11种差异代谢物的积累,包括茶苷C、原飞燕草素A1、茶黄素、茶黄素-3-没食子酸酯、茶黄素-3′-没食子酸酯、茶黄素3,3′-二没食子酸酯、γ-氨基丁酸、丙氨酸、丝氨酸、5-羟基-3′-甲氧基黄酮和没食子酸(图3)。
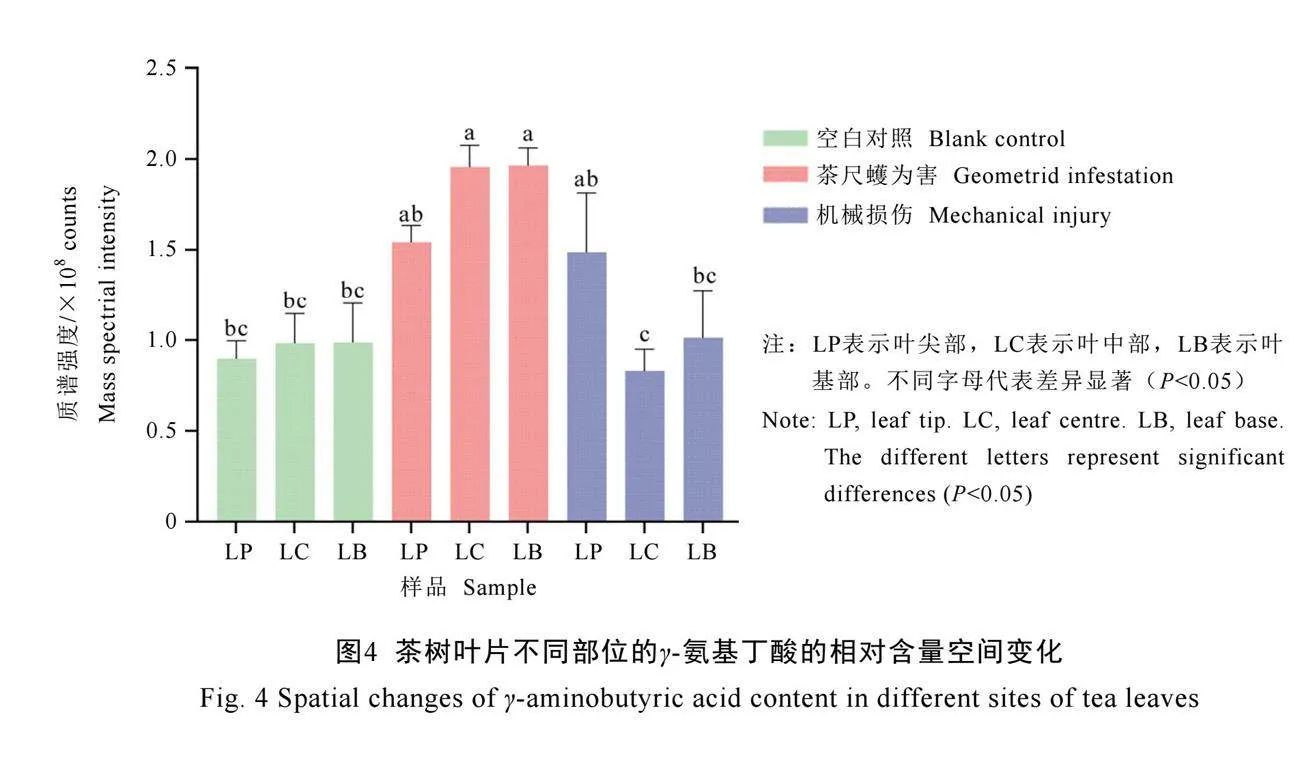
2.2 茶尺蠖为害诱导单片茶树叶片中γ-氨基丁酸的空间变化
茶尺蠖为害后,与空白对照相比,γ-氨基丁酸的相对含量在叶中部和叶基部显著增加(P<0.05),均升高1.99倍,在叶尖部升高1.72倍。茶尺蠖为害后,与机械损伤相比,叶中部与叶基部的γ-氨基丁酸的相对含量显著增加(P<0.05),叶尖部、叶中部、叶基部分别升高1.04倍、2.36倍和1.94倍(图4)。以上结果说明,γ-氨基丁酸是茶尺蠖为害诱导产生的特征代谢物。
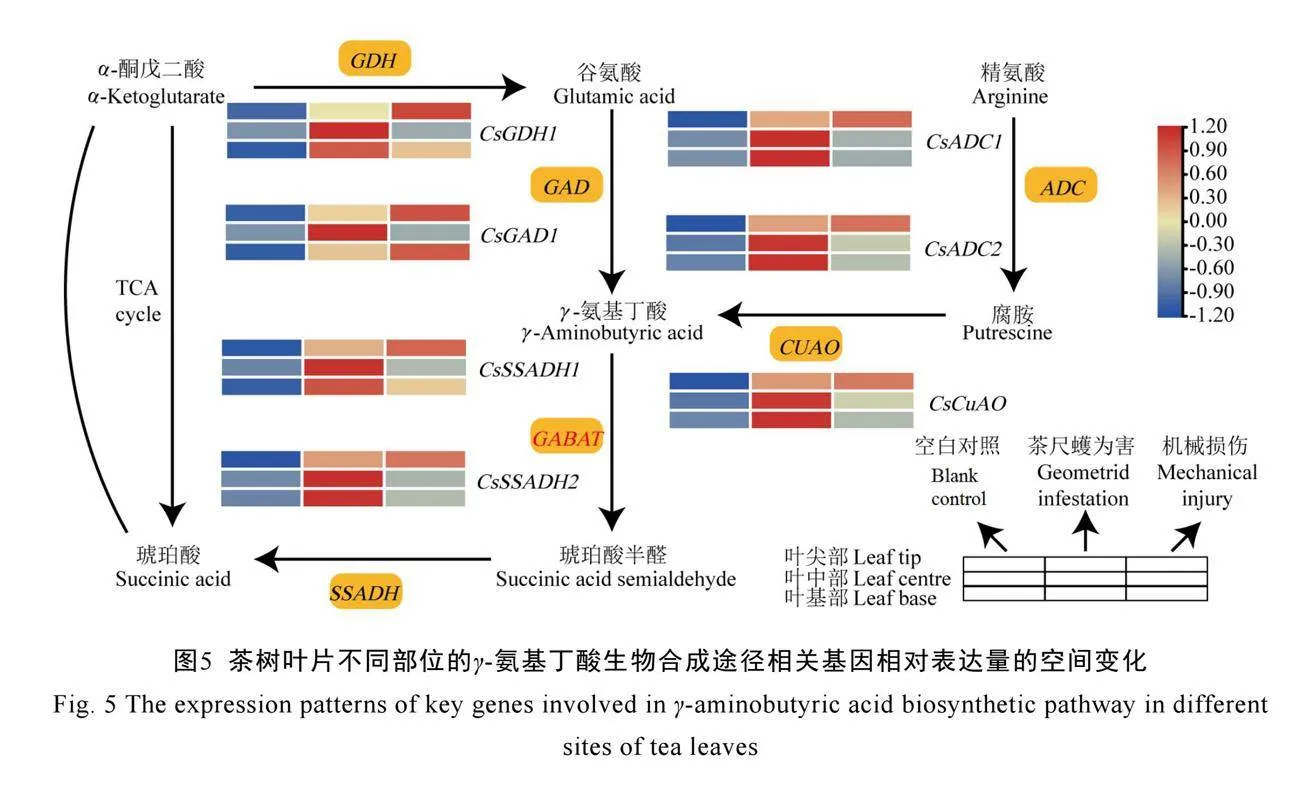
2.3 γ-氨基丁酸生物合成途径关键基因的变化
由图5所示,与空白对照相比,茶尺蠖为害或机械损伤后,γ-氨基丁酸合成途径中关键基因(CsGDH1、CsGAD1、CsCUAO、CsSSADH1、CsSSADH2、CsADC1、CsADC2)的相对表达量在叶尖部、叶中部、叶基部均上调。与机械损伤相比,茶尺蠖为害后,这7个基因的表达量在叶尖部均下调,而在叶中部均上调,其中6个基因在叶基部上调。