显微镜下多血管炎相关间质性肺病合并COVID-19患者疫苗保护作用初探
2024-07-13祁福敏苏睿郝剑苏丽范倩李昕魏蔚
祁福敏 苏睿 郝剑 苏丽 范倩 李昕 魏蔚
作者单位:天津医科大学总医院风湿免疫科(邮编300052)
作者简介:祁福敏(1996),女,医师,主要从事ANCA相关性血管炎方面研究。E-mail:qifumin0607@163.com
△通信作者 E-mail:tjweiwei2003@163.com
摘要:目的 分析显微镜下多血管炎(MPA)相关间质性肺病(ILD)患者合并新型冠状病毒肺炎(COVID-19)的临床特征,初步探究新型冠状病毒(SARS-CoV-2)疫苗的保护作用。方法 纳入确诊的MPA-ILD患者,同时符合《新型冠状病毒感染诊疗方案(第十版)》制定的COVID-19诊断标准,且均有明确SARS-CoV-2抗原或核酸检测阳性证据。收集并分析患者临床资料,ILD影像学分型,SARS-CoV-2疫苗接种情况、临床分型及预后。结果 共纳入37例MPA-ILD患者,女14例,男23例。32例在并发COVID-19时MPA病情处于缓解期,31例同时维持免疫抑制剂或生物制剂治疗。ILD的影像学类型以寻常型间质性肺炎(15例)和非特异性间质性肺炎(14例)为主,剩余8例分型不确定。COVID-19分型中,27例为轻症,5例因COVID-19死亡。11例未接种疫苗,26例接种SARS-CoV-2灭活疫苗,其中11例完成3剂次加强免疫。接种疫苗患者COVID-19轻症比例高于未接种疫苗组(P=0.038)。多因素分析显示接种疫苗为MPA-ILD患者合并COVID-19住院(OR=0.045,95%CI:0.004~0.472,P=0.010)及非轻症感染(OR=0.049,95%CI:0.005~0.517,P=0.012)的独立保护因素。同时MPA处于缓解期为COVID-19非轻症感染的保护因素(OR=0.021,95%CI:0.001~0.459,P=0.014)。结论 接种SARS-CoV-2疫苗及MPA病情处于缓解期有可能减轻MPA患者合并COVID-19的严重程度。
关键词:显微镜下多血管炎;新型冠状病毒肺炎;COVID-19疫苗
中图分类号:R593.2文献标志码:ADOI:10.11958/20231791
Study on the protective effect of vaccine in patients with microscopic polyangiitis-interstitial lung disease complicated with COVID-19 infection
QI Fumin, SU Rui, HAO Jian, SU Li, FAN Qian, LI Xin, WEI Wei△
Department of Rheumatology and Immunology, Tianjin Medical University General Hospital, Tianjin 300052, China
△Corresponding Author E-mail: tjweiwei2003@163.com
Abstract: Objective To analyze the clinical features of microscopic polyangiitis (MPA)-associated interstitial lung disease (ILD) patients infected with coronavirus disease-2019 (COVID-19),and investigate the protective effect of SARS-CoV-2 vaccine. Methods Patients with MPA-ILD in the General Hospital of Tianjin Medical University were included, and patients also met the diagnostic criteria for COVID-19 in accordance with the COVID-19's diagnosis and treatment plan (10th Edition). All of patients had positive evidence of coronavirus antigens or positive nucleic acid tests. Clinical data of patients, ILD imaging typing, COVID-19 vaccination, clinical typing and prognostic results were collected and analyzed. Results Thirty-seven patients with MPA-ILD were included, including 14 females and 23 males. Thirty-two patients with MPA were in remission at the time of infection with COVID-19, and 31 were maintained on concurrent immunosuppressive/biologic therapy. The original imaging type of MPA-ILD was predominantly usual interstitial pneumonia (UIP, 15 cases) and nonspecific interstitial pneumonia (NSIP, 14 cases). The typing of the remaining 8 cases was inconclusive. Of the COVID-19 subtypes, 27 patients were mild infection, and 5 dead due to COVID-19. Eleven patients were unvaccinated, and 26 received inactivated vaccine. Among them, 11 completed 3-dose of booster immunization. The proportion of patients with mild COVID-19 was significantly higher in MPA-ILD patients with vaccinated patient group than that in the unvaccinated patient group (P=0.038). Multifactorial analysis showed that vaccines were an independent protective factor for MPA-ILD patients with COVID-19 infection (OR=0.045, 95%CI: 0.004-0.472, P=0.010) and non-mild infection (OR=0.049, 95%CI: 0.005-0.517, P=0.012). Moreover, MPA in remission was a protective factor for COVID-19 non-mild infections (OR=0.021, 95%CI: 0.001-0.459, P=0.014). Conclusion Vaccination and MPA in remission may reduce the severity of COVID-19 infection in patients with MPA.
