CB2受体激活对慢性PD模型小鼠运动功能和黑质胶质细胞活化影响
2024-10-17刘欣宇张丽马泽刚
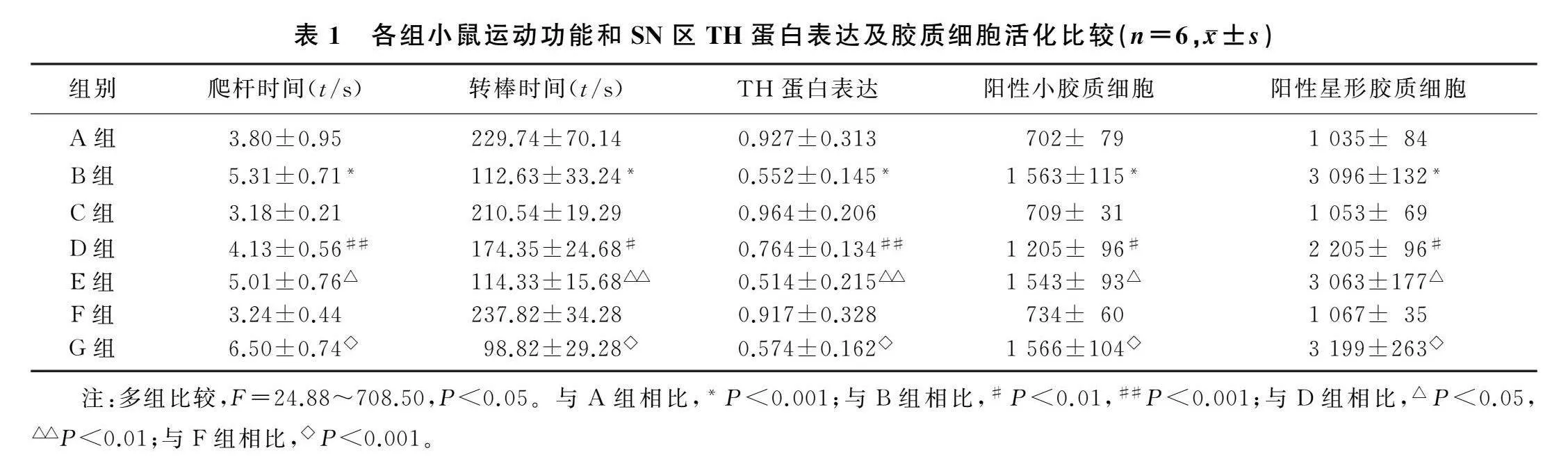
[摘要]目的通过行为学、免疫印迹技术及免疫组织化学技术探讨大麻素Ⅱ型(CB2)受体对1-甲基-4-苯基吡啶(MPTP)诱导的慢性帕金森病(PD)模型小鼠运动功能、黑质(SN)区酪氨酸羟化酶(TH)蛋白表达及小胶质细胞和星形胶质细胞活化的影响。
方法将30只8周龄雄性C57BL/6野生型(WT)小鼠随机分为WT对照组(A组)、WT MPTP组(B组)、WT CB2受体激动剂(JWH133)组(C组)、WT MPTP+JWH133组(D组)和WT MPTP+JWH133+CB2受体拮抗剂(AM630)组(E组),12只8周龄雄性CB2受体敲除(CB2-KO)C57BL/6小鼠随机分为CB2-KO对照组(F组)和CB2-KO MPTP组(G组)。模型组小鼠首先腹腔注射20 μg/(kg·d)AM630和(或)10 μg/(kg·d)JWH133,每天1次,连续注射30 d;然后腹腔注射30 mg/(kg·d)的MPTP,每周2次,持续4周。对照组小鼠腹腔注射等量的生理盐水。应用行为学实验检测各组小鼠爬杆与转棒时间,免疫印迹技术检测SN区TH蛋白的表达,免疫组织化学染色检测SN区小胶质细胞和星形胶质细胞数量和形态变化。
结果与A组相比,B组小鼠爬杆时间增加,转棒时间减少;与B组相比,D组小鼠爬杆时间减少,转棒时间增加;与D组相比,E组小鼠爬杆时间增加,转棒时间减少;与F组相比,G组小鼠爬杆时间增加,转棒时间减少。上述差异具有统计学意义(F=29.70、45.45,q=4.87~18.09,P<0.05)。与A组相比,B组小鼠SN区TH蛋白表达水平下降;与B组相比,D组小鼠SN区TH蛋白表达水平上调;与D组相比,E组小鼠SN区TH蛋白表达水平235a007ab0da0788f67618cbce1e13718f1515b1efc38d204e8c11bfeb149e8f下降;与F组相比,G组小鼠SN区TH蛋白表达水平下降。上述差异具有统计学意义(F=24.88,q=5.09~8.88,P<0.001)。小鼠SN区活化的小胶质细胞和星形胶质细胞计数显示,与A组相比,B组明显增加;与B组相比,D组明显减少;与D组相比,E组明显增加;与F组相比,G组明显增加。上述差异具有统计学意义(F=269.80、708.50,q=13.29~54.78,P<0.01)。
结论激活CB2受体能够改善MPTP诱导的慢性PD模型小鼠的运动功能障碍,抑制小鼠SN区小胶质细胞和星形胶质细胞的活化。
[关键词]受体,大麻酚,CB2;帕金森病;1-甲基-4-苯基吡啶;旋转棒性能试验;黑质;小神经胶质细胞;星形细胞;小鼠,近交C57BL
[中图分类号]R392.1;R742.5
[文献标志码]A
[文章编号]2096-5532(2024)04-0478-05doi:10.11712/jms.2096-5532.2024.60.093
[开放科学(资源服务)标识码(OSID)]
[网络出版]https://link.cnki.net/urlid/37.1517.R.20240726.0931.005;2024-07-2616:43:35
Effect of cannabinoid type 2 receptor activation on motor function and glial cell activation in the substantia nigra in a mouse model of chronic Parkinson’s disease