叶尖部可能因为直接受害,从而产生应激反应,之后防御信号传导至叶中部、叶基部,通过上调γ-氨基丁酸生物合成途径的关键基因,增加γ-氨基丁酸积累来抵御茶尺蠖取食[32]。
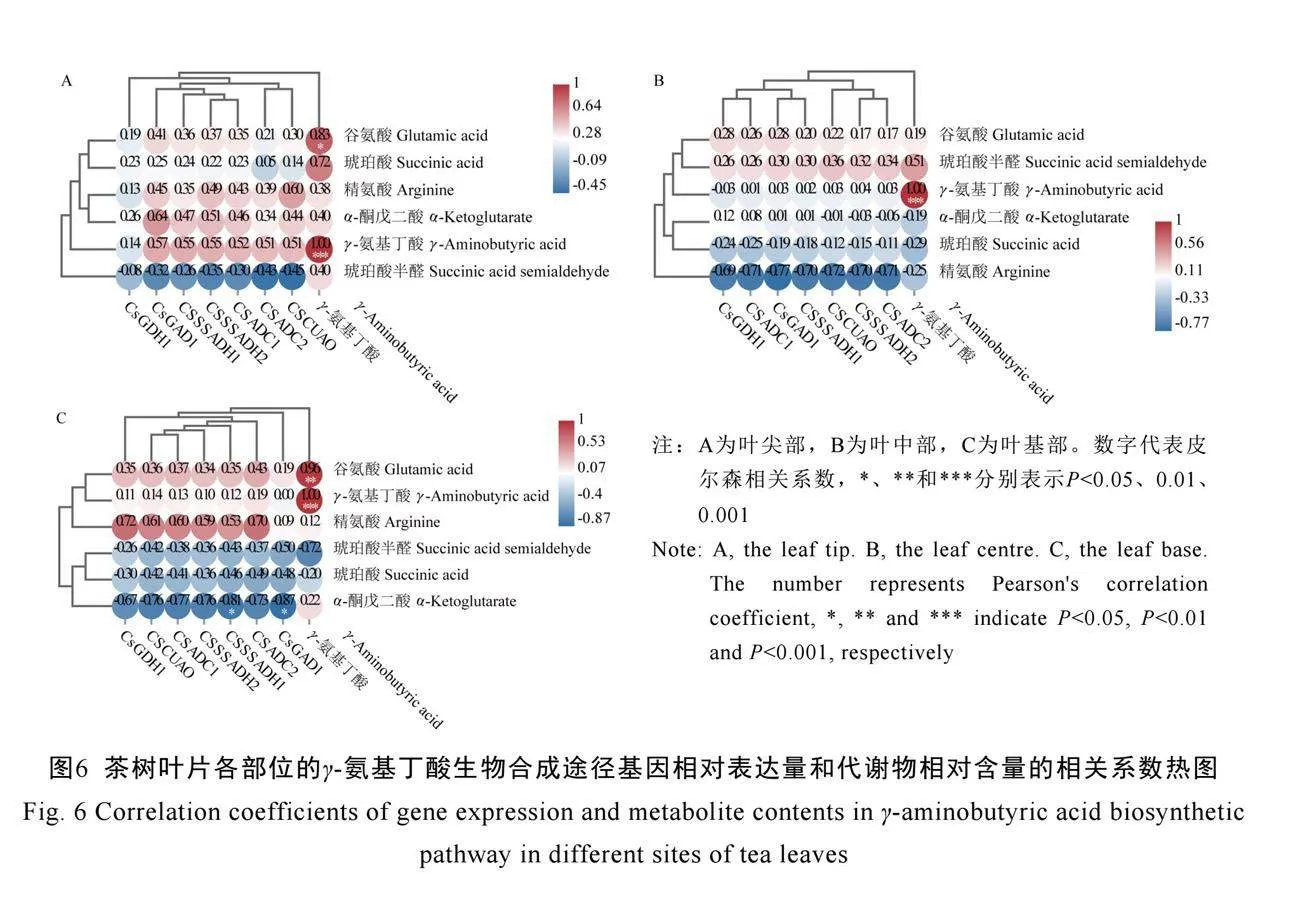
2.4 γ-氨基丁酸生物合成途径相关代谢物和基因的相关性分析
茶尺蠖为害后,受害位点的γ-氨基丁酸相对含量与其前体物质谷氨酸的相对含量呈显著正相关(P<0.05),与合成途径的关键基因CsGAD1、CsSSADH1、CsSSADH2、CsADC1、CsADC2、CsCUAO的相对表达量均呈正相关(图6A)。叶中部的γ-氨基丁酸相对含量仅与琥珀酸半醛的相对含量呈正相关(图6B),而叶基部的γ-氨基丁酸相对含量与琥珀酸半醛的相对含量呈负相关,与谷氨酸的相对含量呈极显著正相关(P<0.01)(图6C)。以上研究结果表明,茶树叶片在茶尺蠖为害后,立即启动防御反应,加快前体物质谷氨酸的合成和转运,进而增加γ-氨基丁酸积累以抵御虫害。
2.5 γ-氨基丁酸对茶尺蠖的生长抑制作用
茶尺蠖分别取食添加了0.2、0.5、2.0 mg·g-1 γ-氨基丁酸的人工饲料后,其体质量与体长均呈下降趋势,且与对照组差异显著(P<0.05,图7)。随着γ-氨基丁酸浓度的升高,其对茶尺蠖幼虫生长抑制作用也逐渐增强。