Key words: microscopic polyangiitis; COVID-19; COVID-19 vaccines
显微镜下多血管炎(MPA)是一种坏死性小血管炎,以多系统受累、血清中存在抗髓过氧化物酶(MPO)阳性的抗中性粒细胞胞质抗体(ANCA)为主要特点,预后不佳[1]。该病易出现肺部受累,其中以间质性肺病(ILD)最为突出,糖皮质激素、免疫抑制剂或生物制剂(如利妥昔单抗)为主要治疗方案。新型冠状病毒病肺炎(COVID-19)是由新型冠状病毒(SARS-CoV-2)引起的感染,发病率和病死率高,临床症状多样[2]。对COVID-19的大规模人群调查结果表明,风湿性疾病(包括类风湿关节炎、系统性红斑狼疮、银屑病等)患者合并COVID-19相关住院或死亡风险升高,并且在接受免疫抑制治疗的群体中出现重症或死亡的风险更高[3]。但尚无数据表明MPA-ILD患者合并COVID-19的情况。本文旨在了解MPA-ILD患者SARS-CoV-2感染的风险及SARS-CoV-2疫苗对其是否具有保护作用,初步探究COVID-19不良预后的预测因素。
1 对象与方法
1.1 研究对象 收集2016年1月—2023年7月于天津医科大学总医院诊断明确的MPA患者224例。MPA诊断需符合2022年美国风湿病学会/欧洲抗风湿病联盟(ACR/EULAR)或2012年国际Chapel Hill共识会议发布的MPA分类标准。排除药物或感染继发的MPA、未合并ILD及合并其他自身免疫性疾病的患者后,MPA合并ILD(MPA-ILD)患者172例。进行电话及门诊随访明确SARS-CoV-2感染及SARS-CoV-2疫苗接种情况,其中19例否认SARS-CoV-2感染史,116例曾于COVID-19大流行期间出现发热、咽痛、咳嗽、咳痰等可疑感染症状,但未进行抗原或核酸检测证实。最终将具有明确SARS-CoV-2感染证据的37例MPA-ILD患者纳入本研究。
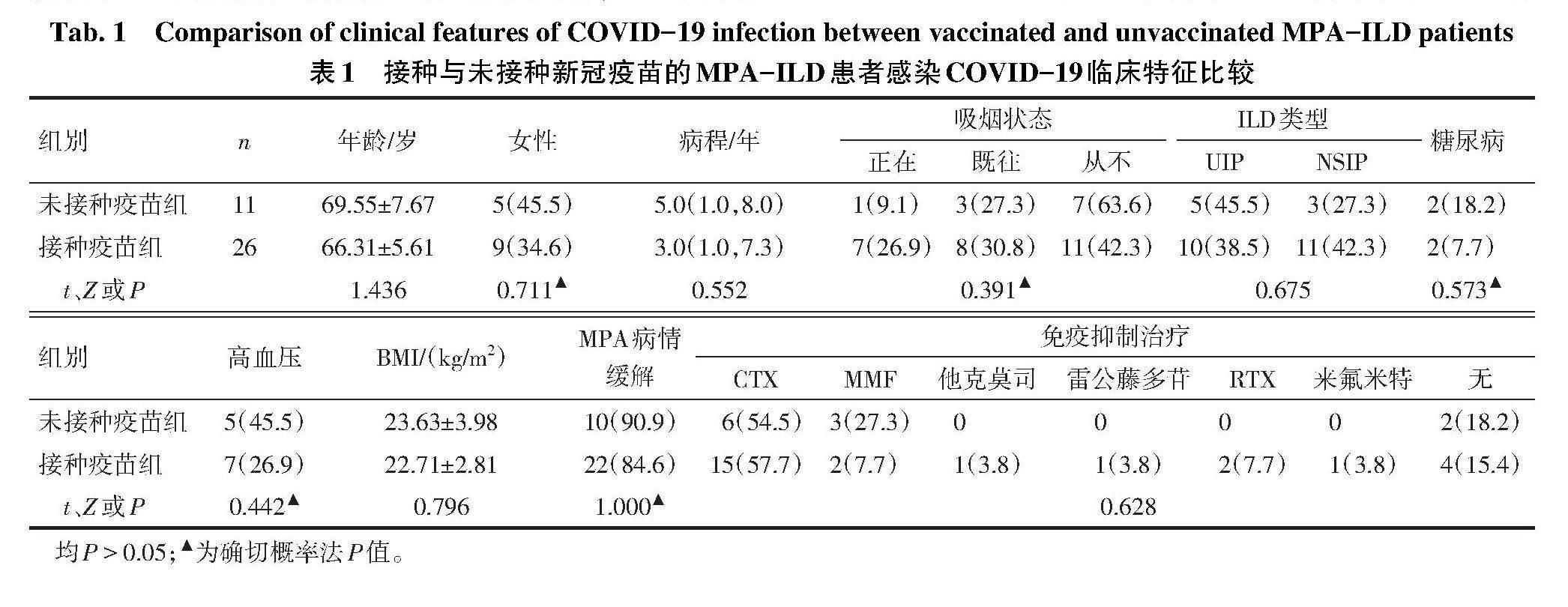
1.2 研究方法 收集并记录患者性别、年龄、吸烟状态、体质量指数(BMI)、是否合并高血压病或糖尿病等临床资料。记录患者合并COVID-19时MPA病情及应用免疫抑制剂或生物制剂的种类。对于MPA病情缓解定义为无任何临床症状,激素用量不超过每日2片,且无新发的器官损害。依据患者患COVID-19前最近一次胸部HRCT影像,结合《2018中国结缔组织病相关间质性肺病诊断和治疗专家共识》进行ILD影像学分类[4]。统计患者SARS-CoV-2疫苗接种情况,并按照是否接种疫苗分组比较。
同时参考《新型冠状病毒感染诊疗方案(试行第十版)》进行临床分型,对于以上呼吸道感染为主要表现的轻型患者定义为轻症;出现特征性新冠病毒感染肺炎表现、呼吸衰竭、休克的中型、重型及危重型患者定义为非轻症。记录COVID-19住院及死亡等不良事件。