LIU Xinyu, ZHANG Li, MA Zegang(Department of Physiology, School of Basic Medicine, Qingdao University, Qingdao 266071, China); [Abstract]ObjectiveTo investigate the effect of cannabinoid type 2 (CB2) receptor on motor function, the protein expression of tyrosine hydroxylase (TH), and the activation of microglial cells and astrocytes in the substantia nigra (SN) in a mouse model of chronic Parkinson’s disease (PD) induced by 1-methyl-4-phenyl-1,2, 3, 6-tetrahydropyridine (MPTP) based on beha-
vioristics, Western blotting, and immunohistochemistry. MethodsA total of 30 wild-type (WT) male C57BL/6 mice, aged 8 weeks, were randomly divided into WT control group (group A), WT MPTP group (group B), WT CB2 receptor agonist JWH133 group (group C), WT MPTP+JWH133 group (group D), and WT MPTP+JWH133+CB2 receptor antagonist AM630 group (group E), and 12 CB2 receptor-knockout (CB2-KO) male C57BL/6 mice were randomly divided into CB2-KO control group (group F) and CB2-KO MPTP group (group G). The mice in the model group were given intraperitoneal injection of 20 μg/(kg·d) AM630 and/or 10 μg/(kg·d) JWH133 once a day for 30 consecutive days, followed by intraperitoneal injection of 30 mg/(kg·d) MPTP twice a week for four weeks, while those in the control group were given intraperitoneal injection ofan equal volume of normal saline. Behavioral experiments were used to measure the time of poleclimbing and the time spent on the rotating rod; Westernblotting was used to measure the protein expression of TH in the
SN; immunohistochemical staining was used to observe changes in the number and morphology of microglial cells and astrocytes in the SN.