3 讨论
茶树能感知由植食性昆虫取食或产卵造成的机械损伤和释放的特定诱导物质,从而引起大量非挥发性代谢物质的变化[33]。本研究以茶树叶片为材料,采用代谢组学方法对不同处理下单片茶树叶片3个不同部位的非挥发性物质进行鉴定和分析,发现与空白对照和机械损伤相比,茶尺蠖为害诱导叶片中11种差异代谢物的显著积累,包括6种二聚儿茶素类(茶苷C、原飞燕草素A1、茶黄素、茶黄素-3-没食子酸酯、茶黄素-3′-没食子酸酯、茶黄素3,3′-二没食子酸酯)、3种氨基酸类(γ-氨基丁酸、
丙氨酸、丝氨酸)、1种黄酮和黄酮苷类(5-羟基-3′-甲氧基黄酮)和1种酚酸类(没食子酸)。研究表明,非挥发性物质参与茶树对多种生物胁迫的响应,例如,槲皮素-3-O-葡萄糖苷、儿茶素、表儿茶素、表没食子儿茶素没食子酸酯和没食子酸是茶尺蠖取食诱导茶树积累的关键非挥发性物质,这些化合物是茶树对茶尺蠖的直接抗性物质[25-26,34-36]。然而,茶尺蠖为害诱导非挥发性物质中的其他组分是否能够激活相似的防御反应有待进一步研究。
γ-氨基丁酸是茶树中高含量非蛋白氨基酸,其对昆虫的神经传递具有负调节功能,例如,γ-氨基丁酸能抑制昆虫神经递质传递[37-40]。