气体扩散电极电合成过氧化氢技术研究进展
2024-06-23杨武霖易可欣袁夏雨黄兴俊倪金元胡君杰王丽君刘盛
杨武霖 易可欣 袁夏雨 黄兴俊 倪金元 胡君杰 王丽君 刘盛
摘要:过氧化氢(H2O2)是一种高效的氧化剂,被广泛应用于化学合成、消毒杀菌和废水处理.通过二电子氧还原反应(2e- ORR)原位电合成H2O2的方法具有高性能和环保性,可作为传统蒽醌工艺的替代策略.气体扩散电极(GDEs)可作为阴极,通过电催化生产H2O2,具有更低的成本、更低的能耗和更高的氧气利用效率等优点.探讨GDEs作为阴极时通过2e- ORR产H2O2的机制;讨论GDEs的基本构型、原理及优化方法;分析GDEs催化剂的种类及优势;展望了GDEs作为阴极原位制备H2O2时存在的挑战,为推动2e- ORR原位电合成H2O2迈向市场化提供借鉴.
关键词:气体扩散电极; 过氧化氢; 碳材料催化剂; 二电子氧还原
中图分类号:O643.36; TM911.3 文献标志码:A 文章编号:1001-8395(2024)05-0596-15
doi:10.3969/j.issn.1001-8395.2024.
过氧化氢(H2O2)是一种重要的化工原料,并且广泛地应用于有机合成[1]、漂白[2]、废水处理[3]、能源转化[4]、医疗消毒[5]等方面.1930 年,德国巴斯夫公司开发了一种使用金属催化剂和氧气生产 H2O2的方法,为大规模蒽醌氧化工艺奠定了基础[6].1939年,Riedl和Pfleiderer开发了蒽醌法制备H2O2,是工业合成H2O2的主要方法,其反应主要涉及以下步骤,分别为:蒽醌在Pd或Pt催化剂上发生H2加氢、O2氧化,随后将H2O2进行提取,最后对工作液进行处理[7].蒽醌法制备H2O2工艺存在着明显的缺点,例如反应会产生大量的废液及废气,不利于环境友好;生成的H2O2需要净化,增加操作和人工成本;已完成的H2O2产品需要储存和运输,具有一定的安全风险[8-9].因此需要开发更绿色、经济、高效、安全的替代方法,以实现环境友好的H2O2制备方法.
近年来,越来越多的学者通过电化学氧还原法合成H2O2,该方法不仅高效,而且安全[10-11].电化学氧还原法通过氧气在阴极发生双电子氧还原反应(2e- ORR)合成H2O2,与传统的蒽醌工艺相比具有许多优点,如合成路线绿色安全、不产生有机副产物、可以原位生产、操作简便,是一种理想的工业制备H2O2方法.电催化方法合成H2O2最早追溯到20世纪30年代[12].到了20世纪80年代,陶氏和休伦科学技术有限公司采用这种方法现场生产稀碱性H2O2,被称作Huron-Dow工艺.该方法生成的稀碱性H2O2被用于纸浆和纸张漂白过程,既不需要中和也不需要蒸馏,因此在工业上被广泛应用[13].由于氧气可分别通过四电子或二电子途径还原为水或H2O2,因此为了提高H2O2的生成效率,应提高2e- ORR的能力.近年来,也有许多学者为提高2e- ORR能力做出了努力[14-18].其中最便捷的手段就是对电极进行改良,如对电极构型进行设计改进,或者选择合适的催化剂并提高催化剂的性能.目前2e- ORR研究的催化剂主要有贵金属基催化剂、单原子催化剂和碳基催化剂[19].也有学者对电极进行设计,如采用气体扩散电极,使得H2O2的产量达到了前所未有的水平[16].
基于此,本文全面回顾GDEs原位电合成H2O2技术的进展,探讨GDEs作为阴极时通过2e- ORR产H2O2的微观机制,详细讨论GDEs的基本原理,探究GDEs反应装置、电极构型和实验条件对H2O2的生成效率的影响;还分析了GDEs电极催化剂的种类及性能;最后总结GDEs作为阴极原位制备H2O2时面临的挑战和前景.