ResultsCompared with group A, group B had a significant increase in the time of poleclimbing and a significant reduction in the time spent on the rotating rod; compared with group B, group D had a significant reduction in the time of poleclimbing and a significant increase in the time spent on the rotating rod; compared with group D, group E had a significant increase in the time of poleclimbing and a significant reduction in the time spent on the rotating rod; compared with group F, group D had a signi-
ficant increase in the time of poleclimbing and a significant reduction in the time spent on the rotating rod (F=29.70,45.45;q=4.87-18.09;P<0.05). Compared with group A, group B had a significant reduction in the protein expression level of TH in the SN of mice; compared with group B, group D had a significant increase in the protein expression level of TH; compared with group D, group E had a significant reduction in the protein expression level of TH; compared with group F, group G had a significant reduction in the protein expression level of TH (F=24.88,q=5.09-8.88,P<0.001). Compared with group A, group B had significant increases in the numbers of activated microglial cells and astrocytes in the SN of mice; compared with B, group D had significant reductions in these numbers; compared with group D, group E had significant increases in these numbers; compared with group F, group G had significant increases in these numbers (F=269.80,708.50;q=13.29-54.78;P<0.01).
ConclusionActivation of CB2 receptor can improve dyskinesia and inhibit the activation of microglial cells and astrocytes in the SN of mice with MPTP-induced chronic PD.
[Key words]receptor, cannabinoid, CB2; Parkinson disease; 1-methyl-4-phenylpyridinium; rotarod performance test; substantia nigra; microglia; astrocytes; mice, inbred C57BL
帕金森病(PD)是一种常见的神经退行性疾病,其临床表现为运动缓慢、静止性震颤、强直和姿势不稳以及其他一些非运动症状[1-2]。PD病理特征是黑质(SN)多巴胺能神经元丢失、α-突触核蛋白聚集以及铁沉积。大麻素Ⅱ型(CB2)受体主要分布在外周组织当中,包括脾脏、免疫细胞、扁桃体、胸腺和肝脏等[3]。近年来研究发现CB2受体在中枢神经系统也有表达,包括SN、海马、腹侧被盖区等[4]。免疫荧光染色证实,在大鼠和小鼠的多个脑区都有CB2受体表达[5]。大脑发生炎症时,活化的胶质细胞上CB2受体上调,并在许多神经系统疾病中起重要作用。有研究表明,在1-甲基-4-苯基吡啶(MPTP)诱导的急性PD模型小鼠中,CB2受体激活对小鼠SN多巴胺能神经元具有保护作用[6],同时可抑制胶质细胞神经毒性递质的产生和外周免疫细胞的浸润[7],从而保护多巴胺能神经元。但是,CB2受体的激活对慢性PD模型小鼠的保护作用及具体机制尚不够清楚,所以本研究通过使用MPTP建立慢性PD小鼠模型[8-9],观察CB2受体激活对模型小鼠的运动功能及SN区小胶质细胞和星形胶质细胞活化的影响。
