缺氧对虹鳟肌肉氧化损伤及葡萄糖代谢的影响
2024-10-09胡澳郭兵兵宋乡月赵曼曼熊光权汪兰陈胜吴文锦石柳乔宇陈朗郭晓嘉李玮
摘 要:研究缺氧引起的虹鳟肌肉氧化损伤,并从葡萄糖代谢角度探讨其导致抗氧化系统改变的可能机制。随机将虹鳟分为6 组,每组9 条,其中,对照组(CG,溶解氧(dissolved oxygen,DO)质量浓度(7.5±0.5)mg/L)、中度缺氧组(MHG,DO质量浓度(5.5±0.5)mg/L)、重度缺氧组(SHG,DO质量浓度(3.0±0.5)mg/L)及3 个复氧组(DO质量浓度(3.0±0.5)mg/L)在各自DO水平下转运3 h后,复氧组在DO质量浓度(7.5±0.5)mg/L条件下恢复12、24、48 h,检测肌肉中的活性氧水平、抗氧化酶活性、脂代谢相关酶活性、葡萄糖代谢指标和相关基因表达。结果表明:SHG虹鳟肌肉活性氧、丙二醛含量较其他组显著升高(P<0.05),复氧24 h后显著降低(P<0.05),MHG虹鳟肌肉过氧化氢酶活性显著高于CG(P<0.05),MHG和SHG总超氧化物歧化酶和谷胱甘肽过氧化物酶活性显著低于CG(P<0.05);与CG相比,MHG和SHG虹鳟肌肉肌糖原含量、磷酸果糖激酶和丙酮酸激酶活性显著降低(P<0.05),皮质醇含量和己糖激酶活性显著升高(P<0.05);SHG虹鳟肌肉脂肪酶活性与其他组相比显著升高(P<0.05),乳酸含量与CG相比显著升高(P<0.05),MHG虹鳟肌肉脂蛋白脂肪酶活性显著低于CG(P<0.05);缺氧处理后低氧诱导因子1α(hypoxia-inducible factor 1α,HIF-1α)通路的HIF-1α、AMPK、SIRT1和PFK基因mRNA相对表达量显著升高(P<0.05),复氧24 h显著降低(P<0.05)。缺氧可引起虹鳟肌肉氧化损伤,并通过激活HIF-1α通路基因转录调节葡萄糖代谢,抵抗氧化损伤。
关键词:缺氧;虹鳟;葡萄糖代谢;氧化损伤;低氧诱导因子1α信号通路
Effects of Hypoxia on Oxidative Damage and Glucose Metabolism in Rainbow Trout Muscle
HU Ao1,2, GUOB9Fh5mWZNOLv0VeTDtqrb7EqeYPXKAcJRudapem3xbI= Bingbing1,2, SONG Xiangyue1,2, ZHAO Manman1,3, XIONG Guangquan2, WANG Lan2,
CHEN Sheng2, WU Wenjin2, SHI Liu2, QIAO Yu2, CHEN Lang2, GUO Xiaojia2, LI Wei1,*
(1. School of Life and Health Sciences, Hubei University of Technology, Wuhan 430068, China;
2. Key Laboratory of Cold Chain Logistics Technology for Agro-product, Ministry of Agriculture and Rural Affairs, Institute of Agricultural Products Processing and Nuclear Agricultural Technology, Hubei Academy of Agricultural Sciences, Wuhan 430064, China;
3. College of Life Sciences, Yangtze University, Jingzhou 434025, China)
Abstract: This study aims to investigate hypoxia-induced oxidative damage in rainbow trout muscle and to explore the mechanism underlying the resulting alterations in the antioxidant defense system from the perspective of glucose metabolism. Rainbow trout were randomly assigned into six groups with nine fish each: the control group (CG) was transported for 3 h
at a dissolved oxygen (DO) level of (7.5 ± 0.5) mg/L; the moderate hypoxia group (MHG) was transported for 3 h at a DO level of (5.5 ± 0.5) mg/L; the severe hypoxia group (SHG) was transported for 3 h at a DO level of (3.0 ± 0.5) mg/L; the three reoxygenated groups were transported for 3 h at a DO level of (3.0 ± 0.5) mg/L and then revived at a DO level of
(7.5 ± 0.5) mg/L for 12 (R12), 24 (R24) and 48 h (R48), respectively. We measured reactive oxygen species (ROS) level, antioxidant enzyme activities, lipid metabolism related enzyme activities, glucose metabolism indexes, and related gene expressions in muscle tissues. The results showed that the levels of ROS and malondialdehyde (MDA) in the muscle of rainbow trout significantly increased in the SHG compared to the other groups (P < 0.05), and significantly decreased after 24 h of reoxygenation (P < 0.05). Catalase (CAT) activity was significantly higher in the MHG than in the CG
(P < 0.05). Total superoxide dismutase (T-SOD) and glutathione peroxidase (GSH-Px) activities were significantly lower in both MHG and SHG compared to the CG (P < 0.05). The MHG and SHG showed a significant decrease in muscle glycogen content as well as phosphofructokinase (PFK) and pyruvate kinase (PK) activities, and a significant increase in cortisol content and hexokinase (HK) activity compared to the CG (P < 0.05). The SHG had significantly higher lipase activity than the other groups and significantly higher lactic acid levels than the CG (P < 0.05). lipoprteinlipase activity was significantly lower in the MHG than in the CG (P < 0.05). The mRNA relative expression of the HIF-1α,
AMPK, SIRT1 and PFK genes in the hypoxia-inducible factor 1α (HIF-1α) signaling pathway was significantly upregulated following hypoxia treatment (P < 0.05) but significantly downregulated after reoxygenation for 24 h
(P < 0.05). Hypoxia-induced oxidative damage in rainbow trout muscle, which is mitigated by the activation of glucose metabolism-related gene transcription in the HIF-1α signaling pathway.