1.3 统计学方法 应用SPSS 25.0进行数据分析。正态分布的计量资料以[x] ±s表示,组间比较采用成组t检验;非正态数据以M(P25,P75)表示,组间比较采用Mann-Whitney U检验。分类变量以例(%)表示,使用Fisher确切概率法进行比较。二元Logistic回归分析预后危险因素。P<0.05为差异有统计学意义。
2 结果
2.1 MPA-ILD合并COVID-19的临床特点 共纳入37例诊断明确的MPA-ILD患者,其中女14例(37.8%),男23例(62.2%),诊断年龄(67.27±6.36)岁,中位病程4.0(1.0,7.5)年,BMI(22.98±3.17)kg/m?。有吸烟史者19例,合并糖尿病4例,合并高血压12例。在并发COVID-19时,32例MPA处于疾病缓解期,31例有免疫抑制剂或生物制剂维持缓解治疗,包括环磷酰胺(CTX)21例,吗替麦考酚酯(MMF)5例,利妥昔单抗(RTX)2例,他克莫司1例,雷公藤多苷1例,来氟米特1例。ILD的影像学分型:寻常型间质性肺炎(UIP)15例,非特异性间质性肺炎(NSIP)14例,剩余8例分型不确定。37例MPA-ILD患者中有26例接种了SARS-CoV-2疫苗(均为灭活疫苗),其中11例完成了3剂次的加强免疫,15例完成2剂次疫苗接种。接种与未接种SARS-CoV-2疫苗患者在年龄、病程、性别比例、BMI、MPA缓解期等方面,差异均无统计学意义(P>0.05),见表1。
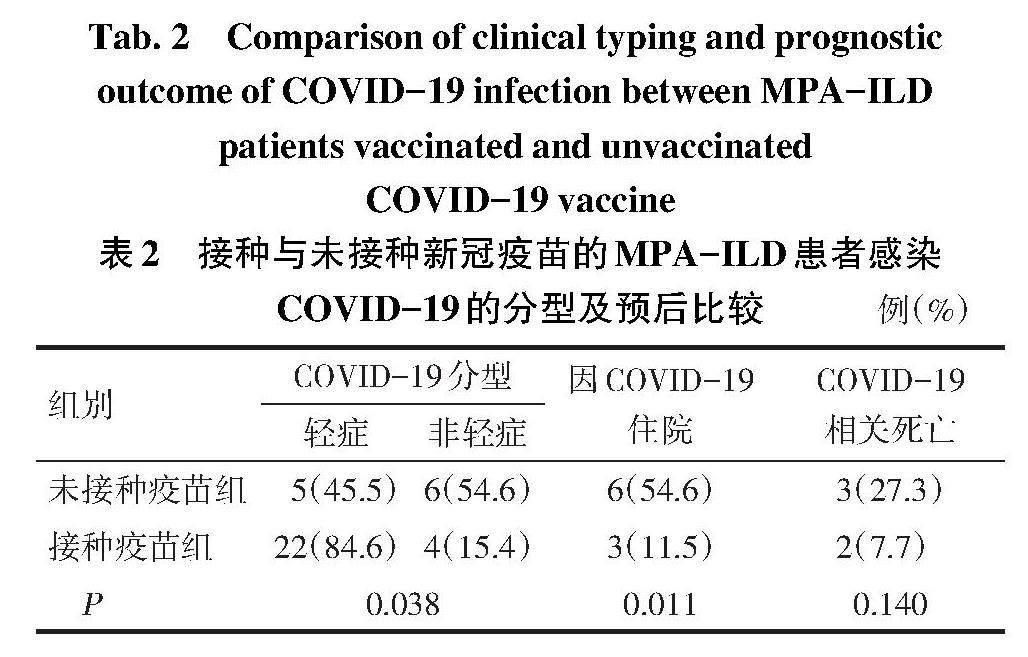
2.2 MPA-ILD患者COVID-19的严重程度分型及预后 COVID-19分型中,轻症27例,非轻症10例,9例因COVID-19住院治疗,其中5例死亡。与未接种疫苗组相比,接种疫苗组不仅COVID-19轻症比例高,且因COVID-19住院患者比例降低。接种疫苗组与未接种疫苗组在COVID-19相关死亡方面,差异无统计学意义,见表2。
2.3 COVID-19不良结局的预测因素分析 结果显示,接种疫苗(未接种=0,接种=1)为MPA-ILD患者因COVID-19住院(未住院=0,住院=1)的独立保护因素,见表3。接种疫苗和MPA处于缓解期(未缓解=0,缓解=1)为COVID-19非轻症感染(轻症=0,非轻症=1)的保护因素。而患者ILD影像学类型(非UIP=0, UIP=1)以及新冠感染时是否应用免疫抑制治疗(否=0,是=1)均不是新型冠状病毒感染不良结局的危险因素,见表4。
3 讨论