1 二电子氧还原产H2O2机制
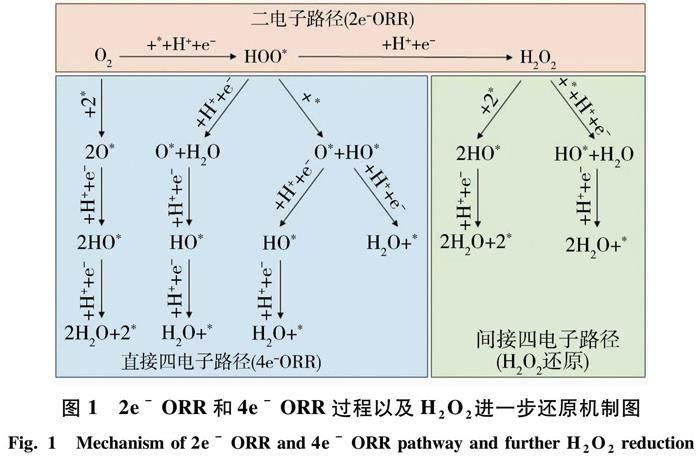
O2可以通过两种途径进行电化学还原:1) 生成H2O的四电子过程((1)式);2) 形成H2O2的二电子过程((6)式).为了使催化剂高效生成H2O2,提高材料的选择性,是抑制氧还原的四电子途径,并最大限度地提高二电子反应的途径[20].图1展示了2e- ORR和4e- ORR过程以及H2O2进一步还原或分解的详细途径[21].(1)~(8)式展示了氧还原的主要反应机制.
4e- ORR的作用机制(*表示未占据的活性位点或吸附在活性位点表面上的物种):O2+4H++4e-→2H2O, E0=1.23 V vs.RHE, (1)
*+O2+(H++e-)→HOO*,(2)
HOO*+(H++e-)→H2O+O*,(3)
O*+(H++e-)→OH*,(4)
HO*+(H++e-)→H2O+*.(5)
2e- ORR的作用机制:
O2+2H++2e-→H2O2, E0=0.70V vs.RHE,(6)
*+O2+(H++e-)→HOO*,(7)
HOO*+(H++e-)→H2O2.(8)
由于4e- ORR与2e- ORR存在竞争,催化剂对二电子产物的选择性是合成H2O2的关键.因此,ORR的最终产物很大程度上取决于所选择的催化剂材料[19].根据图1及(2)和(7)式可以看出,HOO*是两种ORR途径之间的共同中间体,决定了反应产物的最终形态.保护好HOO*中间体的O—O键是使ORR反应倾向于二电子过程形成H2O2的关键.而具有较强氧结合能的催化剂容易分解O—O键,发生4e- ORR生成水为主要产物.因此,想要使2e- ORR发生并生成H2O2,应选择那些结合氧不太强烈的催化剂.
杨武霖,等:气体扩散电极电合成过氧化氢技术研究进展
同样,O2在电极表面的吸附强度也可以解释2e- ORR与4e- ORR之间的选择性.对氧结合能力强的催化剂表面与O2分子O—O键平行,而对氧吸附能力较弱的催化剂表面与O—O键垂直[22].有研究表明,最低的反应势垒对应于O—O键的垂直方向,更有利于解离反应的进行[23];而随着催化剂表面反应活性的增加,氧更有可能平行于催化剂表面,导致O—O键的断裂,使反应向4e- ORR路径进行.
上述所描述的2e- ORR的机制是基于酸性条件的,由两个质子和电子耦合转移到O2分子上,形成OOH*和H2O2.下面将讨论碱性条件下2e- ORR的机制:
O2+H2O+2e-→HO-2+OH-,(9)
*+O2+H2O+e-→HOO*+OH-,(10)
HOO*+e-→HO-2+*.(11)
与酸性机制相似,体系中唯一参与反应的中间体是HOO*.不同之处在于,酸性环境中的质子源是水合氢离子,而碱性环境中的质子源是水.但也有研究报道,吸附能是一种表面性质,不受质子源的影响[19].因此,无论在酸性或是碱性条件下,2e- ORR活性都可以通过HOO*的吸附能进行表征.