昆虫体内的γ-氨基丁酸会与γ-氨基丁酸受体结合[41],如离子型GABAA受体和代谢型GABAB受体等,快速激活相应的氯离子通道,引发氯离子细胞内流,导致细胞质膜产生超极化[12],产生抑制性突触后电位作用,抑制昆虫神经中枢的传导并产生镇静效果[42]。因此,γ-氨基丁酸过度积累会产生持续抑制效应,从而影响昆虫的生长和发育[43]。
昆虫取食会诱导植物启动先于系统反应的局部快速反应机制,包括钙信号的传导,随后激活γ-氨基丁酸代谢途径,导致γ-氨基丁酸的积累[44]。本研究中γ-氨基丁酸在茶树叶片的3个部位持续积累,并且其相对含量与前体物质谷氨酸的相对含量在叶尖部和叶基部显著正相关,说明γ-氨基丁酸代谢途径被激活以抵御茶尺蠖为害[45]。人工饲料饲喂试验也证明了低龄茶尺蠖幼虫的生长发育明显受到γ-氨基丁酸的抑制。
本研究解析了茶尺蠖为害后单片茶树叶片中非挥发性代谢物的相对含量变化和空间变化,并发现昆虫抑制性神经递质γ-氨基丁酸的相对含量与其前体物质谷氨酸显著相关,并对茶尺蠖的生长发育有抑制效果,说明γ-氨基丁酸代谢途径在茶树抵御早期茶尺蠖为害的防御反应中发挥重要作用。
参考文献
[1] Walling L L. The myriad plant responses to herbivores [J]. Journal of Plant Growth Regulation, 2000, 19(2): 195-216.