1材料与方法
1.1实验材料
1.1.1实验动物及饲养选用SPF级雄性健康C57BL/6J野生型(WT)和CB2受体敲除(CB2-KO)小鼠,8周龄,体质量为18~22 g,其中WT小鼠购于北京维通利华实验动物技术有限公司,CB2-KO小鼠由美国巴罗神经研究所赠予。小鼠饲养环境:室温23~26 ℃,湿度40%~60%,12 h-12 h昼夜循环光照,自由饮水进食。每笼3~4只。实验开始前小鼠需要适应饲养环境1周。
1.1.2主要试剂来源CB2受体激动剂JWH133、CB2受体拮抗剂AM630均购于美国APE x BIO生物科技公司;神经胶质酸性蛋白(GFAP)抗体、离子钙结合衔接分子-1(IBA-1)抗体均购于美国Cell Signaling Technology公司,酪氨酸羟化酶(TH)抗体、山羊抗兔荧光二抗均购于美国Thermo Fisher Scientific公司;MPTP购于Sigma公司。其他试剂均为国产分析纯。
1.2实验方法
1.2.1动物分组及处理将30只野生型小鼠随机分为对照组(A组)、MPTP组(B组)、JWH133组(C组)、MPTP+JWH133组(D组)和MPTP+JWH133+AM630组(E组)共5组,12只CB2基因敲除小鼠随机分为对照组(F组)和MPTP组(G组)两组。根据小鼠体质量,模型组小鼠首先腹腔注射20 μg/(kg·d) 的AM630和(或)10 μg/(kg·d) 的JWH133,每天1次,连续注射30 d;然后腹腔注射30 mg/(kg·d) 的MPTP,每周2次,持续4周。对照组小鼠腹腔注射等量的生理盐水。
1.2.2运动行为学检测①爬杆实验:将一根直径1 cm、高60 cm的木杆垂直放置在笼中,杆的顶部有一个直径2 cm的球形突起,将小鼠头朝上放置在球形突起的顶部,记录小鼠从转头到头朝下爬到杆底部时间。②转棒实验:首先将小鼠放在旋转棒上
适应2 min,打开转棒,在5 min内转速从4 r/min匀速增加到40 r/min。小鼠随转棒开始爬行,小鼠脱落转棒,系统会自动停止计时,并记录小鼠在转棒上停留的时间。上述两个实验均为每只小鼠进行3次测试并取平均值进行统计分析。每只小鼠每次测量的时间间隔不少于1 h。
1.2.3免疫印迹检测将含有10 μg蛋白质的样品通过100 g/L十二烷基硫酸钠聚丙烯酰胺凝胶进行电泳分离,随后转移到聚偏二氟乙烯膜(PVDF)上。将PVDF置于50 g/L脱脂牛奶中室温封闭2 h,加TH(1∶2 000)和β-actin (1∶10 000)一抗于4 ℃孵育过夜。然后使用TBST洗涤目的条带3次,每次10 min,加山羊抗兔IgG(H+L)(1∶10 000)二抗在室温下共同孵育1 h。用ECL检测试剂盒显现蛋白质条带,以Image J软件分析TH蛋白的表达。
1.2.4免疫组织化学染色检测将各组小鼠灌注取脑,先后用200和300 g/L的蔗糖溶液对其脱水沉糖,应用切片机对小鼠SN区进行连续切片。将脑片置于40 g/L甲醛溶液中固定10 min;用PBS漂洗 3次,每次10 min;用PBST稀释的体积分数0.05驴血清溶液封闭脑片1 h后,将脑片置于一抗(IBA-1(1∶200),GFAP(1∶300))中4 ℃摇床孵育过夜;PBS漂洗3次,每次10 min;加入二抗室温避光摇床孵育2 h后,每孔中加入DAPI染色液50 μL继续孵育 5 min;PBS漂洗 3次,每次 10 min。将脑片平铺至洁净的病理防脱载玻片上,适当干燥后用体积分数0.70的甘油封片,使用Olympus数字病理切片扫描系统进行观察并扫描。分别计数每个高倍视野(400倍)内SN区IBA-1和GFAP阳性细胞总数并取平均值。
1.3统计学处理
应用Graph Pad Prism 8.0统计软件进行分析。实验所得计量资料数据以±s表示,多组均数比较采用单因素方差分析(One-way ANOVA),并应用Turkey法进行两两比较。以P<0.05表示差异有统计学意义。
2结果
2.1CB2受体激活对小鼠运动功能影响
与A组相比,B组小鼠爬杆时间显著增加(F=45.45,q=8.37,P<0.001),D组可抑制MPTP所引起小鼠爬杆时间显著增加(q=6.55,P<0.001),E组可以阻断JWH133的作用从而使小鼠爬杆时间显著增加(q=4.87,P<0.05);与F组相比,G组小鼠爬杆时间显著增加(q=18.09,P<0.001)。与A组相比,B组小鼠转棒时间显著减少(F=29.70,q=10.86,P<0.001),D组可以抑制MPTP所引起小鼠转棒时间显著减少(q=5.84,P<0.01),E组可以阻断JWH133的作用从而使小鼠转棒时间显著减少(q=5.48,P<0.01);与F组相比,G组小鼠爬杆时间显著增加(q=12.62,P<0.001)。见表1。