3.1 自身免疫病患者合并COVID-19情况 自身免疫性疾病的患者通常感染风险增加。接受免疫抑制剂或生物制剂治疗的自身免疫病患者免疫原性受损,尤其在接受利妥昔单抗或吗替麦考酚酯治疗的群体中[5-6]。但意大利的一项研究发现,与一般人群相比,自身免疫性疾病患者合并COVID-19的风险似乎并没有增加[7]。糖皮质激素或免疫抑制剂的长期治疗与COVID-19预后较差无关[7-8],这与本研究结果一致。但也有研究表明,在因突破性SARS-CoV-2感染住院的疫苗接种患者中,免疫抑制剂的使用占比更高[9]。这可能与疫苗接种时免疫反应受损,导致中和抗体滴度产生降低相关。但本研究中并未收集到抗体滴度这部分数据,接种疫苗患者的抗体滴度变化是否与突破性感染相关,其保护性抗体滴度最低阈值的确定仍有待进一步研究。
已有ILD的患者在SARS-CoV-2感染的大流行背景下面临着更高的风险。既往研究发现,与年龄、性别和(或)种族匹配的对照受试者相比,ILD患者在合并COVID-19后比没有ILD的匹配患者有更高的死亡风险[10-11]。COVID-19大流行持续影响公共健康,但是仍然缺乏关于MPA患者合并COVID-19的病死率及特定危险因素的数据。在MPA患者中可以参考与一般人群类似的危险因素,包括高龄、合并症和男性,似乎可以预测COVID-19的不良预后结局[7-8]。有研究人员发现COVID-19感染与原有的结缔组织病相关ILD恶化无关[12]。在COVID-19感染后,肺间质纤维化的出现与高龄、急性呼吸窘迫综合征、住院时间延长、机械通气相关[13]。但本研究未发现ILD的影像学类型与COVID-19预后有关,而控制原发病使其处于病情缓解期与更好的COVID-19结局相关。
3.2 SARS-CoV-2疫苗对自身免疫病患者SARS-CoV-2感染的影响及意义 SARS-CoV-2的疫苗接种有效遏制了疾病大流行,在系统性风湿病患者中同样有效,疫苗安全性与耐受性良好,并且与原发病活动度显著增加无关[14-16]。但已有研究报道在医护群体中观察到接种新冠疫苗后的突破性SARS-CoV-2感染,同样突破性SARS-CoV-2感染可能发生在系统性风湿性疾病患者中[17]。与未接种疫苗的系统性风湿性疾病患者相比,无论风湿性疾病的类型如何(类风湿关节炎、系统性红斑狼疮、系统性硬化症、ANCA相关性血管炎),接种SARS-CoV-2 mRNA疫苗的患者总体COVID-19结局更好[18]。不同类型的SARS-CoV-2疫苗在普通人群中具有公认的安全性和有效性,虽然本研究中MPA患者接种的新冠疫苗均为灭活疫苗,但是研究结果同样显示接种新冠灭活疫苗可能改善COVID-19的结局。德国的一项研究发现,在2 314例风湿病患者中,接种了3剂次疫苗的COVID-19病死率为0.6%,加强免疫与较低的COVID-19相关死亡风险显著相关[9]。
本研究有一定的局限性。首先,本研究为单中心回顾性研究,且样本量小。其次,回顾性研究避免不了选择偏倚的产生,缺乏明确SARS-CoV-2感染证据的患者有可能被遗漏。未来需要更长时间的随访以及更大的样本量去评估COVID-19及新冠疫苗的接种对MPA-ILD或自身免疫性疾病相关ILD群体的长期影响。
