UCH-L1和GFAP对硬膜外血肿进展的预测价值
2024-10-17刘俊汝张广宇邓明铷聂安格袁海成


[摘要]目的探讨泛素C末端水解酶-L1(UCH-L1)和胶质纤维酸性蛋白(GFAP)在预测硬膜外血肿(EDH)进展中的价值。
方法将雄性新西兰白兔随机分为假手术组(SH组)和EDH 0.1、0.2、0.3 mL组,每组各24只。于术后1、3、6、24 h采血,采用酶联免疫吸附试验法测定血浆中UCH-L1、GFAP的水平。术后24 h进行神经行为学评分、脑含水量检测,采用免疫组织化学法检测大脑皮质中UCH-L1及GFAP的表达。
结果与SH组相比,EDH各组的神经行为学评分降低(F=290.10,P<0.001)、脑含水量增加(F=33.64,P<0.001)。4组兔大脑皮质中UCH-L1和GFAP的差异有统计学意义(F=45.90、118.03,P<0.001)。EDH各组血浆UCH-L1水平在术后1、3、6 h高于SH组(F=3.15~47.93,P<0.001),血浆GFAP水平在术后各个时间点均高于SH组(F=29.17~332.00,P<0.001)。
结论EDH体积增大可以促进损伤侧大脑皮质中UCH-L1和GFAP的表达,上调血浆中二者的水平。大脑皮质和血浆中的UCH-L1和GFAP可作为预测EDH进展的有效生物标志物。
[关键词]血肿,硬膜外,颅内;生物标记;泛素C末端水解酶-L1;神经胶质原纤维酸性蛋白质
[中图分类号]R651.15;R34-33
[文献标志码]A
[文章编号]2096-5532(2024)04-0496-05doi:10.11712/jms.2096-5532.2024.60.110
[开放科学(资源服务)标识码(OSID)]
[网络出版]https://link.cnki.net/urlid/37.1517.R.20240830.1034.001;2024-08-3019:01:13
Value of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein in predicting the progression of epidural hematoma
LIU Junru, ZHANG Guangyu, DENG Mingru, NIE Ange, YUAN Haicheng
(Clinical Medicine School of Weifang Medical University, Weifang 261000, China); [Abstract]ObjectiveTo investigate the value of ubiquitin C-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP) in predicting the progression of epidural hematoma (EDH).
MethodsMale New Zealand white rabbits were randomly divided into sham-operation group (SH group), EDH 0.1 mL group, EDH 0.2 mL group, and EDH 0.3 mL group. Blood samples were collected at 1, 3, 6, and 24 h after surgery, and enzyme-linked immunosorbent assay was used to measure the levels of UCH-L1 and GFAP in plasma. Neurobehavioral scoring and brain water content measurement were performed at 24 h after surgery, and immunohistochemistry was used to measure the expression of UCH-L1 and GFAP in cerebral cortex.
Results
Compared with the SH group, the three EDH groups had a significant reduction in neurobehavioral score (F=290.10,P<0.001) and a significant increase in brain water content (F=33.64,P<0.001). There were significant differences in UCH-L1 and GFAP in cerebral cortex between the four groups (F=45.90,118.03;P<0.001). Compared with the SH group, the three EDH groups had a significantly higher level of UCH-L1 in plasma at 1, 3, and 6 h after surgery (F=3.15-47.93,P<0.001) and a significantly higher level of GFAP in plasma at each time point after surgery (F=29.17-332.00,P<0.001).
ConclusionAn increase in EDH volume can promote the expression of UCH-L1 and GFAP in the injured cortex and upregulate the levels of UCH-L1 and GFAP in plasma. UCH-L1 and GFAP in cerebral cortex and plasma can be used as effective biomarkers for predicting the progression of EDH.