[2] 谢辉, 王燕, 刘银泉, 等. 植物组成型防御对植食性昆虫的影响[J]. 植物保护, 2012, 38(1): 1-5.
Xie H, Wang Y, Liu Y Q, et al. The influence of plant constitutive defense system on phytophagous insects [J]. Plant Protection, 2012, 38(1): 1-5.
[3] Agrawal A A. Induced responses to herbivory and increased plant performance [J]. Science, 1998, 279(5354): 1201-1202.
[4] Akula R, Mukherjee S. New insights on neurotransmitters signaling mechanisms in plants [J]. Plant Signaling & Behavior, 2020, 15(6): 1737450. doi: 10.1080/15592324.2020.1737450.
[5] Kessler A, Baldwin I T. Plant responses to insect herbivory: the emerging molecular analysis [J]. Annual Review of Plant Biology, 2002, 53: 299-328.
[6] Köllner T G, Lenk C, Schnee C, et al. Localization of sesquiterpene formation and emission in maize leaves after herbivore damage [J]. BMC Plant Biology, 201psSEPEtj12RDx5q2sPXeqJV52JcCqZwpP09JcbqzNJw=3, 13(1): 15. doi: 10.1186/1471-2229-13-15.
[7] Malook S U, Qi J F, Hettenhausen C, et al. The oriental armyworm (Mythimna separata) feeding induces systemic defence responses within and between maize leaves [J]. Philosophical Transactions of the Royal Society B, 2019, 374(1767): 20180307. doi: 10.1098/rstb.2018.0307.
[8] Li L, Dou N, Zhang H, et al. The versatile GABA in plants [J]. Plant Signaling & Behavior, 2021, 16(3): 1862565. doi:10.1080/15592324.2020.1862565.
[9] Macgregor K B, Shelp B J, Peiris S, et al. Overexpression of glutamate decarboxylase in transgenic tobacco plants deters feeding by phytophagous insect larvae [J]. Journal of Chemical Ecology, 2003, 29(9): 2177-2182.
[10] Scholz S S, Malabarba J, Reichelt M, et al. Evidence for GABA-induced systemic GABA accumulation in Arabidopsis upon wounding [J]. Frontiers in Plant Science, 2017, 8: 388. doi: 10.3389/fpls.2017.00388.
[11] Bown A W, Hall D E, MacGregor K B. Insect footsteps on leaves stimulate the accumulation of 4-aminobutyrate and can be visualized through increased chlorophyll fluorescence and superoxide production [J]. Plant Physiology, 2002, 129(4): 1430-1434.
[12] Bown A W, MacGregor K B, Shelp B J. Gamma-aminobutyrate: defense against invertebrate pests? [J]. Trends in Plant Science, 2006, 11(9): 424-427.
[13] Scholz S S, Reichelt M, Mekonnen D W, et al. Insect herbivory-elicited gaba accumulation in plants is a wound-induced, direct, systemic, and jasmonate-independent defense response [J]. Frontiers in Plant Science, 2015, 6: 1128. doi: 10.3389/fpls.2015.01128.
[14] Zhou H L, Chen H Y, Bao D P, et al. Recent advances of γ-aminobutyric acid: physiological and immunity function, enrichment, and metabolic pathway [J]. Frontiers in Nutrition, 2022, 9: 1076223. doi: 10.3389/fnut.2022.1076223.
[15] 周俊萍, 徐玉娟, 温靖, 等. γ-氨基丁酸(GABA)的研究进展[J]. 食品工业科技, 2024, 45(5): 393-401.