2 气体扩散电极制备H2O2研究进展
2.1 气体扩散电极
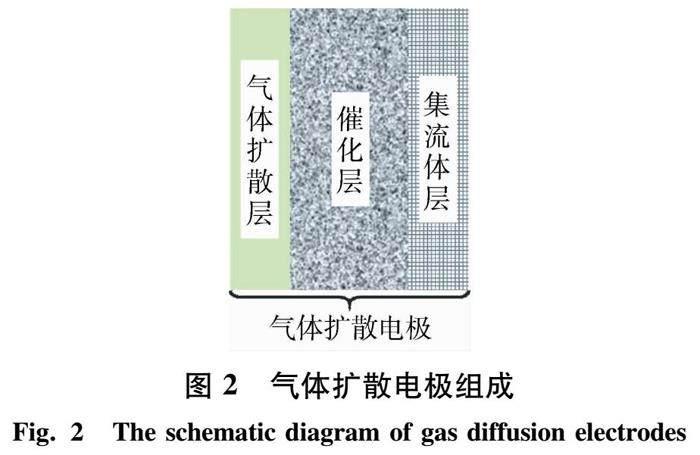
气体扩散电极(GDEs)是一种多孔膜电极,其中固体催化剂同时与液相(电解液)和气相(空气或氧气)接触,这种结构可以组成一种稳定的固、液、气三相界面(TPI)[24].如图2所示,GDEs主要由气体扩散层(GDL)、催化层(CL)以及集流体层(CC)组成.其中,GDL的主要作用是让反应气体顺利地通过,并且为催化层输送其所需要的气体.同时,GDL必须要防止因电解液的迁移导致气体扩散通道被掩没的状况.因此,GDL一般需要既要具有透气性,又要具有疏水性.CL是氧气发生还原反应的场所,通常由含或不含金属掺杂的碳基材料和黏结剂(如:疏水聚四氟乙烯(PTFE))组成.由于从GDL输送过来的气体应与该层中的催化剂、电解液一起形成电化学反应活性位点,进而将反应气体还原,所以CL应该具有一定的亲水性.CC主要由多孔碳纤维、碳布、碳纸或金属网构成,主要作用是让电流更均匀地分布在电极,并提供通向外部电路的导电路径,同时还起到支撑催化层的作用[25].因此,CC应该具备导电性优良、化学稳定性高、机械强度高、成本低的特点[26].
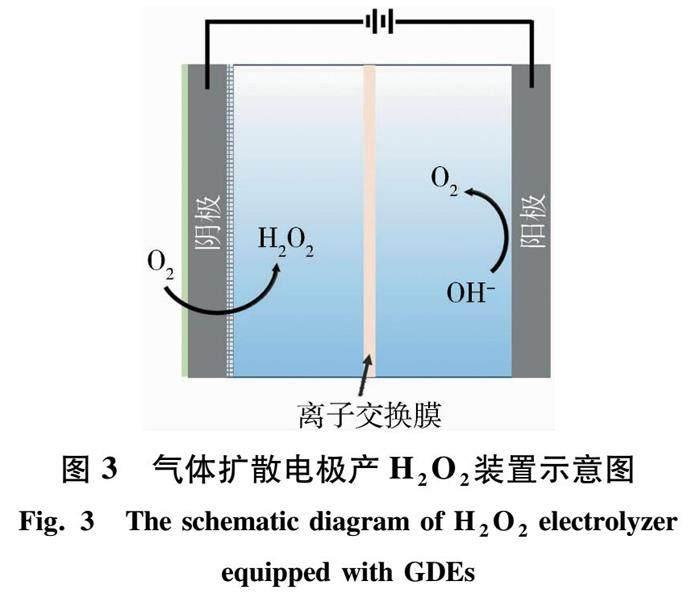
有研究表明,GDEs能直接原位制备H2O2.因为GDEs能提高二电子氧还原反应(2e- ORR)效率,具有更高的原位H2O2生成率.由于CL面向电解质溶液,GDL面向氧气或空气,O2从GDL表面扩散,使阴极上的氧还原反应成为可能,同时也为气体扩散电极产H2O2提供了基础[11].GDEs作为阴极产H2O2反应如图3所示.另外,由于气体扩散电极具有优良的导电性和高表面积,能加速O2还原成H2O2;它的多孔结构及疏水通道,允许O2无限地传递到电极和电解液表面,在这种条件下,O2传递限制被消除,二电子氧还原效率和H2O2生成率增加.有研究表明,采用GDEs制备H2O2效率比采用普通溶解氧的高0.9~1.2倍[27].因此,GDEs是一种很好的原位制备H2O2的阴极材料[25].
2.2 气体扩散电极的优化
为了提高气体扩散电极制备H2O2的产量,需对其进行优化改性,其中包括:1) 对反应装置进行设计;2) 对电极进行改良,如对电极构型进行设计改进;3) 选择合适的催化剂,提高电极二电子氧还原的能力;4) 优化反应参数.
2.2.1 设计气体扩散电极反应装置 设计低能耗高性能的气体扩散电极反应装置,对于电化学制备H2O2的规模化应用具有重要意义.对于空气阴极而言,如何将氧气快速输送到阴极附近是设计反应器时要考虑关键因素[28].气体扩散电极反应器构型主要分为2种,分别为浸没式和气体扩散式反应器(图4).