2.2CB2受体激活对小鼠SN区TH蛋白表达影响
免疫印迹检测结果显示,与A组相比,B组小鼠SN区TH蛋白表达显著下降(F=24.88,q=8.88,P<0.001),D组抑制了MPTP诱导的小鼠SN区TH蛋白表达下降(q=5.09,P<0.01),E组阻断了JWH133的作用从而使TH蛋白表达下降(q=5.96,P<0.01);与F组相比,G组小鼠SN
区TH蛋白表达下降(q=7.58,P<0.001)。见表1。
2.3CB2受体激活对小鼠SN区胶质细胞活化影响
免疫荧光染色结果显示,与A组小鼠SN区小胶质细胞和星形胶质细胞阳性细胞数相比,B组两者阳性细胞数增加(F=269.80、708.50,q=33.46、52.96,P<0.01),D组抑制了MPTP诱导的两种阳性细胞数的增加(q=13.90、22.90,P<0.01),E组阻断了JWH133的作用从而使两种阳性细胞数量增多(q=13.29、22.06,P<0.01);与F组相比,G组小鼠SN区活化的小胶质细胞和星形胶质细胞数量显著增加(q=32.32、54.78,P<0.01)。见表1。
3讨论
PD是继阿尔茨海默病(AD)之后第二常见的慢性神经退行性疾病,其病理学特征是中脑SN区多巴胺能神经元的进行性丢失[10]。PD病人以运动和非运动症状为特征,通常表现出静止性震颤、强直、运动迟缓和弯腰姿势等。PD还可能与神经行为障碍(抑郁、焦虑)、认知障碍(痴呆)和自主神经功能障碍(例如多汗症)等密切相关[11-13]。目前,左旋多巴(L-dopa)替代疗法是临床治疗PD的重要手段,但却不能抑制PD的进展。因此,探究导致多巴胺能神经元死亡的确切机制,针对性地寻找治疗靶点,是目前改善PD疗效的关键。
近年越来越多的研究显示,大麻素具有神经保护和调节运动的作用[14-26]。CB2受体是内源性大麻素系统(ECS)的组成部分之一,ECS由两种内源性大麻素和大麻素Ⅰ及Ⅱ型(CB1和CB2)受体以及合成和降解它们的酶组成[14-15]。不同于CB1受体的激活,CB2受体的激活没有精神副作用,而且广泛地分布在神经胶质细胞中[16]。因此,CB2受体及其特定配体近年来获得更多的关注。JWH133是一种合成激动剂,对CB2受体具有高选择性[17],能够特异性地激活CB2受体,可以减少MPTP引起的小鼠SN多巴胺能神经元的死亡[18],以及抑制MPP+诱导的星形胶质细胞炎症反应和影响铁离子的转运[19]。同时,JWH133还具有抗癌、心脏保护、抗炎和免疫调节活性的作用[20-23],其还被证实对缺血性卒中、抑郁症、焦虑症、AD、癫痫和神经性疼痛等疾病具有神经保护作用[24-26]。因此,这提示JWH133可能具有很好的药用前景4892674c46cce019ca367a4f78057125ca4b8b343c00178f511bcbf8f7e98992。
神经炎症是促进PD进展的重要病理因素,是由中枢神经系统中的免疫细胞活化引发的,神经炎症是中枢神经系统损伤、感染、毒性或自身免疫的结果。有研究表明,神经胶质细胞的异常激活可能介导神经炎症,导致神经退行性疾病。在PD病人尸体解剖的SN中,除了多巴胺能神经元丢失之外,还检测到活化的胶质细胞和大量的炎症因子[27],这表明神经炎症参与了PD的发病。因此,有效抑制胶质细胞介导的炎症反应,可能有助于PD的治疗。研究表明,未活化M0小胶质细胞中CB2受体的激活与细胞迁移密切相关,因为BV2细胞中CB2受体激活会触发细胞迁移,这可能与小胶质细胞板状伪足前缘的CB2受体表达有关[28]。脂多糖/干扰素γ刺激的原代小胶质细胞与CB2受体的配体AEA共同治疗,可以剂量依赖性方式增加白细胞介素-10的表达,而白细胞介素-10是M2极化的标志物[29]。提示CB2受体的激活不仅能够减少促炎型小胶质细胞的激活,而且还能增加抗炎型小胶质细胞的表达。以上研究提示,激活CB2受体与神经炎症的治疗存在正向关系。
本文课题组前期研究已经证实,激活CB2受体可抑制MPP+处理的BV2小胶质细胞M1极化,并且促进BV2小胶质细胞从M1型转化为M2型[30]。所以,我们选择IBA-1和GFAP特异性地标记小胶质细胞和星形胶质细胞,在小鼠SN区进行免疫组织化学分析,检测了小胶质细胞和星形胶质细胞激活的数量变化,并且通过观察小鼠的运动行为学实验指标变化来进一步佐证我们的观点。本文研究结果显示,JWH133能够抑制MPTP诱导的小鼠SN区胶质细胞的激活并且改善模型鼠的运动行为障碍,而进一步使用CB2受体抑制剂AM630验证的结果显示JWH133作用被阻断。
综上所述,激活CB2受体能够改善MPTP诱导的慢性PD模型小鼠的运动功能障碍,抑制小鼠SN区小胶质细胞和星形胶质细胞的活化。CB2受体激活在PD发病中发挥了抗炎作用,但其涉及到的机制仍未明确,有待进一步探究。由于CB2受体在神经胶质细胞中广泛表达,而作为CB2受体特异性激动剂的JWH133,可能对治疗PD等神经退行性疾病的药物开发具有很好前景,值得进一步研究。