[Key words]hematoma, epidural, cranial; biomarkers; ubiquitin C-terminal hydrolase-L1; glial fibrillary acidic protein
硬膜外血肿(EDH)是颅脑创伤的常见类型之一。颅脑损伤病人中EDH的发生率在2.7%~4.0%之间[1]。当EDH体积增大时,及时的手术干预可以降低病死率[2]。CT扫描被广泛用于判断血肿体积的变化情况,但有研究认为多次重复CT扫描不能改变EDH的治疗策略及预后[3-4]。循环生物标志物因标本易获得、无辐射暴露、成本低廉等优点,有望作为CT扫描诊断的补充手段。神经元来源的泛素C末端水解酶-L1(UCH-L1)和星形胶质细胞来源的胶质纤维酸性蛋白(GFAP)的串联使用近来已被美国食品和药物管理局批准用于评估病人的颅内损伤[5]。本研究探讨兔EDH模型中血肿体积改变对UCH-L1和GFAP表达的影响,为UCH-
L1和GFAP用于临床预测EDH的进展提供参考依据。现将结果报告如下。
1材料与方法
1.1实验动物
体质量为2 000~2 500 g的雄性新西兰白兔96只购自南通大学动物实验中心,在恒温(21±2)℃、恒湿(50±10)%、12 h-12 h昼夜交替光照下饲养,可自由饮水与取食。将兔随机分为假手术组(SH组)以及EDH 0.1、0.2、0.3 mL组,每组各24只。
1.2动物模型制备
静脉注射30 g/L戊巴比妥钠(30 mg/kg)麻醉兔。动物以俯卧位固定,头部局部消毒后备皮。以中线左侧1.0 cm为轴,纵向切开头皮露出矢状线左侧骨面。使用牙科磨钻磨开0.5 cm×0.5 cm的骨窗,充分暴露硬脑膜,并保持硬脑膜的结构完整。将尖端附有乳胶可膨胀性球囊的麻醉用硬膜外导管(长10 cm,直径1 mm)置入硬膜外腔,连接外部压力泵后妥善固定。分别注入0.1、0.2、0.3 mL生理盐水使球囊扩张,每注入0.01 mL生理盐水稍停片刻,SH组在不注射生理盐水的情况下放置硬膜外导管。保持20 mim,当同侧瞳孔扩大、呼吸深缓、心率减慢时,缓慢解压,实验立即中止。实验过程中保持环境温度为24 ℃并保证动物生命体征完整。
1.3血浆和脑组织标本采集
解压后24 h,麻醉兔并仰卧位固定。将静脉采血针垂直刺入心脏,用肝素抗凝管收集血液标本,低温高速离心15 min。将上清液-80 ℃冻存备用。在采集血浆后,自剑突下沿胸骨两侧旁开1.5 cm向上剪开胸腔及心包,充分暴露兔的心脏,剪断右心耳。将带输液装置的针尖刺入心尖部,先快速灌注500 mL的生理盐水,待右心流出液接近无色时立即改为灌注1 000 mL的40 g/L多聚甲醛固定液(PFA)。灌注完成即刻断头取脑组织,用40 g/L浓度的PFA 4 ℃固定24 h,300 g/L蔗糖溶液脱水,以备免疫组化检测。从兔脑血肿侧皮质取体积约为8 mm×5 mm×5 mm的脑组织用于下一步检测。
1.4神经功能评分
手术后24 h,密切观察动物功能,进行兔神经功能评分。评分系统覆盖精神状态、进食及活动情况,评分标准参见文献[6]。
1.5脑含水量测定
采用干湿质量法测定脑含水量。用电子天平(±0.01 mg)测量脑组织质量,记为湿质量(M)。将
脑组织置于100 ℃烘箱中烘干24 h后称质量,记为干质量(m)。脑含水量计算公式:脑含水量(%)=(M-m)/M×100%。
1.6免疫荧光染色
