甲状腺乳头状癌转移性淋巴结大小与FNA-Tg测值的关系
2024-10-31陈易来陈煜东詹维伟周伟
摘要:目的 "探究甲状腺乳头状癌颈部转移性淋巴结大小与穿刺洗脱液甲状腺球蛋白(FNA-Tg)测值的关系。方法 "连续收集2018年4月~2019年1月上海交通大学医学院附属瑞金医院的96例疑似甲状腺乳头状癌颈部淋巴结转移或复发患者的临床资料,共纳入疾病相关的颈部淋巴结136枚,以穿刺细胞学结果或石蜡病理为金标准,将颈部淋巴结分为良性和恶性。对患者的颈部可疑淋巴结进行超声评估,测量淋巴结在最大切面上的长轴和短轴,对淋巴结进行穿刺细胞学检查和FNA-Tg测定,FNA-Tg样本使用ECL分析仪(Cobase 602,瑞士罗氏)和ELEXSYS TG II试剂盒测定Tg数值。所有患者穿刺前2周内均完成甲状腺功能的血清学检测,分析甲状腺乳头状癌转移性淋巴结大小与FNA-Tg测值之间的关系。结果 "136枚可疑淋巴结中,89枚(65.44%)和47枚(34.56%)分别诊断为转移性淋巴结和良性淋巴结。转移性淋巴结FNA-Tg的测值水平高于非转移性淋巴结(中位数631.550 ng/mL vs 0.056 ng/mL,Plt;0.001),FNA-Tg诊断转移性淋巴结的截值为2.71 ng/mL,FNA-Tg/sTg诊断转移性淋巴结的截值为6.50 ng/mL,淋巴结大小与FNA-Tg的测值及诊断结果无相关性(Pgt;0.05)。结论 "甲状腺乳头状癌转移性淋巴结的FNA-Tg测值结果显著高于非转移性淋巴结,淋巴结的大小本身不影响FNA-Tg的测值及诊断结果。
关键词:甲状腺球蛋白;甲状腺癌;淋巴结;超声
Relationship between and FNA-Tg and the size of metastatic lymph nodes from papillary thyroid carcinoma
CHEN Yilai1, CHEN Yudong1, ZHAN Weiwei1, ZHOU Wei1, 2
1Department of Ultrasound, Ruijin Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200025, China; 2Department of Ultrasound, Ruijin Hospital/Lu Wan Branch, School of Medicine, Shanghai Jiaotong University, Shanghai 200025, China
Abstract: Objective To analyze the relationship between and the value of FNA-Tg and the size of metastatic lymph nodes from papillary thyroid carcinoma (PTC). Methods The clinical data of 96 patients with suspected cervical lymph node metastasis or recurrence of thyroid papillary carcinoma in Ruijin Hospital affiliated to Medical College of Shanghai Jiaotong University from April 2018 to January 2019 were collected. A total of 136 suspicious cervical lymph nodes from 96 PTC patients were prospectively included, and the lymph nodes were divided into benign and malignant according to the results of aspiration cytology or paraffin pathology. The long and short axes of each lymph node on the largest section were measured by ultrasound, each suspected lymph node was aspirated with a 22-gauge needle, then FNA-Tg was measured. ECL analyzer (Cobas E602, Roche, Switzerland) and ELEXSYS TG II kit were used to determine the Tg value of FNA-Tg samples. Serological examination of thyroid function was performed in all patients within 2 weeks before FNA. The relationship between metastatic lymph node size and FNA-Tg in PTC patients was analyzed. Results Among the 136 lymph nodes, 89 (65.44%) were diagnosed as metastatic lymph nodes and 47 (34.56%) were benign. The level of FNA-Tg in metastatic lymph nodes was significantly higher than that in non-metastatic lymph nodes (median 631.550 ng/mL vs 0.056 ng/mL). The cut-off value of FNA-Tg and FNA-Tg/sTg in the diagnosis of metastatic lymph nodes was 2.71 ng/mL and 6.50 ng/mL separately. There was no significant correlation between the size of lymph nodes and FNA-Tg (Pgt;0.05). Conclusion The FNA-Tg level of metastatic lymph nodes in PTC patients is significantly higher than that of non-metastatic lymph nodes. The size of lymph nodes alone can not predict the level of FNA-Tg.