Keywords: hypoxia; rainbow trout; glucose metabolism; oxidative damage; hypoxia-inducible factor 1α signaling pathway
DOI:10.7506/rlyj1001-8123-20240618-145
中图分类号:TS254.4 文献标志码:A 文章编号:1001-8123(2024)09-0001-07
引文格式:
胡澳, 郭兵兵, 宋乡月, 等. 缺氧对虹鳟肌肉氧化损伤及葡萄糖代谢的影响[J]. 肉类研究, 2024, 38(9): 1-7. DOI:10.7506/rlyj1001-8123-20240618-145. http://www.rlyj.net.cn
HU Ao, GUO Bingbing, SONG Xiangyue, et al. Effects of hypoxia on oxidative damage and glucose metabolism in rainbow trout muscle[J]. Meat Research, 2024, 38(9): 1-7. (in Chinese with English abstract) DOI:10.7506/rlyj1001-8123-20240618-145.
http://www.rlyj.net.cn
虹鳟(Oncorhynchus mykiss)是一种具有高营养价值的特种经济鱼类,含有丰富的氨基酸,不饱和脂肪酸及被称为脑黄金的二十二碳六烯酸和二十碳五烯酸含量是其他鱼类的数倍[1],是联合国粮食及农业组织向世界推广的产量高且品质优良的四大淡水养殖鱼类品种之一[2]。
溶解氧(dissolved oxygen,DO)是水产养殖和运输过程中的关键因素之一。运输过程中缺氧极易引起鱼类产生应激损伤,导致鱼类死亡,造成经济损失。特别是急性缺氧,可能由运输密度、水温和氧气注入
量等引起[3]。大量文献报道显示,急性缺氧可能导致新鲜鱼生理紊乱、组织损伤、肌肉质量下降,如团头鲂(Megalobrama amblycephala)[4]、斑点叉尾鮰(Ictalurus punctatus)[5]、中国花鲈(Lateolabrax maculates)[6]。研究发现,虹鳟对缺氧敏感,缺氧极易造成其肝脏氧化损伤和转录水平变化[7-8]。本团队前期研究也报道了缺氧可能导致虹鳟在短期运输过程中肌肉品质劣化和代谢紊乱[9]。然而,缺氧是否会对虹鳟肌肉造成氧化损伤及其抗氧化系统如何抵抗氧化损伤等尚不清楚。
缺氧条件下,生物体会发生能量代谢转变,葡萄糖代谢和脂代谢是影响鱼体能量代谢的关键[10]。其中葡萄糖代谢与氧化还原信号传导密切相关,氧化还原代谢降低是细胞过氧化物增加的结果[11]。为抵抗氧化损伤,糖酵解途径被激活,为机体产生更多的能量,以糖原分解和乳酸积累为代表[12]。丙酮酸激酶(pyruvate kinase,PK)、磷酸果糖激酶(phosphofructokinase,PFK)和己糖激酶(hexokinase,HK)是糖酵解限速酶,其活性水平表征导致乳酸水平增加的厌氧糖酵解程度[13]。有研究发现,靶向糖酵解限速酶可以缓解缺氧引起的氧化应激和线粒体损伤[14]。然而,由缺氧引起的虹鳟肌肉葡萄糖代谢紊乱研究少见报道。鱼类在长期或急性缺氧情况下,脂质代谢会成为主要的能量代谢方式,且以脂质分解代谢为主[10]。脂解的酶类如脂肪酶(lipase,LPS)和脂蛋白脂肪酶(lipoprteinlipase,LPL)会将甘油三酯(triglyceride,TG)水解,为鱼体提供能量[15]。因此,可通过脂解酶活性探讨脂质代谢程度。