收集的血肿侧脑皮质组织切片(切片厚度为15 μm)用缓冲液PBS洗涤3次,共15 min,并在PBS中孵育15 min。切片用山羊血浆在37 ℃下封闭15 min。随后,用UCH-L1和GFAP一抗孵育过夜。PBS冲洗3次共15 min后,用结合荧光染料的二抗(UCH-L1∶羊抗小鼠IgG=1∶100;GFAP∶羊抗兔IgG=1∶100)孵育20 min,PBS冲洗。之后进行DAPI (Sigma-Aldrich)染色。使用安装Image J软件的共聚焦扫描显微镜(Leica,Wetzlar,Germany)拍摄照片并计数UCH-L1和GFAP阳性细胞,阳性细胞比例=阳性细胞数/细胞核数×100%。
1.7酶联免疫吸附试验(ELISA)
采用商品化的ELISA试剂盒检测血浆UCH-L1、GFAP水平。以加入稀释缓冲液的样品的光密度(OD)值为基线。按顺序测量每孔,根据血浆样品OD值取3个重复孔平均值。
1.8统计学处理
所有数据采用统计学软件SPSS 22.0分析处理。正态分布的计量资料用±s表示,多组比较采用单因素方差分析,两两比较采用LSD检验。各组术后各时间点血浆中UCH-L1和GFAP的表达比较采用重复测量设计的方差分析。P<0.05为差异有统计学意义。
2结果
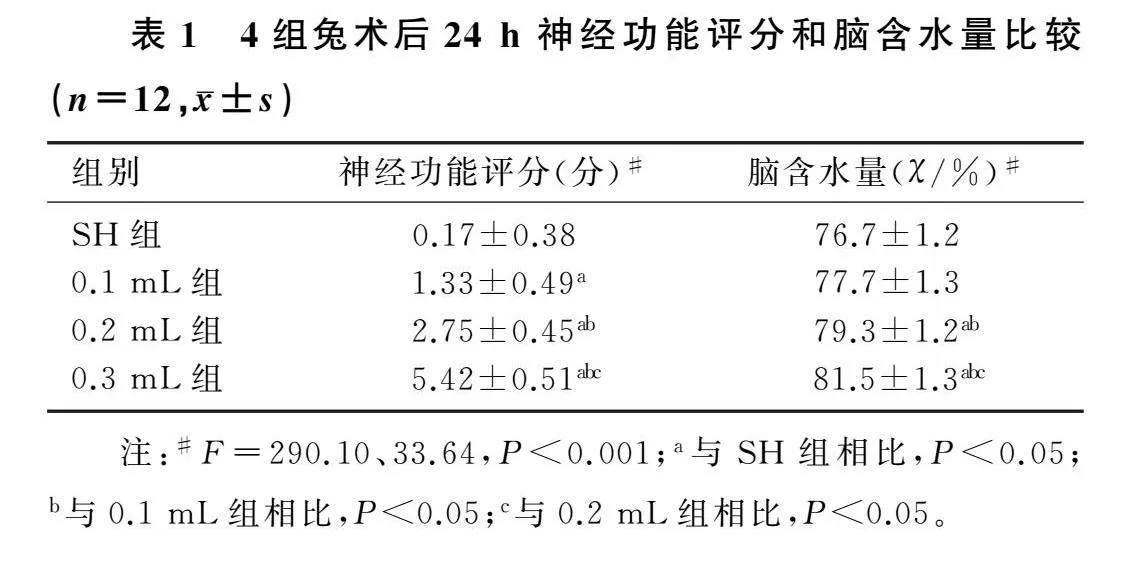
2.1各组兔神经功能评分和脑含水量比较
本文4组兔神经功能评分和脑含水量差异有统计学意义(F=290.10、33.64,P<0.001)。与SH组相比,EDH各组表现出更差的神经功能评分(P<0.05);相对于SH组,EDH 0.2 mL组和0.3 mL组的脑含水量均显著增加(P<0.05)。见表1。
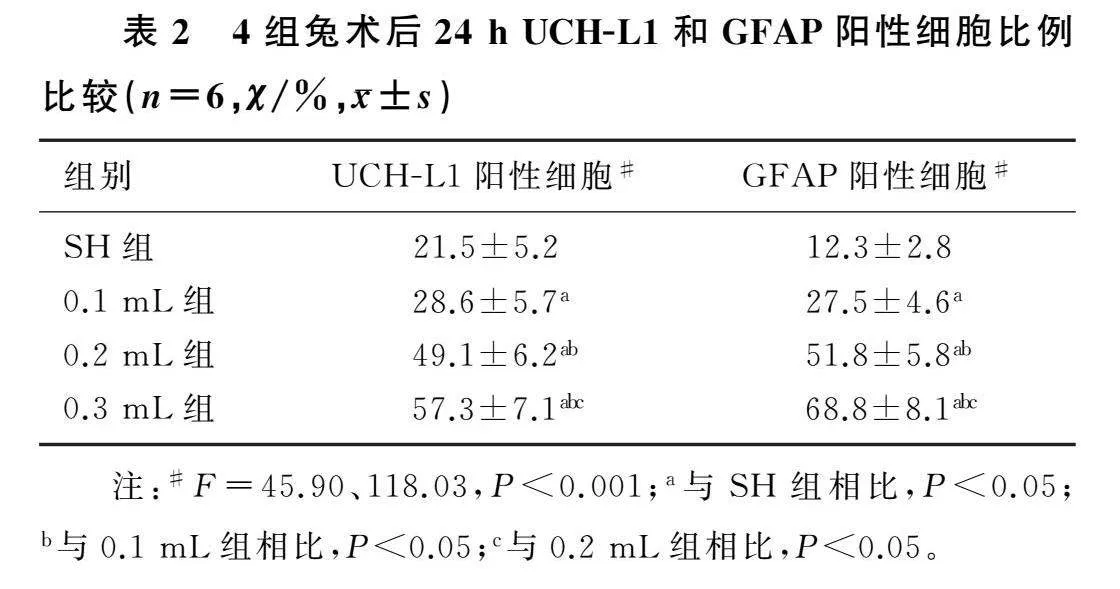
2.2各组兔术后24 h大脑皮质UCH-L1和GFAP表达比较
各组兔术后24 h损伤侧大脑皮质UCH-L1和GFAP阳性表达的免疫荧光染色结果见图1。4组兔UCH-L1阳性细胞比例和GFAP阳性细胞比例比较差异均有统计学意义(F=45.90、118.03,P<0.001);相较于SH组,EDH各组UCH-L1阳性细胞比例和GFAP阳性细胞比例均有不同程度增加(P<0.05)。见表2。
2.3各组兔术后不同时间点血浆中UCH-L1和GFAP表达比较
EDH各组血浆UCH-L1水平在术后1、3、6、12 h均高于SH组(F=3.15~47.93,P<0.001)。与SH组相比,EDH各组血浆GFAP水平在术后各时间点均显著升高,差异均具有统计学意义(F=29.17~332.00,P<0.001)。0.2 mL组术后6、12、24 h GFAP水平显著高于0.1 mL组(P<0.05),0.3 mL组术后6、12、24 h的GFAP水平显著高于0.2 mL组(P<0.05)。见表3。
3讨论
EDH是指位于颅骨内板与硬脑膜之间的血肿,交通事故和意外跌倒则分别是成人和儿童人群中EDH最常见的原因[7]。脑膜中动脉、脑膜中静脉、双侧静脉或静脉窦损伤均可导致EDH[8]。血肿体积被认为是EDH病人预后的独立预测因子,并在治疗决策的制定中起着核心作用[9]。目前,临床上对EDH的诊断及病情评估主要依靠CT扫描[10-11]。然而,频繁辐射暴露[12]、基层地区缺乏CT设备等问题限制了CT的应用。近年来,医学界逐渐重视生物标记物在预测EDH损伤中的作用,为解决这些问题提供了新的思路[13]。