Keywords: thyroglobulin; thyroid carcinoma; lymph node; ultrasonography
甲状腺癌是最常见的内分泌恶性肿瘤,其发病率呈逐年上升趋势[1],其中最为常见的类型即甲状腺乳头状癌(PTC),目前PTC的颈部淋巴结转移率高达20%~50%[2]。PTC的颈部淋巴结转移的诊断对临床手术决策起到至关重要的作用[3-6],而在临床实践中,对于部分存在囊性变或体积较小的淋巴结确诊较为困难,细胞样本不足和假阴性率分别为5%~20%和6%~8%[7],且对细胞病理学专家的经验和技术有一定的依赖性。近年有研究提出应用FNA洗脱液中甲状腺球蛋白(FNA-Tg)来诊断PTC转移性淋巴结,文献报道FNA-Tg诊断转移性淋巴结的敏感度为81%~100%,特异度为95%~100%[8-11]。FNA-Tg的检测可能受到各种因素的影响,如血清甲状腺球蛋白(sTg)、血清抗甲状腺球蛋白(TgAb)、血清促甲状腺素(TSH)、Tg检测试剂盒性能以及穿刺技术等因素相关。这些影响因素的存在最终可能影响FNA-Tg的测值乃至诊断结果,从而给研究间的对比增加了难度[12-13]。尽管既往研究分析了上述各种因素对FNA-Tg结果可能造成的影响,然而淋巴结大小对FNA-Tg的测值的影响尚待探索。本文旨在分析甲状腺乳头状癌颈部转移性淋巴结大小与穿刺洗脱液甲状腺球蛋白(FNA-Tg)测值的关系,为FNA-Tg在小体积淋巴结诊断中的应用提供理论依据。
1 "资料与方法
1.1 "一般资料
本研究连续性收集了2018年4月~2019年1月上海交通大学医学院附属瑞金医院的96例疑似甲状腺乳头状癌颈部淋巴结转移或复发的患者,本研究通过医院伦理学审批(2023临伦审第386号),所有纳入患者均已签署知情同意书。其中男性患者41例,女性患者55例,年龄16~82(39±12.4)岁,纳入标准:已行甲状腺功能血清学检查及颈部超声检查,颈部淋巴结出现至少1个可疑特征;细胞学或病理学结果诊断为良性或恶性;甲状腺切除术后(甲状腺部分切除或甲状腺全切术后)。排除标准:无法耐受或拒绝穿刺者;未取得甲状腺功能血清学检查者。最终纳入淋巴结136枚,对可疑的颈部淋巴结均行超声引导下细针穿刺活检(US-FNA)和FNA-Tg测定。对淋巴结FNA结果提示转移的患者行手术治疗,并根据术前定位取得相应淋巴结的石蜡病理结果;对于部分术前US-FNA结果阴性而无需手术的患者,以术前FNA结果为金标准。
1.2 "方法
1.2.1 "仪器 " 采用Esaote MyLab 90型彩色多普勒超声诊断仪和高频线阵探头。准备22 G针头,5 mL针筒,2 mL离心管及0.9%生理盐水等;采用Beckmam-Coulter Microfuge 18离心机,设置为3000 r/5 min处理穿刺洗脱液样本。FNA-Tg标本及sTg测定采用电化学免疫发光法,检测范围0.04~500 ng/mL。检测时采用用欧洲共同体标准物质局的有证参考物质457标准品校正以降低检测间偏倚[14]。