[参考文献]
[1]GONALVES E D, DUTRA R C. Cannabinoid receptors as therapeutic targets for autoimmune diseases: where do we stand[J]? Drug Discovery Today, 2019,24(9):1845-1853.
[2]GRECO R, DEMARTINI C, ZANABONI A M, et al. The endocannabinoid system and related lipids as potential targets for the treatment of migraine-related pain[J]. Headache, 2022,62(3):227-240.
[3]HOWLETT A C, ABOOD M E. CB1 and CB2 receptor pharmacology[J]. Advances in Pharmacology (San Diego, Calif), 2017,80:169-206.
[4]BOROWSKA M, CZARNYWOJTEK A, SAWICKA-GUTAJ N, et al. The effects of cannabinoids on the endocrine system[J]. Endokrynologia Polska, 2018,69(6):705-719.
[5]LEUNG K. 1-(2,4-Dichlorophenyl)-4-cyano-5-(4-[11C]methoxyphenyl)-N-(pyrrolidin-1-yl)-1H-pyrazole-3-carboxamide[M/OL]// Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda
(MD): National Center for Bio-
technology Information (US); 2004-2013. https://www.ncbi.nlm.nih.gov/books/NBK5923/.
[6]GARCA C, PALOMO-GARO C, GMEZ-GALVEZ Y, et al. Cannabinoid-dopamine interactions in the physiology and physiopathology of the basal Ganglia[J]. British Journal of Pharmacology, 2016,173(13):2069-2079.
[7]PRICE D J, KENNEDY H, DEHAY C, et al. The development of cortical connections[J]. The European Journal of Neuroscience, 2006,23(4):910-920.
[8]PETROSKE E, MEREDITH G E, CALLEN S, et al. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment[J]. Neuroscience, 2001,106(3):589-601.
[9]SELVAKUMAR G P, JANAKIRAMAN U, ESSA M M, et al. Escin attenuates behavioral impairments, oxidative stress and inflammation in a chronic MPTP/probenecid mouse model of Parkinson’s disease[J]. Brain Research, 2014,1585:23-36.
[10]PAKKENBERG B, MLLER A, GUNDERSEN H J, et al. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson’s disease estimated with an unbiased stereological method[J]. Journal of Neurology, Neurosurgery, and Psychiatry, 1991,54(1):30-33.
[11]RAY S, AGARWAL P. Depression and anxiety in parkinson disease[J]. Clinics in Geriatric Medicine, 2020,36(1):93-104.
[12]GOLDMAN J G, SIEG E. Cognitive impairment and dementia in parkinson disease[J]. Clinics in Geriatric Medicine, 2020,36(2):365-377.