研究发现,创伤性脑损伤(TBI)时,血管撕裂、压迫等机械损伤,致受损脑组织神经元变性坏死,邻近区域神经胶质细胞功能异常[14],特异性标志物UCH-L1和GFAP会释放进入脑脊液、血浆等体液中[15],并与颅内损伤的严重程度具有良好的相关性[16-18]。作为TBI的常见类型,预后不良EDH病人血清UCH-L1、GFAP水平明显高于预后良好病人[15],但其血清水平升高是否可进一步提升血肿体积预测效能以指导临床治疗,尚鲜见报道。本研究通过构建兔EDH模型,模拟了EDH血肿体积改变对生物体的影响,以期为EDH诊疗工作提供临床前数据参考。
UCH-L1是富有多种功能的去泛素化酶,在神经元中特异表达[19],通过维持泛素蛋白酶系统稳定蛋白质降解,在轴突运输当中起着关键的作用[20-21]。作为星形胶质细胞特异性标志物的GFAP,是细胞骨架蛋白的中间丝蛋白单体[22],参与维持细胞内骨架重组、细胞黏附,维持细胞形态与功能[23-24],在星形胶质细胞中由神经损伤诱导,并在细胞骨架崩解时释放[25]。研究表明,实验性EDH可以导致受压大脑皮质的早期细胞应激、组织坏死以及神经元变性[26],引起严重的神经元缺失,显著诱导胶质细胞的明显激活[27]。
本研究结果显示,随着EDH体积的增大,动物模型表现出神经功能评分进行性恶化,脑水肿程度加重,模型较好地模拟了不同EDH血肿量对动物机体的影响。通过免疫荧光染色发现,相较于SH组,EDH组的UCH-L1及GFAP阳性细胞比例均显著增加,且这一差异与血肿体积呈正相关,这与PAN等[27]的研究结果相似。应用ELISA法检测不同时间点血浆中的UCH-L1及GFAP水平发现,SH组大多数血浆标本中GFAP水平低于ELISA检测的下限值,说明生理情况下GFAP不在血浆中表达,这与HUANG等[28]的研究报道一致。此外,血浆UCH-L1水平在术后1、3、6 h对EDH体积改变较为敏感,而EDH组血浆GFAP水平在术后24 h内各个时间点均保持增长态势,这意味着不同生物标志物有着不同的监测窗口期,GFAP的窗口期可能长于UCH-L1。
综上所述,本研究结果表明,EDH体积的增加上调了兔EDH模型大脑皮质和血浆中UCH-L1及GFAP的表达,这为UCH-L1和GFAP用于临床监测EDH进展提供了证据。
[参考文献]
[1]WANG X Z, GE R X, YUAN J L, et al. Risk factors and prognostic value of swirl sign in traumatic acute epidural hematoma[J]. Frontiers in Neurology, 2020,11:543536.
[2]SOON W C, MARCUS H, WILSON M. Traumatic acute extradural haematoma-Indications for surgery revisited[J]. British Journal of Neurosurgery, 2016,30(2):233-234.
[3]FLAHERTY B F, LOYA J, ALEXANDER M D, et al. Utility of clinical and radiographic findings in the management of traumatic epidural hematoma[J]. Pediatric Neurosurgery, 2013,49(4):208-214.