1.2.2 "超声诊断颈部可疑PTC转移性淋巴结的声像图标准 (1)出现钙化;(2)出现囊性区;(3)出现团状高回声区;(4)边缘型或混合型血供;(5)淋巴结形态趋圆(纵横比≤2);(6)淋巴门结构消失。出现(1)~(4)项任意1个声像图特征或同时具备(5)、(6)2项声像图特征时诊断为可疑淋巴结[8]。所有超声图像均由1位有20年以上超声诊断经验的超声专家采集,根据每名患者情况调节仪器获得最佳图像,并在二维图像上测量淋巴结大小。最后由2位医生在未知病理诊断结果的情况下对图像资料进行回顾性分析,并对每个指标评图,若结果不一致则协商一致后纳入。
1.2.3 "穿刺方法 " 超声引导下穿刺细胞学检查由1位具有7年穿刺操作经验的超声科医师完成,穿刺采取细针负压抽吸技术,每枚淋巴结穿刺2~3次,超声科护士随即将针芯内的组织推注至载玻片上完成涂片和固定,最后将所取得的穿刺细胞学涂片和穿刺洗脱液标本分别标记并送细胞学检查和FNA-Tg测定。
1.3 "统计学分析
所有数据采用SPSS19.0软件处理。正态分布的计量资料以均数±标准差表示,非正态分布的计量资料以中位数表示。计量资料组间比较采取秩和(Mann Whitney U)检验,采用Spearman秩相关分析相关性(居于-1~1之间);二分类变量采用配对卡方检验进行两两比较。计算FNA-Tg诊断颈部转移性淋巴结的敏感度、特异度和约登指数等。绘制ROC曲线,计算曲线下面积(AUC),根据约登指数最高的临界点确定FNA-Tg及FNB-Tg/sTg的最佳诊断截值。以Plt;0.05为差异有统计学意义。
2 "结果
2.1 "临床特征及细胞病理结果
96例患者的136枚颈部可疑淋巴结中,92枚经手术病理证实,以细胞或病理学结果为金标准,最终89枚被诊断为淋巴结转移阳性,47枚被诊断为淋巴结转移阴性。在47枚阴性淋巴结中包括44例反应性淋巴结,2例结核淋巴结,1例异位甲状腺组织。
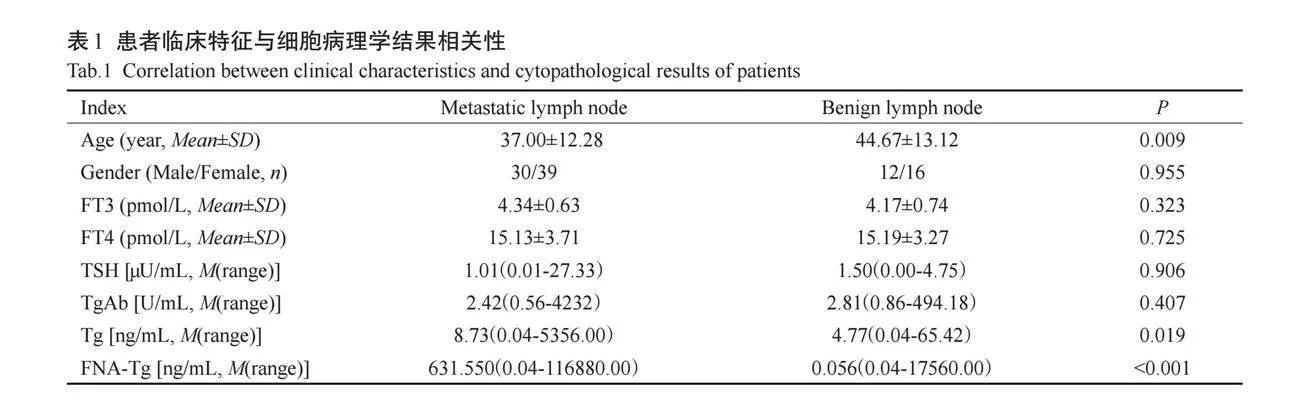
淋巴结良恶性与患者年龄和sTg水平有关(Plt;0.05),PTC患者颈部淋巴结转移的患者较非淋巴结转移的患者更年轻(Plt;0.01);淋巴结良恶性与患者性别、血清FT3、FT4、TSH、sTgAb无关(Pgt;0.05);转移性淋巴结FNA-Tg测值水平高于非转移性淋巴结(Plt;0.001),剔除3例假阳性病例后,非转移性淋巴结FNA-Tg的中位数为0.04 ng/mL,差异有统计学意义(Plt;0.001,表1)。
2.2 "FNA-Tg诊断转移性淋巴结的截值
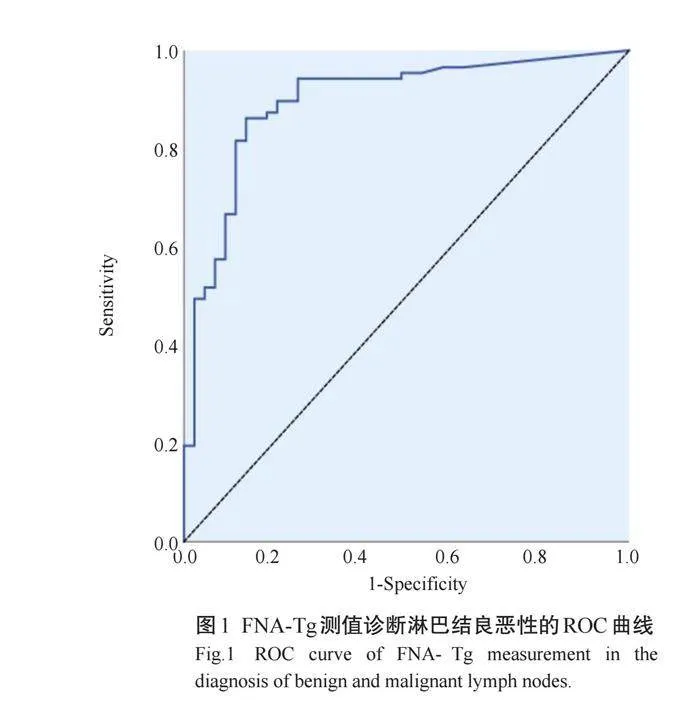
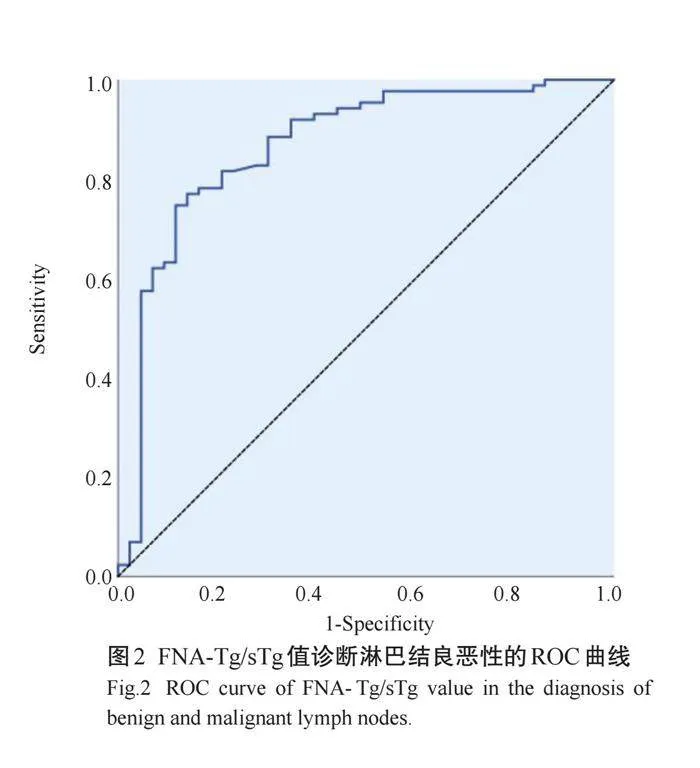
ROC曲线显示,FNA-Tg诊断转移性淋巴结的截值为2.71 ng/mL,敏感度为88.5%,特异度为83.0%,约登指数为71.8%,AUC为0.897(95%CI: 0.836~0.966,Plt;0.001,图1);FNA-Tg/sTg诊断转移性淋巴结的截值为6.50 ng/mL,敏感度为74.7%,特异度为88.4%,约登指数为63.1%,AUC为0.866(95% CI: 0.794~0.938,Plt;0.001,图2)。
2.3 "淋巴结大小与淋巴结良恶性的关系