在缺氧环境中,鱼类的氧传感器和能量传感器被激活,调节能量代谢从有氧糖酵解向厌氧糖酵解转变[16-17]。
在缺氧条件下,低氧诱导因子1α(hypoxia-inducible factor 1α,HIF-1α)的稳定性被破坏,通过与启动子区域的缺氧响应元件结合,激活基因转录,从而调节缺氧诱导的代谢和损伤[18]。本研究基于前期缺氧导致虹鳟肌肉质地软化、色泽下降、营养成分减少等品质劣变结果[8],进一步分析缺氧对虹鳟鱼肌肉氧化损伤、脂代谢酶活性和葡萄糖代谢的影响,并通过测定HIF-1α通路的相关基因转录水平,初步探讨缺氧对虹鳟肌肉糖代谢不良影响的潜在机制及复氧对虹鳟运输过程中缺氧不良影响的改善作用,确定最佳复氧时间,为虹鳟活体运输复氧时间提供一定参考。
1 材料与方法
1.1 材料与试剂
鲜活虹鳟(平均体质量(2.0±0.2)kg)购于湖北咸宁青山水库虹鳟养殖基地。
超氧化物歧化酶(superoxide dismutase,SOD)、过氧化氢酶(catalase,CAT)、谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px)、丙二醛(malondialdehyde,MDA)、总蛋白(total protein,TP)、LPL、LPS、肌糖原、皮质醇、乳酸含量、PK、PFK、HK检测试剂盒 南京建成生物工程研究所;二氢乙啶(dihydroethidium,DHE) 美国Sigma公司;总RNA提取商用试剂盒(R701)、SYBR Green PCR Master Mix试剂盒 南京碧云天生物技术有限公司;间氨基苯甲酸乙酯甲磺酸盐(tricaine methane-sulfonate,MS-222)(纯度98%) 上海麦克林生化科技有限公司。
1.2 仪器与设备
L5S紫外分光光度计 上海仪电分析仪器有限
公司;NanoDrop 2000分光光度计 美国Thermo Fisher Scientific公司;G90B便携式氨氮检测仪 山东格林凯瑞精密仪器有限公司;HQ40D哈希水质仪 上海哈希水质分析仪器有限公司;EclipseTi-SR倒置荧光显微镜 日本尼康株式会社;HH-6数显式恒温水浴锅 常州国华电器有限公司;AD500S-H均质机 上海昂尼仪器仪表有限公司。
1.3 方法
1.3.1 样品采集和缺氧设计
运输前,虹鳟禁食24 h。将54 条鱼随机分为6 组,每组9 条。对照组(CG):培养池DO质量浓度(7.5±0.5)mg/L;中等缺氧组(MHG):培养池DO质量浓度(5.5±0.5)mg/L;重度缺氧组(SHG):培养池DO质量浓度(3.0±0.5)mg/L。在培养池运输过程中,鱼水比为1∶9(kg/L),加入冰袋以保持水温在(18.5±0.7)℃范围内。每0.5 h监测水质(温度、DO和pH值)。运输卡车配备3 个储罐(240 cm×55 cm×60 cm)和氧气泵,每个氧气泵以不同功率运行,确保各组DO水平。以上3 个组分别在各自DO水平下转运3 h。复氧组虹鳟鱼在DO质量浓度为(3.0±0.5)mg/L条件下转运3 h后,随即在DO质量浓度为(7.5±0.5)mg/L的养殖池复氧12、24、48 h(分别记为R12、R24、R48)。