Zhou J P, Xu Y J, Wen J, et al. Research progress of γ-aminobutyric acid (GABA) [J]. Science and Technology of Food Industry, 2024, 45(5): 393-401.
[16] 程永祥. 1969—2019年临安茶尺蠖发生特点调查与分析[J]. 中国茶叶, 2020, 42(4): 55-56, 59.
Cheng Y X. Investigation and analysis on the occurrence characteristics of tea geometrid in Lin'an from 1969 to 2019 [J]. China Tea, 2020, 42(4): 55-56, 59.
[17] Liu G H, Wang Q, Chen H, et al. Plant-derived monoterpene S-linalool and β-ocimene generated by CsLIS and CsOCS-SCZ are key chemical cues for attracting parasitoid wasps for suppressing Ectropis obliqua infestation in Camellia sinensis L [J]. Plant, Cell & Environment, 2024, 47(3): 913-927.
[18] Liao Y Y, Tan H B, Jian G T, et al. Herbivore-induced (Z)-3-Hexen-1-ol is an airborne signal that promotes direct and indirect defenses in tea (Camellia sinensis) under light [J]. Journal of Agricultural and Food Chemistry, 2021, 69(43): 12608-12620.
[19] Liu G H, Yang M, Fu J Y. Identification and characterization of two sesquiterpene synthase genes involved in volatile-mediated defense in tea plant (Camellia sinensis) [J]. Plant Physiology and Biochemistry, 2020, 155: 650-657.
[20] Ye M, Liu M M, Erb M, et al. Indole primes defence signalling and increases herbivore resistance in tea plants [J]. Plant, Cell & Environment, 2021, 44(4): 1165-1177.
[21] Qian J J, Liao Y Y, Jian G T, et al. Light induces an increasing release of benzyl nitrile against diurnal herbivore Ectropis grisescens Warren attack in tea (Camellia sinensis) plants [J]. Plant, Cell & Environment, 2023, 46(11): 3464-3480.
[22] Jing T T, Qian X N, Du W K, et al. Herbivore-induced volatiles influence moth preference by increasing the β-ocimene emission of neighbouring tea plants [J]. Plant, Cell & Environment, 2021, 44(11): 3667-3680.
[23] Chen Y F, Wang Z Y, Gao T, et al. Deep learning and targeted metabolomics-based monitoring of chewing insects in tea plants and screening defense compounds [J]. Plant, Cell & Environment, 2023, 47: 698-713.
[24] Wang W W, Zheng C, Hao W J, et al. Transcriptome and metabolome analysis reveal candidate genes and biochemicals involved in tea geometrid defense in Camellia sinensis [J]. Plos One, 2018, 13(8): e0201670. doi: 10.1371/journal.pone.0201670.
[25] Jing T T, Du W K, Qian X N, et al. UGT89AC1-mediated quercetin glucosylation is induced upon herbivore damage and enhances Camellia sinensis resistance to insect feeding [J]. Plant, Cell & Environment, 2024, 47(2): 682-697.
[26] Li X W, Zhang J, Lin S B, et al. (+)-Catechin, epicatechin and epigallocatechin gallate are important inducible defensive compounds against Ectropis grisescens in tea plants [J]. Plant, Cell & Environment, 2021, 45: 496-511.
[27] Zhu J Y, He Y X, Yan X M, et al. Duplication and transcriptional divergence of three Kunitz protease inhibitor genes that modulate insect and pathogen defenses in tea plant (Camellia sinensis) [J]. Horticulture Research, 2019, 6(1): 126. doi:10.1038/s41438-019-0208-5.
[28] Yang Z W, Duan X N, Jin S, et al. Regurgitant derived from the tea geometrid Ectropis obliqua suppresses wound-induced polyphenol oxidases activity in tea plants [J]. Journal of Chemical Ecology, 2013, 39(6): 744-751.
[29] Gao J J, Zhou M X, Chen D, et al. High-throughput screening and investigation of the inhibitory mechanism of α-glucosidase inhibitors in teas using an affinity selection-mass spectrometry method [J]. Food Chemistry, 2023, 422: 136179. doi: 10.1016/j.foodchem.2023.136179.