[13]CHEN Z C, LI G L, LIU J. Autonomic dysfunction in Parkinson’s disease: implications for pathophysiology, diagnosis, and treatment[J]. Neurobiology of Disease, 2020,134:104700.
[14]DI MARZO V, PISCITELLI F. The endocannabinoid system and its modulation by phytocannabinoids[J]. Neurotherapeutics, 2015,12(4):692-698.
[15]BISOGNO T, MACCARRONE M. Endocannabinoid signaling and its regulation by nutrients[J]. BioFactors, 2014,40(4):373-380.
[16]NAGOOR MEERAN M F, SHARMA C, GOYAL S N, et al. CB2 receptor-selective agonists as candidates for targeting infection, inflammation, and immunity in SARS-CoV-2 infections[J]. Drug Development Research, 2021,82(1):7-11.
[17]CHEN M, YAN X T, YE L, et al. Dexmedetomidine ameliorates lung injury induced by intestinal ischemia/reperfusion by upregulating cannabinoid receptor 2, followed by the activation of the phosphatidylinositol 3-kinase/akt pathway[J]. Oxidative Medicine and Cellular Longevity, 2020, 2020:6120194.
[18]CHUNG Y C, SHIN W H, BAEK J Y, et al. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease[J]. Experimental & Molecular Medicine, 2016,48(1):e205.
[19]JIA Y, DENG H, QIN Q Y, et al. JWH133 inhibits MPP+-induced inflammatory response and iron influx in astrocytes[J]. Neuroscience Letters, 2020,720:134779.
[20]QAMRI Z, PREET A, NASSER M W, et al. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer[J]. Molecular Cancer Therapeutics, 2009,8(11):3117-3129.
[21]SERVETTAZ A, KAVIAN N, NICCO C, et al. Targeting the cannabinoid pathway limits the development of fibrosis and autoimmunity in a mouse model of systemic sclerosis[J]. The American Journal of Pathology, 2010,177(1):187-196.
[22]AKR M, TEKIN S, OKAN A, et al. The ameliorating effect of cannabinoid type 2 receptor activation on brain, lung, liver and heart damage in cecal ligation and puncture-induced sepsis model in rats[J]. International Immunopharmacology, 2020,78:105978.
[23]ZHU M, YU B Q, BAI J X, et al. Cannabinoid receptor 2 agonist prevents local and systemic inflammatory bone destruction in rheumatoid arthritis[J]. Journal of Bone and Mineral Research, 2019,34(4):739-751.
[24]KRUK-SLOMKA M, MICHALAK A, BIALA G. Antidepressant-like effects of the cannabinoid receptor ligands in the forced swimming test in mice: mechanism of action and possible interactions with cholinergic system[J]. Behavioural Brain Research, 2015,284:24-36.
[25]SHENG W S, CHAUHAN P, HU S X, et al. Antiallodynic effects of cannabinoid receptor 2 (CB2R) agonists on retrovirus infection-induced neuropathic pain[J]. Pain Research & Ma-
nagement, 2019,2019:1260353.
[26]CAO Q J, YANG F H, WANG H. CB2R induces a protective response against epileptic seizures through ERK and p38 signaling pathways[J]. The International Journal of Neuroscience, 2021,131(8):735-744.
[27]XU S B, LU J N, SHAO A W, et al. Glial cells: role of the immune response in ischemic stroke[J]. Frontiers in Immu-
nology, 2020,11:294.
[28]WALTER L, FRANKLIN A, WITTING A, et al. Nonpsy-
chotropic cannabinoid receptors regulate microglial cell migration[J]. The Journal of Neuroscience, 2003,23(4):1398-1405.
[29]CORREA F, HERNANGMEZ M, MESTRE L, et al. Anandamide enhances IL-10 production in activated microglia by targeting CB(2) receptors: roles of ERK1/2, JNK, and NF-kappaB[J]. Glia, 2010,58(2):135-147.
[30]王梦雅,刘曼,马泽刚. 激活CB2受体对MPP+诱导BV2小胶质细胞iNOS和Arg-1表达的影响[J]. 青岛大学学报(医学版), 2023,59(2):195-198.
(本文编辑于国艺)