鱼运输至实验室后,在屠宰前迅速用50 mg/L MS-222麻醉。在冷室中进行背最长肌取样。将肌肉用铝箔纸包装并用液氮快速冷冻,立即保存于-80 ℃,以供活性氧(reactive oxygen species,ROS)、抗氧化酶活性、糖脂代谢指标及逆转录实时荧光定量聚合酶链式反应(reverse transcription-quantitative polymerase chain reaction,RT-qPCR)分析。所有的采样工作在1 h内完成。
1.3.2 ROS含量和抗氧化酶活性测定
ROS含量测定参考Baker等[19]方法并略有修改。首先加入DHE至覆盖样品,37 ℃暗箱孵育30 min,用磷酸盐缓冲液(pH 7.4)洗涤,然后加入4,6-二氨基-2-苯基吲哚染色液至覆盖样品,室温暗箱孵育10 min。使用显微镜相机控制器采集图像,采用ImageJ Pro Plus 6.0软件分析相对荧光强度(光密度总和与面积总和的比值),以此表示ROS含量。虹鳟鱼背肌在0.9 g/100 mL氯化钠缓冲溶液中按料液比1∶9(g/mL)均质,3 000×g、4 ℃离心10 min。收集上清液,采用试剂盒测定MDA水平和总超氧化物歧化酶(total superoxide dismutase,T-SOD)、CAT和GSH-Px活性。
1.3.3 葡萄糖代谢指标测定
采用试剂盒测定肌糖原、皮质醇、乳酸含量及PK、PFK、HK活性。
1.3.4 脂代谢相关酶活性测定
采用试剂盒测定LPL、LPS活性。
1.3.5 RNA分离和qRT-PCR分析
从虹鳟肌肉中分离总RNA,用分光光度计检测RNA的完整性和浓度,然后采用逆转录方法合成cDNA。在NCBI Nucleotide BLAST进行同源性比对,确定同源性较高的基因序列,使用Primer Premier 5.0软件设计引物(表1)。采用SYBR Green PCR Master Mix试剂盒进行RT-qPCR分析。PCR程序设置为95 ℃、10 min,40 个循环(95 ℃、15 s,60 ℃、30 s)。每个反应包括3 个技术重复。采用2−ΔΔCt法和归一化法计算靶基因的相对表达量。
1.4 数据处理
采用Excel 2020软件进行数据处理,所有数据均以平均值±标准差表示。采用SPSS 25软件进行方差分析,以
P<0.05表示差异显著,采用Pad Prism 5.0软件进行绘图。
2 结果与分析
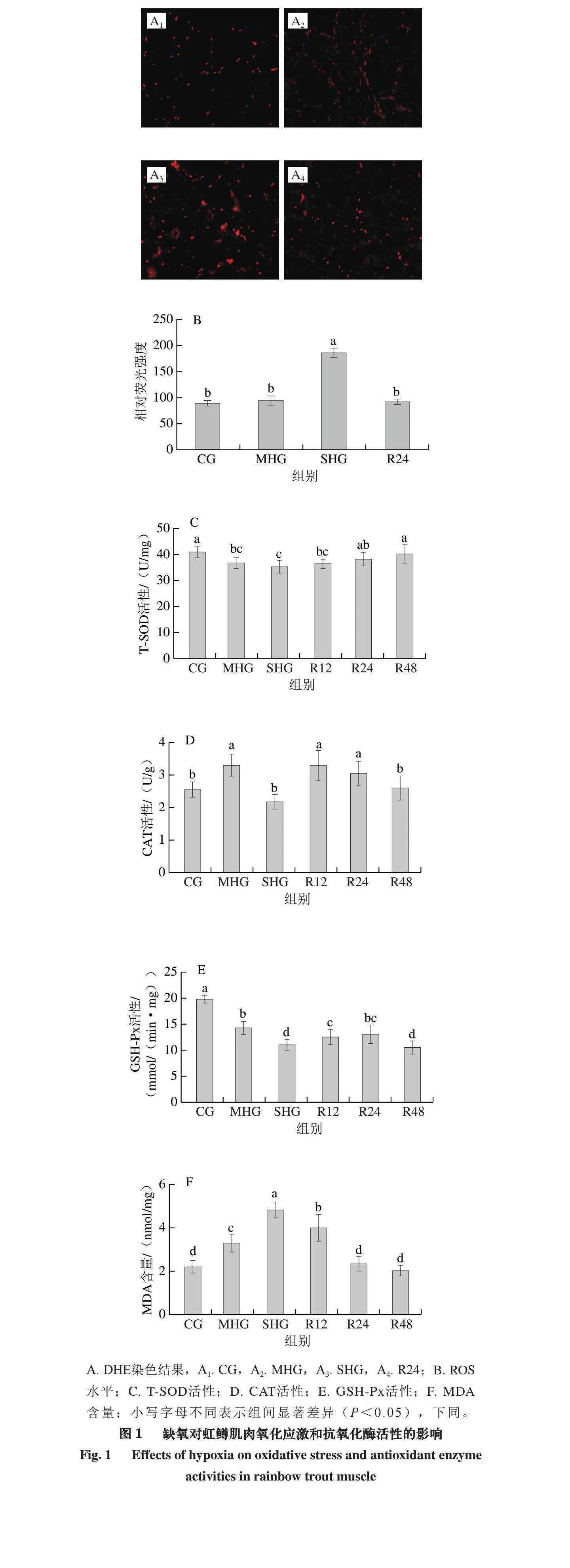
2.1 缺氧对虹鳟肌肉氧化应激的影响
由图1A、B可知,SHG虹鳟肌肉ROS含量较CG显著升高(P<0.05),复氧24 h后ROS含量显著降低(P<0.05);MHG和R24组与CG均无显著差异(P>0.05)。由图1C~F可知,缺氧组(MHG和SHG)T-SOD和GSH-Px活性显著低于CG(P<0.05),复氧后,T-SOD可恢复到对照组水平;MHG虹鳟鱼肌肉CAT活性较CG显著升高(P<0.05),SHG虹鳟肌肉CAT活性较CG无显著差异(P>0.05)。SHG虹鳟肌肉MDA含量显著高于其他组(P<0.05),复氧24 h后恢复到CG水平。
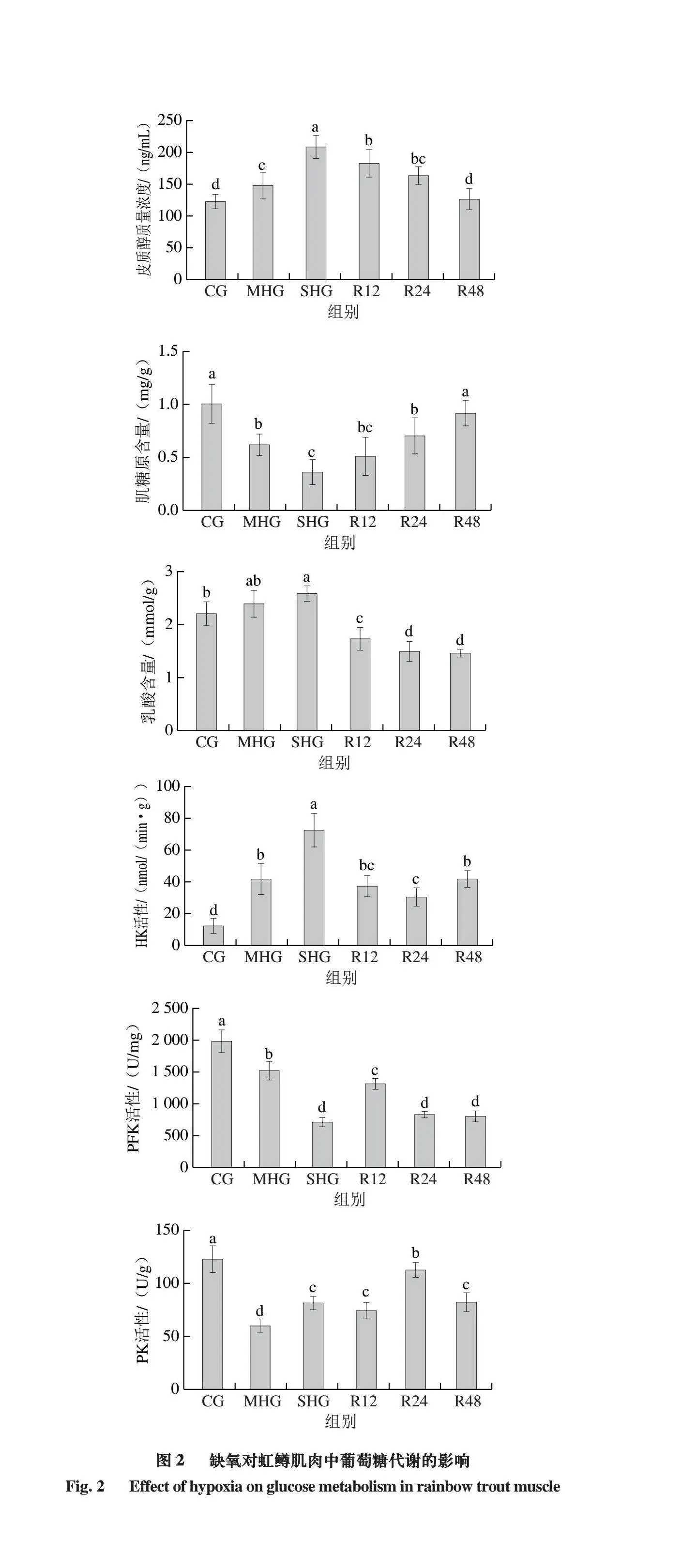
2.2 缺氧对虹鳟肌肉中葡萄糖代谢的影响