136枚可疑的PTC转移性颈部淋巴结中,130枚位于颈侧区,另有6枚来自甲状腺切除术后患者的颈部VI区淋巴结,其中非转移性淋巴结大小为5.58±2.63 mm,转移性淋巴结大小为5.60±2.71 mm。淋巴结大小与淋巴结良恶性之间的关系结果显示,淋巴结大小不能预测淋巴结转移(Z=-0.213,P=0.831)。
2.4 "淋巴结大小与FNA-Tg测值及诊断结果的关系
FNA-Tg测值与淋巴结大小无相关性(rs=0.022,P=0.804)。
3 "讨论
目前PTC的颈部淋巴结转移率达20%~50%[15],准确判断淋巴结的转移情况对确定TNM分期、手术方案制定及预后判断等具有重要意义[2]。近年来,诸多研究证实FNA-Tg作为一种特异性的生物学标记物在提高诊断准确性方面的重要作用,因其标本获取便捷,FNA-Tg亦被各指南建议作为细胞学检查的补充,尤其是在FNA阴性、无法诊断或不确定时应用,从而提高检查准确性[2, 8, 16]。一项纳入22项研究共2670个淋巴结的荟萃分析显示,FNA-Tg诊断转移性淋巴结的敏感度为91%,特异度为94%[10]。目前FNA-Tg对PTC转移性淋巴结的最佳诊断临界值尚无统一标准。一项大样本的回顾性研究表明,当临界值为1.0 ng/mL时,FNA-Tg鉴别淋巴结是否转移敏感度最高,当临界值为30 ng/mL时,特异度最高[10]。
血清Tg水平被认为对FNA-Tg的测定结果有重要影响[17],另外,血清TgAb[18-19]及血清TSH水平[13, 20]对FNA-Tg的影响亦存在争议。sTg及sTgAb等血清学因素影响FNA-Tg结果的主要原因是对于有甲状腺组织存在的患者,若淋巴结穿刺过程中抽吸了过多血液污染了穿刺针,则淋巴结穿刺洗脱液可能受sTg的污染,导致FNA-Tg假阳性[9],Jeon等[17]的研究认为甲状腺术前患者的FNA-Tg诊断应根据sTg水平使用不同诊断截值,在sTg水平较低的患者中,FNA-Tg的最佳临界值为1.0 ng/mL,而在sTg水平较高的患者中,无论sTgAb状态如何,选用FNA-Tg/sTggt;0.5诊断转移性淋巴结。sTgAb的存在则可能降低FNA-Tg测值,故有研究认为应降低诊断截值,从而提高sTgAb阳性患者FNA-Tg的诊断准确性[21]。此外,有学者推测血清TSH水平对FNA-Tg存在影响,且独立于sTg水平,即甲状腺切除术后血清TSH抑制可能导致sTg和FNA-Tg的降低[20]。 故本研究对纳入患者的血清甲状腺功能等指标进行测定,结果提示淋巴结良恶性与血清FT3、FT4、TSH、sTgAb无关(Pgt;0.05)。排除干扰因素后,本研究分别采用ROC曲线阈值[22]和洗脱液中的Tg浓度与血清的比率(FNA-Tg/sTg)[23]两种常用方法分析FNA-Tg诊断转移性淋巴结的临界值,提高了诊断临界值的可信度。