参考文献
[1] W?JCIK K,ASIAK A,JELENIEWICZ R,et al. Association of antineutrophil cytoplasmic antibody(ANCA)specificity with the demographic and clinical characteristics of patients with ANCA-associated vasculitides[J]. Pol Arch Intern Med,2022,132(3):16187. doi:10.20452/pamw.16187.
[2] COVID is here to stay:countries must decide how to adapt[J]. Nature,2022,601(7892):165-165. doi:10.1038/d41586-022-00057-y.
[3] KRONBICHLER A,GEETHA D,SMITH R M,et al. The COVID-19 pandemic and ANCA-associated vasculitis-reports from the EUVAS meeting and EUVAS education forum[J]. Autoimmun Rev,2021,20(12):102986. doi:10.1016/j.autrev.2021.102986.
[4] 中国医师协会风湿免疫科医师分会风湿病相关肺血管/间质病学组,国家风湿病数据中心. 2018中国结缔组织病相关间质性肺病诊断和治疗专家共识[J]. 中华内科杂志,2018,57(8):558-565. Group of Pulmonary Vascular and Interstitial Diseases Associated with Rheumatic Diseases,Chinese Association of Rheumatology and Immunology Physicians,Chinese Rheumatic Disease Data Center. 2018 Chinese expert-based consensus statement regarding the diagnosis and treatment of interstitial lung disease associated with connective tissue diseases[J]. Chin J Intern Med,2018,57(8):558-565. doi:10.3760/cma.j.issn.0578-1426.2018.08.005.