由图2可知,缺氧组(MHG和SHG)皮质醇含量相较于CG显著增加(P<0.05),并随着复氧时间延长而逐渐恢复。缺氧组(MHG和SHG)肌糖原含量相较于CG显著降低(P<0.05),乳酸含量随着DO水平降低呈现上升趋势,而复氧后显著下降(P<0.05)。缺氧组(MHG和SHG)HK活性相较于CG显著升高(P<0.05),而PFK和PK活性显著降低(P<0.05)。
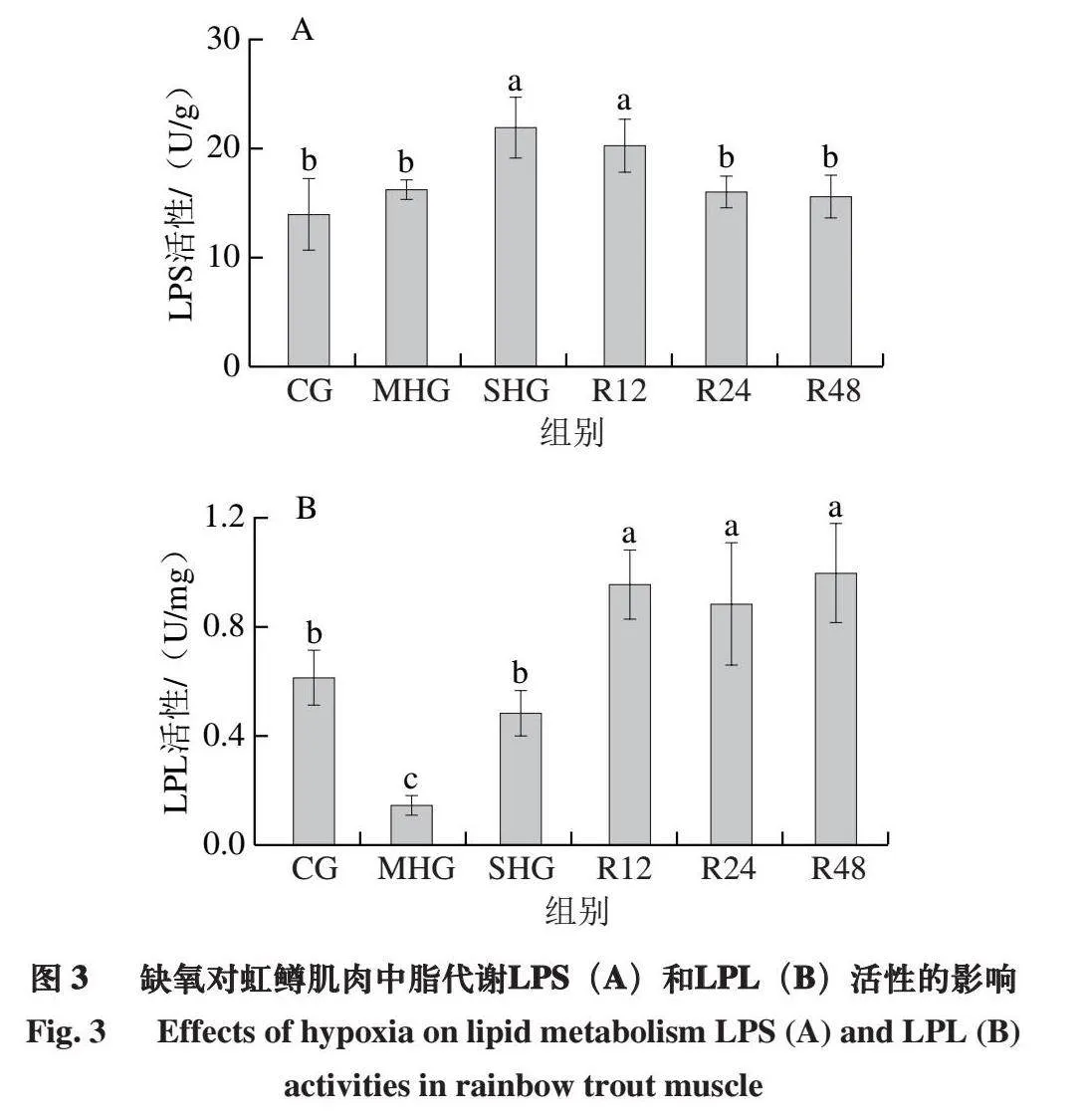
2.3 缺氧对虹鳟肌肉中脂代谢的影响
如图3A所示,与CG相比,缺氧组(MHG和SHG)随着DO水平降低,LPS活性呈现上升趋势,SHG肌肉LPS活性显著高于CG和MHG(P<0.05),而复氧24 h后显著下降至CG水平(P<0.05)。由图3B可知,与CG相比,缺氧组(MHG和SHG)随着DO水平降低,LPL活性呈现先下降后上升趋势,MHG肌肉LPL活性显著低于其他组(P<0.05),3 个复氧组间无显著差异(P>0.05),均显著高于缺氧组和CG(P<0.05)。
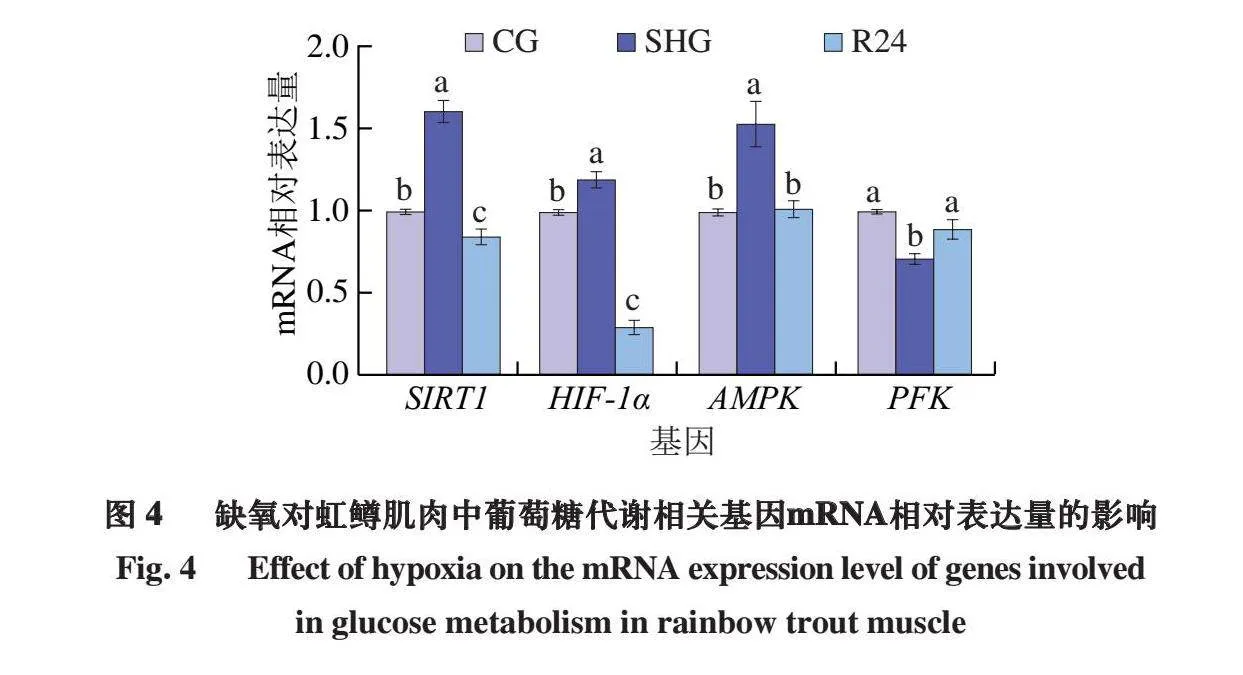
2.4 缺氧对虹鳟肌肉中葡萄糖代谢相关基因表达量的影响
如图4所示,在缺氧条件下,虹鳟鱼肌肉HIF-1α、AMPK和SIRT1 mRNA相对表达量显著升高(P<0.05),但均在复氧24 h后显著下降(P<0.05);PFK mRNA相对表达量显著下降(P<0.05),而在复氧24 h后,mRNA相对表达量又显著升高至CG水平(P<0.05)。
3 讨 论
3.1 缺氧诱导虹鳟肌肉氧化应激
研究显示,缺氧可以通过调节细胞内ROS水平诱导氧化应激[19]。本团队前期研究结果显示,24 h为最佳复氧时间,可有效缓解氧化应激[20],故本研究只观测了复氧24 h时的虹鳟肌肉ROS水平,其中,SHG虹鳟肌肉ROS含量较CG显著升高,随后在复氧24 h后显著降低。类似的结果在小黄鱼(Larimichthys polyactis)中也有报道,缺氧导致其细胞ROS产生和氧化还原稳态失调[21]。
T-SOD、CAT和GSH-Px作为抗氧化防御系统的关键酶,可有效清除ROS,保护细胞和组织[22-23]。缺氧转运3 h后,缺氧组(MHG和SHG)T-SOD和GSH-Px活性显著低于CG,复氧后T-SOD恢复到CG水平。缺氧组T-SOD和GSH-Px活性降低的部分原因是其被消耗以清除ROS,这与缺氧对大口黑鲈(Micropterus salmoides)肌肉影响的研究结果[24]一致。一般情况下,SOD催化超氧阴离子自由基分解为过氧化氢,CAT与GSH-Px可以清除过氧化氢,平衡细胞氧化还原代谢[25]。当虹鳟肌肉轻度缺氧时,CAT活性显著升高,表明轻度缺氧可激活CAT活性,而严重缺氧将大量消耗CAT,甚至损害抗氧化系统。这一结果与Zhao Liulan等[24]的报道相一致。虹鳟肌肉中MDA含量被认为是氧化损伤的生物标志物[26],与CG相比,缺氧组(MHG和SHG)MDA含量显著增加,复氧24 h后恢复到CG水平。因此,急性缺氧应激可导致虹鳟肌肉氧化损伤,24 h复氧可有效缓解缺氧应激引起的肌肉损伤。
3.2 缺氧干扰虹鳟肌肉葡萄糖代谢