对于可疑淋巴结穿刺时是否应受大小限制仍存在争议,既往有学者认为小体积淋巴结的临床复发率低[24],另有研究提出仅大于30 mm的淋巴结才提高疾病复发率[25];也有学者认为当淋巴结最短径线小于5 mm时可能导致细胞样本量不足而无法诊断[26]。基于这些研究,2015年美国甲状腺学会指南建议仅对最短径线大于10 mm的颈侧区淋巴结进行穿刺[2],然而在实际临床工作中,这是术前患者难以接受的建议。相反,2017AACE/ACE指南建议,不论大小如何,均应对可疑淋巴结进行穿刺细胞学检查[16]。研究显示[27],47%的小淋巴结(≤10 mm)存在淋巴结外侵犯,反映了其侵袭性生物学风险,对于出现可疑超声征象的淋巴结,即使其体积小也可能已经出现转移。尽管小体积转移性淋巴结的的临床意义尚待进一步研究,然而不可否认早期发现小体积转移性淋巴结能够为临床医生提供更多有价值的信息,避免重复手术[28],并为患者早期制定治疗策略或选择其他治疗方案提供了机会,如经皮激光消融[29]或术后I131治疗。基于此,本研究纳入可疑淋巴结时未对大小做限制,一旦出现可疑的淋巴结超声征象,结合临床要求及患者意愿进行FNA检查,本研究结果显示转移性淋巴结与非转移性淋巴结大小的差异无统计学意义,这可能与近年来超声设备的更新、高频超声的泛普及相关,转移性淋巴结可以在更早期被发现[2]。基于既往研究认为淋巴结体积过小可能导致细胞学诊断效能减低,因此,FNA-Tg在小淋巴结中的应用价值更加值得探讨。本研究显示,淋巴结大小不影响FNA-Tg测值结果,即FNA-Tg对小体积淋巴结的诊断效果依然很好。
但本研究仍存在局限性:本研究为单中心研究,样本量较小,且只对出现可疑超声特征的淋巴结进行了穿刺检查,可能存在选择偏倚;尽管大多数淋巴结均获得了手术病理结果,但只能核对病理报告的位置和大小来确定超声检查时诊断的淋巴结,可能存在潜在偏差,且穿刺细胞学阴性的颈侧区淋巴结因无需手术故难以避免的缺乏病理结果;本研究为获取FNA-Tg的具体测值,对高出检测范围的样本进行了不同程度的稀释(10~80倍),可能导致稀释后部分样本测值偏低。但这些样本的FNA-Tg测值均显著高于诊断截值,故不影响诊断结果。
综上,本研究首次分析了淋巴结大小和FNA-Tg测值及诊断效能之间的关系,初步证实了FNA-Tg对小体积淋巴结的诊断效能可靠性,后续拟行更大样本研究以进一步证实。
参考文献:
[1] "Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424.
[2] " Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer[J]. Thyroid, 2016, 26(1): 1-133.
[3] " Ito Y, Fukushima M, Tomoda C, et al. Prognosis of patients with papillary thyroid carcinoma having clinically apparent metastasis to the lateral compartment[J]. Endocr J, 2009, 56(6): 759-66.
[4] " Kim DW, Choo HJ, Lee YJ, et al. Sonographic features of cervical lymph nodes after thyroidectomy for papillary thyroid carcinoma[J]. J Ultrasound Med, 2013, 32(7): 1173-80.