[5] RUDDY J A,CONNOLLY C M,BOYARSKY B J,et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases[J]. Ann Rheum Dis,2021,80(10):1351-1352. doi:10.1136/annrheumdis-2021-220656.
[6] BOYARSKY B J,RUDDY J A,CONNOLLY C M,et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases[J]. Ann Rheum Dis,2021,80(8):1098-1099. doi:10.1136/annrheumdis-2021-220289.
[7] EMMI G,BETTIOL A,MATTIOLI I,et al. SARS-CoV-2 infection among patients with systemic autoimmune diseases[J]. Autoimmun Rev,2020,19(7):102575. doi:10.1016/j.autrev.2020.102575.
[8] GALLAY L,UZUNHAN Y,BORIE R,et al. Risk factors for mortality after COVID-19 in patients with preexisting interstitial lung disease[J]. Am J Respir Crit Care Med,2021,203(2):245-249. doi:10.1164/rccm.202007-2638LE.
[9] HASSELI R,RICHTER J G,HOYER B F,et al. Characteristics and outcomes of SARS-CoV-2 breakthrough infections among double-vaccinated and triple-vaccinated patients with inflammatory rheumatic diseases[J]. RMD Open,2023,9(2):e002998. doi:10.1136/rmdopen-2023-002998.
[10] DRAKE T M,DOCHERTY A B,HARRISON E M,et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An international multicenter study[J]. Am J Respir Crit Care Med,2020,202(12):1656-1665. doi:10.1164/rccm.202007-2794OC.
[11] ESPOSITO A J,MENON A A,GHOSH A J,et al. Increased odds of death for patients with interstitial lung disease and COVID-19:A case-control study[J]. Am J Respir Crit Care Med,2020,202(12):1710-1713. doi:10.1164/rccm.202006-2441LE.
[12] PANOPOULOS S,TZILAS V,BOURNIA V K,et al. COVID-19 and protection of vaccination in patients with systemic sclerosis–associated interstitial lung disease[J]. J Scleroderma Relat Disord,2023,8(2):113-119. doi:10.1177/23971983221143252.
[13] HAN X,FAN Y,ALWALID O,et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia[J]. Radiology,2021,299(1):E177-E186. doi:10.1148/radiol.2021203153.
[14] FURER V,EVIATAR T,ZISMAN D,et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population:a multicentre study[J]. Ann Rheum Dis,2021,80(10):1330-1338. doi:10.1136/annrheumdis-2021-220647.
[15] FRAGOULIS G E,BOURNIA V K,MAVREA E,et al. COVID-19 vaccine safety and nocebo-prone associated hesitancy in patients with systemic rheumatic diseases:a cross-sectional study[J]. Rheumatol Int,2022,42(1):31-39. doi:10.1007/s00296-021-05039-3.
[16] SIMONCELLI E,CONTICINI E,COLAFRANCESCO S,et al. Multicentre case-control study evaluating the safety of anti-SARS-CoV-2 vaccines in a cohort of patients with systemic vasculitis[J]. Clin Exp Rheumatol,2023,41(4):922-927. doi:10.55563/clinexprheumatol/if8nka.
[17] BERGWERK M,GONEN T,LUSTIG Y,et al. Covid-19 breakthrough infections in vaccinated health care workers[J]. N Engl J Med,2021,385(16):1474-1484. doi:10.1056/NEJMoa2109072.
[18] PAPAGORAS C,FRAGOULIS G E,ZIOGA N,et al. Better outcomes of COVID-19 in vaccinated compared to unvaccinated patients with systemic rheumatic diseases[J]. Ann Rheum Dis,2022,81(7):1013-1016. doi:10.1136/annrheumdis-2021-221539.
(2023-11-19收稿 2024-01-09修回)
(本文编辑 李鹏)