研究证实,环境压力可激活鱼体肌肉的糖酵解途径,以满足其抵抗氧化应激的能量需求[7]。皮质醇可调节糖原、蛋白质和脂肪代谢,加速糖异生以缓解环境压力[27],缺氧组(MHG和SHG)皮质醇含量显著增加,并随着复氧时间延长而逐渐恢复。由此可知,复氧处理可有效缓解缺氧导致的肌糖原含量降低与乳酸积累。此现象在大口黑鲈和鲫鲤(Carassius auratus)[28-29]中也有报道,表明缺氧可能加速鱼体肌肉糖酵解,为抵抗环境压力提供能量。为证实这一假设,本研究对HK、PFK和PK等糖酵解酶活性进行评价,结果显示,在缺氧胁迫下,HK活性显著升高,而PFK和PK活性显著降低。此外,经复氧处理后,这些酶的活性也可得到不同程度地恢复,表明糖酵解过程加速产生能量,缓解急性缺氧压力,但随着乳酸不断积累,PK活性降低,糖异生过程被激活,糖酵解过程被抑制[24,30]。
3.3 缺氧加速虹鳟肌肉脂肪分解
脂肪是鱼类重要的营养物质,对鱼类生长、发育和营养代谢均具有重要作用[31]。长期缺氧时,鱼类主要依靠脂肪分解代谢获得能量,LPS和LPL是将TG降解为非酯化脂肪酸的关键酶[32]。本研究以低氧胁迫下虹鳟肌肉中的酶(LPL和LPS)活性表征能量产生能力。与CG相比,缺氧组(MHG和SHG)LPL活性降低,LPS活性升高。大口黑鲈肝脏的LPL和LPS活性也有类似的变化[32]。这可能是由急性低氧应激时脂肪分解加速所致。复氧24 h后,LPL和LPS活性逐渐恢复,说明复氧对抑制脂肪分解有一定作用。SHG虹鳟肌肉LPL活性与CG无显著差异,可能是缺氧暂养时间不充足导致鱼体仍以葡萄糖代谢供能为主[9]。
3.4 HIF-1α信号通路调控揭示缺氧及复氧虹鳟肌肉的葡萄糖代谢机制为探讨缺氧对虹鳟鱼肌肉葡萄糖代谢影响的可能机制,本研究检测了HIF-1α信号通路相关基因表达量。鱼体可以通过主动氧传感器和能量传感器,在缺氧环境中调节葡萄糖代谢从有氧糖酵解转变为无氧糖酵解[16]。HIF-1α作为缺氧时基因表达的重要调控因子,已被认为是可靠的缺氧应激生物标志物[33-34]。5’-腺苷酸活化蛋白激酶(5’-adenosine monophosphate-activated protein kinase,AMPK)也在系统能量平衡中发挥关键作用[35]。
在本研究中,缺氧条件下,虹鳟肌肉HIF-1α和AMPK mRNA相对表达量显著升高,但在复氧24 h后显著下降。类似的结果在斑马鱼(Danio rerio)[36]和鲫鱼(Carassius carassiu)[35]中也有报道,表明缺氧可以诱导AMPK表达,刺激HIF-1α活性,调节机体产生一系列抗应激反应。SIRT1可以调节应激反应,激活AMPK,刺激机体代谢[37],在缺氧条件下,虹鳟肌肉SIRT1 mRNA相对表达量也显著增加,24 h复氧后也显著降低。结果表明,严重缺氧后糖酵解功能增强,复氧24 h后糖酵解功能被抑制。此外,在缺氧条件下,PFK mRNA相对表达量显著降低,而在复氧24 h后显著增加,这与PFK活性结果相一致,表明缺氧促进了厌氧代谢,与乳酸积累结果相一致[16]。
4 结 论
在运输过程中,缺氧可通过增加虹鳟肌肉ROS水平诱导氧化应激,此时,肌肉抗氧化系统被激活,以保护鱼体免受ROS损害。抵抗氧化应激的过程需要更多的能量,这可能由糖酵解途径提供。糖代谢水平和脂代谢酶活性及相关基因mRNA相对表达量结果表明,缺氧可激活HIF-1α信号通路,调节虹鳟肌肉糖代谢,这可能是抗氧化应激的有效途径。此外,复氧24 h可能是调节虹鳟运输过程中缺氧引起的氧化损伤的好方法。
参考文献:
[1] 吴昕宁, 李鸣泽, 梁释介, 等. 茶多酚-壳聚糖复合液对虹鳟鱼肉贮藏品质和菌群结构的影响[J]. 肉类研究, 2023, 37(2): 20-25. DOI:10.7506/rlyj1001-8123-20221008-132.