[5] "Wu MH, Shen WT, Gosnell J, et al. Prognostic significance of extranodal extension of regional lymph node metastasis in papillary thyroid cancer[J]. Head Neck, 2015, 37(9): 1336-43.
[6] "Cracchiolo JR, Wong RJ. Management of the lateral neck in well differentiated thyroid cancer[J]. Eur J Surg Oncol, 2018, 44(3): 332-7.
[7] "Xu YX, Wu DP, Wu WT, et al. Diagnostic value of cytology, thyroglobulin, and combination of them in fine-needle aspiration of metastatic lymph nodes in patients with differentiated thyroid cancer: a systematic review and network meta‑analysis[J]. Medicine, 2019, 98(45): e17859.
[8] " Leenhardt L, Erdogan MF, Hegedus L, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer[J]. Eur Thyroid J, 2013, 2(3): 147-59.
[9] " Boi F, Baghino G, Atzeni F, et al. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-tg antibodies[J]. J Clin Endocrinol Metab, 2006, 91(4): 1364-9.
[10] Zhu XH, Zhou JN, Qian YY, et al. Diagnostic values of thyroglobulin in lymph node fine-needle aspiration washout: a systematic review and meta-analysis diagnostic values of FNA-Tg[J]. Endocr J, 2020, 67(2): 113-23.
[11] Grani G, Fumarola A. Thyroglobulin in lymph node fine-needle aspiration washout: a systematic review and meta-analysis of diagnostic accuracy[J]. J Clin Endocrinol Metab, 2014, 99(6): 1970-82.