[30] Dai W D, Hu Z Y, Xie D C, et al. A novel spatial-resolution targeted metabolomics method in a single leaf of the tea plant (Camellia sinensis) [J]. Food Chemistry, 2020, 311: 126007. doi:10.1016/j.foodchem.2019.126007.
[31] 孙美莲, 王云生, 杨冬青, 等. 茶树实时荧光定量PCR分析中内参基因的选择[J]. 植物学报, 2010, 45(5): 579-587.
Sun M L, Wang Y S, Yang D Q, et al. Selection of reference genes in real time fluorescence quantitative PCR analysis of tea plants [J]. Chinese Bulletin of Botany, 2010, 45(5): 579-587.
[32] Bown A W, Shelp B J. Plant GABA: not just a metabolite [J]. Trends in Plant Science, 2016, 21(10): 811-813.
[33] Zhang J, Yu Y C, Qian X N, et al. Recent advances in the specialized metabolites mediating resistance to insect pests and pathogens in tea plants (Camellia sinensis) [J]. Plants, 2024, 13(2): 323. doi: 10.3390/plants13020323.
[34] Lin S B, Ye M, Li X W, et al. A novel inhibitor of the jasmonic acid signaling pathway represses herbivore resistance in tea plants [J]. Horticulture Research, 2022, 9: uhab038. doi: 10.1093/hr/uhab038.
[35] 冉伟, 张瑾, 张新, 等. 茶尺蠖幼虫取食提高茶树儿茶素代谢响应强度[J]. 茶叶科学, 2018, 38(2): 133-139.
Ran W, Zhang J, Zhang X, et al. Infestation of Ectropis obliqua affects the catechin metabolism in tea plants [J]. Journal of Tea Science, 2018, 38(2): 133-139.
[36] Zhang X, Ran W, Li X W, et al. Exogenous application of gallic acid induces the direct defense of tea plant against Ectropis obliqua caterpillars [J]. Frontiers in Plant Science, 2022, 13: 833489. doi: 10.3389/fpls.2022.833489.
[37] Huang T F, Jander G, Vos M D. Non-protein amino acids in plant defense against insect herbivores: representative cases and opportunities for further functional analysis [J]. Phytochemistry, 2011, 72(13): 1531-1537.
[38] Mithöfer A, Boland W. Plant defense against herbivores: chemical aspects [J]. Annual Review of Plant Biology, 2012, 63: 431-450.
[39] Seifikalhor M, Aliniaeifard S, Hassani B, et al. Diverse role of γ-aminobutyric acid in dynamic plant cell responses [J]. Plant Cell Reports, 2019, 38(8): 847-867.
[40] Tarkowski Ł P, Signorelli S, Höfte M. γ-Aminobutyric acid and related amino acids in plant immune responses: emerging mechanisms of action [J]. Plant, Cell & Environment, 2020, 43(5): 1103-1116.
[41] 筱禾. 作用于GABA受体杀虫剂的代谢、作用机制及开发研究[J]. 世界农药, 2019, 41(2): 18-28.
Xiao H. Study on metabolism, mechanism of action and development of insecticides acting on GABA receptors [J]. World Pesticide, 2019, 41(2): 18-28.
[42] Irving S N, Osborne M P, Wilson R G. Studies on L-glutamate in insect haemolymph [J]. Physiological Entomology, 1979, 4(2): 139-146.
[43] Hosie A M, Aronstein K, Sattelle D B, et al. Molecular biology of insect neuronal GABA receptors [J]. Trends in Neurosciences, 1997, 20(12): 578-583.
[44] Kiep V, Vadassery J, Lattke J, et al. Systemic cytosolic Ca2+ elevation is activated upon wounding and herbivory in Arabidopsis [J]. The New Phytologist, 2015, 207(4): 996-1004.
[45] 余光辉, 涂奕霏, 李承龙, 等. 植物GABA信号途径研究[J]. 中南民族大学学报(自然科学版), 2021, 40(5): 472-477.
Yu G H, Tu Y F, Li C L, et al. GABA signaling pathway research in plant kingdoms [J]. Journal of South-central Minzu University (Natural Science Edition), 2021, 40(5): 472-477.