[2] 张素珍, 励建荣, 刘小红, 等. 青海省养殖三倍体虹鳟呈味核苷酸和挥发性风味物质的研究[J]. 食品工业科技, 2022, 43(20): 310-318. DOI:10.13386/j.issn1002-0306.2021120293.
[3] 彭玲, 尤娟, 熊光权, 等. 物流运输对鱼类肌肉品质影响研究进展[J].
肉类研究, 2021, 35(12): 54-63. DOI:10.7506/rlyj1001-8123-20210531-164.
[4] XIAO K, WANG X, DAI Y J, et al. Hypoxia mediates HIF-1α to affect myofiber development and VC regulates the influence by activating Shh-Gli pathway in channel catfish (Ictalurus punctatus)[J]. Aquaculture, 2023, 562: 738849. DOI:10.1016/j.aquaculture.2022.738849.
[5] SUN Y X, DONG H B, ZHAN A J, et al. Protection of teprenone against hypoxia and reoxygenation stress in stomach and intestine of Lateolabrax maculatus[J]. Fish Physiology and Biochemistry, 2020, 46(2): 575-584. DOI:10.1007/s10695-019-00732-4.
[6] 林基亮, 于朝磊, 曹学彬, 等. 不同打包运输方式对花鲈幼鱼存活率的影响[J]. 水产养殖, 2022, 43(3): 46-48. DOI:10.3969/j.issn.1004-2091.2022.03.010.
[7] WU S J, HUANG J Q, LI Y J, et al. Dynamic and systemic regulatory mechanisms in rainbow trout (Oncorhynchus mykiss) in response to acute hypoxia and reoxygenation stress[J]. Aquaculture, 2023, 572: 739540. DOI:10.1016/j.aquaculture.2023.739540.
[8] WU Y W, ZHAO M M, XIA Y T, et al. Deterioration of muscle quality caused by ammonia exposure in rainbow trout (Oncorhynchus mykiss)[J]. Food Bioscience, 2023, 53: 102609. DOI:10.1016/j.fbio.2023.102609.
[9] 李娇. 长期低氧应激对三倍体虹鳟能源物质代谢影响机制的研究[D].
西宁: 青海大学, 2023.
[10] SALVEMINI F, FRANZE A, LERVOLINO A, et al. Enhanced glutathione levels and oxidoresistance mediated by increased glucose-6-phosphate dehydrogenase expression[J]. Biological Chemistry, 1999, 274(5): 2750-2757. DOI:10.1074/jbc.274.5.2750.
[11] WU Y W, YOU X P, SUN W Q, et al. Insight into acute heat stress on meat qualities of rainbow trout (Oncorhynchus mykiss) during short-time transportation[J]. Aquaculture, 2021, 543: 737013. DOI:10.1016/j.aquaculture.2021.737013.
[12] GAO Y S, DONG Y X, GUANG Z Y, et al. Overexpression of metabolic markers HK1 and PKM2 contributes to lymphatic metastasis and adverse prognosis in Chinese gastric cancer[J]. International Journal of Clinical and Experimental Pathology, 2015, 8(8): 9264. DOI:10.1016/ijcep.2015.9264.
[13] WANG D H, FENG Y L, WEI J Y, et al. Meldonium ameliorates hypoxia-induced lung injury and oxidative stress by regulating platelet-type phosphofructokinase-mediated glycolysis[J]. Frontiers in Pharmacology, 2022, 13: 863451. DOI:10.3389/fphar.2022.863451.
[14] LI M, WANG X, QI C, et al. Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress[J]. Aquaculture, 2018, 495: 187-195. DOI:10.1016/j.aquaculture.2018.05.031.
[15] FU C, JIE L, SHAO T Y, et al. Integrated lung and tracheal mRNA-Seq and miRNA-Seq analysis of dogs with an avian-like H5N1 canine influenza virus infection[J]. Frontiers in Microbiology, 2018, 9: 303. DOI:10.3389/fmicb.2018.00303.
[16] OLSVIK, PAL A, VIBEKE V, et al. Transcriptional responses to temperature and low oxygen stress in Atlantic salmon studied with next-generation sequencing technology[J]. BMC Genomics, 2013, 14: 817. DOI:10.1186/1471-2164-14-817.
[17] GERALD D, EDURNE B, YVES, et al. JunD reduces tumor angiogenesis by protecting cells from oxidative stress[J]. Cell, 2004, 118(6): 781-794. DOI:10.1016/j.cell.2004.08.025.
[18] LIN W, QI X Y, GUO W J, et al. A barrier against reactive oxygen species: chitosan/acellular dermal matrix scaffold enhances stem cell retention and improves cutaneous wound healing[J]. Stem Cell Research and Therapy, 2020, 11(1): 383. DOI:10.1186/s13287-020-01901-6.