[4]NAGESH M, PATEL K R, MISHRA A, et al. Role of repeat CT in mild to moderate head injury: an institutional study[J]. Neurosurgical Focus, 2019,47(5):E2.
[5]WANG K K W, KOBEISSY F H, SHAKKOUR Z, et al. Thorough overview of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein as tandem biomarkers recently cleared by US Food and Drug Administration for the evaluation of intracranial injuries among patients with traumatic brain injury[J]. Acute Medicine & Surgery, 2021,8(1):e622.
[6]CHEN J H, YANG L K, CHEN L, et al. Atorvastatin ame-
liorates early brain injury after subarachnoid hemorrhage via inhibition of AQP4 expression in rabbits[J]. International Journal of Molecular Medicine, 2016,37(4):1059-1066.
[7]李奇,李扩,王宁,等. 硬膜外血肿的手术治疗[J]. 中华神经创伤外科电子杂志,2016,2(5):316-317.
[8]CHARCOS I B, WONG T W, LARSEN B R, et al. Location of traumatic cranial epidural hematoma correlates with the source of hemorrhage: a 12-year surgical review[J]. World Neurosurgery, 2021,152:e138-e143.
[9]LIN H, WANG W H, HU L S, et al. Novel clinical scale for evaluating pre-operative risk of cerebral herniation from traumatic epidural hematoma[J]. Journal of Neurotrauma, 2016,33(11):1023-1033.
[10]XIAO B, MA M Y, DUAN Z X, et al. Could a traumatic epi-dural hematoma on early computed tomography tell us aboutits future development? A multi-center retrospective study in China[J]. Journal of Neurotrauma, 2015,32(7):487-494.
[11]范国锋,秦虎,杨柳,等. 赫尔辛基CT评分判断创伤性脑损伤患者预后的价值[J]. 中华创伤杂志,2019,5(12):1087-1092.