[12] Zhao H, Wang Y, Wang MJ, et al. Influence of presence/absence of thyroid gland on the cutoff value for thyroglobulin in lymph-node aspiration to detect metastatic papillary thyroid carcinoma[J]. BMC Cancer, 2017, 17(1): 296.
[13] Duval MADS, Zanella AB, Cristo AP, et al. Impact of serum TSH and anti-thyroglobulin antibody levels on lymph node fine-needle aspiration thyroglobulin measurements in differentiated thyroid cancer patients[J]. Eur Thyroid J, 2017, 6(6): 292-7.
[14] Netzel BC, Grebe SKG, Carranza Leon BG, et al. Thyroglobulin (tg) testing revisited: tg assays, TgAb assays, and correlation of results with clinical outcomes[J]. J Clin Endocrinol Metab, 2015, 100(8): E1074-83.
[15]Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013[J]. JAMA, 2017, 317(13): 1338-48.
[16]Gharib H, Papini E, Garber JR, et al. American association of clinical endocrinologists, American college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: 2016 update[J]. Endocr Pract, 2016, 22(5): 622-39.
[17] Jeon MJ, Kim WG, Jang EK, et al. Thyroglobulin level in fine-needle aspirates for preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid carcinoma: two different cutoff values according to serum thyroglobulin level[J]. Thyroid, 2015, 25(4): 410-6.
[18]Wang JH, Jiang XF, Xiao GZ, et al. Excellent diagnostic performance of FNA-Tg in detecting lymph nodes metastases from papillary thyroid cancer[J]. Future Oncol, 2020, 16(33): 2735-46.
[19] Shin HJ, Lee HS, Kim EK, et al. A study on serum antithyroglobulin antibodies interference in thyroglobulin measurement in fine-needle aspiration for diagnosing lymph node metastasis in postoperative patients[J]. PLoS One, 2015, 10(6): e0131096.
[20] Moon JH, Kim YI, Lim JA, et al. Thyroglobulin in washout fluid from lymph node fine-needle aspiration biopsy in papillary thyroid cancer: large‑scale validation of the cutoff value to determine malignancy and evaluation of discrepant results[J]. J Clin Endocrinol Metab, 2013, 98(3): 1061-8.
[21]Cappelli C, Pirola I, De Martino E, et al. Thyroglobulin measurement in fine-needle aspiration biopsy of metastatic lymph nodes after rhTSH stimulation[J]. Head Neck, 2013, 35(1): E21-3.
[22]Giovanella L, Suriano S, Ceriani L, et al. Undetectable thyroglobulin in patients with differentiated thyroid carcinoma and residual radioiodine uptake on a postablation whole-body scan[J]. Clin Nucl Med, 2011, 36(2): 109-12.
[23]Chung J, Kim EK, Lim H, et al. Optimal indication of thyroglobulin measurement in fine-needle aspiration for detecting lateral metastatic lymph nodes in patients with papillary thyroid carcinoma[J]. Head Neck, 2014, 36(6): 795-801.
[24]Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension[J]. Thyroid, 2012, 22(11): 1144-52.
[25] Barbosa MP, Momesso D, Bulzico DA, et al. Metastatic lymph node characteristics as predictors of recurrence/persistence in the neck and distant metastases in differentiated thyroid cancer[J]. Arch Endocrinol Metab, 2017, 61(6): 584-9.
[26]Layfield LJ. Fine‑needle aspiration in the diagnosis of head and neck lesions: a review and discussion of problems in differential diagnosis[J]. Diagn Cytopathol, 2007, 35(12): 798-805.
[27]Alpert EH, Wenig BM, Dewey EH, et al. Size distribution of metastatic lymph nodes with extranodal extension in patients with papillary thyroid cancer: a pilot study[J]. Thyroid, 2015, 25(2): 238-41.
[28]Siddiqui S, White MG, Antic T, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma[J]. Thyroid, 2016, 26(6): 807-15.
[29] Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation[J]. Cardiovasc Intervent Radiol, 2016, 39(7): 1023-30.
(编辑:林 "萍)