[19] BAKER A, BOKEMEYER D, KRAMER H J. Endothelin synthesis and receptors in porcine kidney[J]. Acta Physiologica Scandinavica, 2001, 171(1): 105-112. DOI:10.1046/j.1365-201X.2001.00789.x
[20] WU Y W, ZHAO M M, SHI G P, et al. Effects of hypoxia on meat qualities and muscle metabolism in rainbow trout (Oncorhynchus mykiss) during short-time transportation and its relief by reoxygenation[J]. Aquaculture, 2023, 570: 739404. DOI:10.1016/j.aquaculture.2023.739404.
[21] WANG J Q, GAO X M, WANG L, et al. Establishment of the first cell line from the small yellow croaker (Larimichthys polyactis) and its application in unraveling the mechanism of ROS-induced apoptosis under hypoxia[J]. Aquaculture, 2023, 563(Part 1): 738900. DOI:10.1016/j.aquaculture.2022.738900.
[22] JOHANNSSON E O, GIACOMIN M, SADAUSKAS H, et al. Does hypoxia or different rates of re-oxygenation after hypoxia induce an oxidative stress response in Cyphocharax abramoides (Kner 1858), a Characid fish of the Rio Negro?[J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2018, 224: 53-67. DOI:10.1016/j.cbpa.2018.05.019.
[23] SHUANG L, CHEN S L, REN C, et al. Effects of hypoxia and reoxygenation on oxidative stress, histological structure, and apoptosis in a new hypoxia-tolerant variety of blunt snout bream (Megalobrama amblycephala)[J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2022, 278: 111358. DOI:10.1016/j.cbpa.2022.111358.
[24] ZHAO L L, WU H, SUN J L, et al. MicroRNA-124 regulates lactate transportation in the muscle of largemouth bass (Micropterus salmoides) under hypoxia by targeting MCT1[J]. Aquatic Toxicology, 2020, 218: 105359. DOI:10.1016/j.aquatox.2019.105359.
[25] BAL A, PATI S G, PANDA F, et al. Dehydration induced hypoxia and its role on mitochondrial respiratory enzymes and oxidative stress responses in liver of Asian stinging catfish Heteropneustes fossilis[J]. Comparative Biochemistry and Physiology Part C: Toxicology Pharmacology, 2022, 256: 109300. DOI:10.1016/j.cbpc.2022.109300.
[26] NAIEL M A E, ABD E, AHMED H A, et al. Gum Arabic-enriched diet modulates growth, antioxidant defenses, innate immune response, intestinal microbiota and immune related genes expression in tilapia fish[J]. Aquaculture, 2022, 556: 738249. DOI:10.1016/j.aquaculture.2022.738249.
[27] PFALZGRAFF T, LUND I, PETER V S. Prolonged cortisol elevation alters whole body and tissue metabolism in rainbow trout (Oncorhynchus mykiss)[J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2022, 263: 111098. DOI:10.1016/j.cbpa.2021.111098.
[28] GENZ J, JYDE M B, SVENDSEN J C, et al. Excess post-hypoxic oxygen consumption is independent from lactate accumulation in two cyprinid fishes[J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2013, 165: 54-60. DOI:10.1016/j.cbpa.2013.02.002.
[29] YANG S, WU H, HE K, et al. Response of AMP-activated protein kinase and lactate metabolism of largemouth bass (Micropterus salmoides) under acute hypoxic stress[J]. Science of the Total Environment, 2019, 666: 1071-1079. DOI:10.1016/j.scitotenv.2019.02.236.
[30] MAHFOUZ M E, HEGAZI M M, EL-MAGD M A, et al. Metabolic and molecular responses in Nile tilapia, Oreochromis niloticus during short and prolonged hypoxia[J]. Marine and Freshwater Behaviour and Physiology, 2015, 48(5): 319-340. DOI:10.1080/10236244.2015.1055915.
[31] 石钢鹏, 高天麒, 钱晓庆, 等. 不同速冻处理方式对大口黑鲈鱼肉冻藏期间品质变化影响[J]. 肉类研究, 2020, 34(12): 68-74. DOI:10.7506/rlyj1001-8123-20201113-264.
[32] SUN J L, ZHAO L L, WU H, et al. Acute hypoxia changes the mode of glucose and lipid utilization in the liver of the largemouth bass (Micropterus salmoides)[J]. Science of the Total Environment, 2020, 713: 135157. DOI:10.1016/j.scitotenv.2019.135157.
[33] 方玲玲, 王忠良, 陈刚, 等. 卵形鲳鲹肉碱棕榈酰基转移酶I全长cDNA序列的克隆及生物信息学分析[J]. 广东海洋大学学报, 2015, 35(3): 7-15. DOI:10.3969/j.issn.1673-9159.2015.03.002.
[34] TEROVA G, RIMOLDI S, CORA S, et al. Acute and chronic hypoxia affects HIF-1α mRNA levels in sea bass (Dicentrarchus labrax)[J]. Aquaculture, 2008, 279(1/4): 150-159. DOI:10.1016/j.aquaculture.2008.03.041.
[35] STENSLOKKEN K O, ELLEFSEN S, STECYK J A, et al. Differential regulation of AMP-activated kinase and AKT kinase in response to oxygen availability in crucian carp (Carassius carassius)[J]. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 2008, 295(6): R1803-14. DOI:10.1152/ajpregu.90590.2008.
[36] RYTKONEN K T, PROKKOLA J M, SALONEN V, et al. Transcriptional divergence of the duplicated hypoxia-inducible factor α
genes in zebrafish[J]. Gene, 2014, 541(1): 60-66. DOI:10.1016/j.gene.2014.03.007.
[37] NAGAPPAN A, KIM J H, JUNG D Y, et al. Cryptotanshinone from the Salvia miltiorrhiza Bunge attenuates ethanol-induced liver injury by activation of AMPK/SIRT1 and Nrf2 signaling pathways[J]. International Journal of Molecular Sciences, 2019, 21(1): 265. DOI:10.3390/ijms21010265.