[12]KALRA M K, SODICKSON A D, MAYO-SMITH W W. CT radiation: key concepts for gentle and wise use[J]. Radiographics: a Review Publication of the Radiological Society of North America, Inc, 2015,35(6):1706-1721.
[13]HUIBREGTSE M E, BAZARIAN J J, SHULTZ S R, et al. The biological significance and clinical utility of emerging blood biomarkers for traumatic brain injury[J]. Neuroscience and Biobehavioral Reviews, 2021,130:433-447.
[14]GHAITH H S, NAWAR A A, GABRA M D, et al. A literature review of traumatic brain injury biomarkers[J]. Molecular Neurobiology, 2022,59(7):4141-4158.
[15]RAHIMI A, CORLEY J A, AMMAR A, et al. The unmet global burden of cranial epidural hematomas: a systematic review and meta-analysis[J]. Clinical Neurology and Neurosurgery, 2022,219:107313.
[16]BOGOSLOVSKY T, WILSON D, CHEN Y, et al. Increases of plasma levels of glial fibrillary acidic protein, tau, and amyloid β up to 90 days after traumatic brain injury[J]. Journal of Neurotrauma, 2017,34(1):66-73.
[17]RAHEJA A, SINHA S, SAMSON N, et al. Serum biomar-
kers as predictors of long-term outcome in severe traumatic brain injury: analysis from a randomized placebo-controlled Phase Ⅱ clinical trial[J]. Journal of Neurosurgery, 2016,125(3):631-641.
[18]VISSER K, KOGGEL M, BLAAUW J, et al. Blood-based biomarkers of inflammation in mild traumatic brain injury: a systematic review[J]. Neuroscience and Biobehavioral Reviews, 2022,132:154-168.
[19]MI Z P, GRAHAM S H. Role of UCHL1 in the pathogenesis of neurodegenerative diseases and brain injury[J]. Ageing Research Reviews, 2023,86:101856.
[20]POSTI J P, TAKALA R S, RUNTTI H, et al. The levels of glial fibrillary acidic protein and ubiquitin C-terminal hydrolase-L1 during the first week after a traumatic brain injury: correlations with clinical and imaging findings[J]. Neurosurgery, 2016,79(3):456-464.
[21]CHMIELEWSKA N, SZYNDLER J, MAKOWSKA K, et al. Looking for novel, brain-derived, peripheral biomarkers of neurological disorders[J]. Neurologia i Neurochirurgia Polska, 2018,52(3):318-325.
[22]MUOZ-BALLESTER C, ROBEL S. Astrocyte-mediated mechanisms contribute to traumatic brain injury pathology[J]. WIREs Mechanisms of Disease, 2023,15(5):e1622.
[23]BUTTON E B, CHENG W H, BARRON C, et al. Development of a novel, sensitive translational immunoassay to detect plasma glial fibrillary acidic protein (GFAP) after murine traumatic brain injury[J]. Alzheimer’s Research & Therapy, 2021,13(1):58.
[24]VAN GEEL W J, DE REUS H P, NIJZING H, et al. Measurement of glial fibrillary acidic protein in blood: an analytical method[J]. Clinica Chimica Acta; International Journal of Clinical Chemistry, 2002,326(1-2):151-154.
[25]ABDELHAK A, FOSCHI M, ABU-RUMEILEH S, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders[J]. Nature Reviews Neurology, 2022,18(3):158-172.
[26]BALIKCI M, KOC K, ANIK I, et al. Biochemical effects of experimental epidural hematoma on brain parenchyma of rats[J]. Neurological Research, 2008,30(5):450-456.
[27]PAN A H, LI M, GAO J Y, et al. Experimental epidural hematoma causes cerebral infarction and activates neocortical glial and neuronal genesis in adult guinea pigs[J]. Journal of Neuroscience Research, 2013,91(2):249-261.
[28]HUANG X J, GLUSHAKOVA O, MONDELLO S, et al. Acute Temporal Profiles of Serum Levels of UCH-L1 and GFAP and Relationships to Neuronal and Astroglial Pathology following Traumatic Brain Injury in Rats[J]. Journal of Neurotrauma, 2015,32(16):1179-1189.
(本文编辑周晓彬)