传统的浸没式反应器如图4(a)所示,主要是将电极浸入溶液中,并在阴极周围通过空气/氧气泵加压,以提高阴极周围溶解氧的浓度,从而增加H2O2生成效率.但该方式由于外部曝气需要通过压缩机对反应器进行加压,可能会增加反应能耗和运营维护成本.基于此,研究人员进一步优化了一些浸没式反应器,如图4(b)所示的文丘里射流曝气反应器.文丘里射流曝气反应器是一种有效的液流曝气供氧装置,它可以通过高速流体的引射作用
将被携带的气体打成微小气泡,并随着液体流动进入反应器,向电极提供连续充足的氧气的同时,减少能量消耗[29].Pérez等[30]采用碳毡作为阴极的文丘里射流曝气反应器,在180 min可获得960 mg·L-1的H2O2.但浸没式反应器由于直接将电极浸入电解液,一方面水中溶解氧浓度有限,另一方面,电极输气通道易被水淹,氧气传质效率受限,从而导致H2O2合成效率低.
为了改善可被利用的氧气浓度及传质效率,研究者们提出将电极置于气液交界面,即电极一侧接触气体,另一侧接触液体.气体从电极一侧通过疏水通道到达固液气三相界面,在气体高效传质的同时避免了电解液泄漏,从而高效合成H2O2[31].有研究对比了以CB/PTFE作为气体扩散层的空气阴极在20 mL·min-1的O2流速下,气体扩散式反应器(图4(c))与传统浸没式反应器(图4(a))下产H2O2浓度.采用传统浸没式反应器的H2O2产量为220 mg·L-1,氧气利用效率为0.7%;而气体扩散电极反应器可产生1 160 mg·L-1的H2O2,氧气利用效率为3.5%,是传统反应器的5倍[32].除此之外,研究者们研发了一种空气自驱动气体扩散电极的反应装置(图4(d)),该装置可直接利用空气中的氧气,不需要额外曝气,在高效产H2O2的同时降低反应能耗[33].Zhao等[34]用CB/PTFE气体扩散电极作为阴极,无需氧气曝气,最高可获得(4 300±160) mg·L-1的H2O2.Zhang等[35]制备了一种可放大漂浮式气体扩散电极反应器(图4(e)),通过在相转化空气阴极上施加1.8 V vs. Ag/AgCl的电压,可得到200 mg·L-1的H2O2.总体而言,气体扩散式反应器避免了氧气传质通道被堵塞,增强了O2利用效率,从而提高了H2O2的产率.
2.2.2 改良气体扩散电极构型 在传统的固-液两相催化模式中,O2在溶液中溶解度低、扩散速率慢,导致O2向电极界面的传输受限,极大地限制了H2O2的产率[36].为了减轻气体输运的限制,研究人员在研究固-液-气三相催化模式方面做出了努力,开发了气体扩散电极.通过疏水气体扩散层将O2从气相导入,直接到达与电解液面对的催化层,形成固-液-气三相催化界面[37].形成的三相界面为反应提供了充足的电活性区域,从而显著提高了H2O2的生成速率[38].然而,当电流增大时,由于电润湿的作用导致催化层疏水性降低,气体向催化位点传质阻力增大,O2浓度不足,从而造成与氧还原反应相关的H2O2的生成速率较差[39],并且在持续通电的情况下,电极被电解液浸润,导致气体扩散通道堵塞和三相界面被破坏,加剧了H2O2生成速率进一步降低[40].因此,改善气体扩散电极构型,稳定三相界面,在加速O2传质的同时有效防止水淹,对于长时间稳定合成H2O2的电化学系统是十分必要的.
2.2.3 改性催化层 电极催化材料在产H2O2中起着至关重要的作用,它与电流效率 (CE)、氧还原选择性、H2O2的生产息息相关.因此,许多研究人员也通过不同的方法改进GDEs[41-42].总体而言,理想的2e- ORR催化剂应该具有良好的活性,优异的二电子选择性,长时间操作下的稳定性,对环境友好并且成本低廉.本文后续会对气体扩散电极催化剂进行详细分析.
2.2.4 GDEs反应参数优化 除了优化GDEs自身结构及反应器会影响GDEs电产H2O2效率,还有多种参数如电流密度、电解液的 pH 值、空气/O2流速、电解时间、电解质溶液的种类和浓度等,均会对GDEs电合成H2O2的浓度产生影响[36, 43].表1总结了多种参数对GDEs产H2O2的效率的影响.Luo等[44]将PTFE混合在炭黑和石墨中制备催化层,探究了pH值、电流密度、空气流速对H2O2产量的影响.通过优化实验条件,发现在空气流速为55 mL·min-1,电流密度为52 mA·cm-2,pH值为4.0的条件下,可得到H2O2产量约为10 500 mg·L-1. Yu 等[42]用7~35 mg·cm-2的炭黑采用涂覆法制备GDEs作为CL.通过优化实验条件,发现在电流密度为7.1 mA·cm-2,空气流速为0.5 L·min-1时,3 h后0.15 g炭黑制备的GDEs产H2O2质量浓度最高达到566 mg·L-1.
3 GDEs催化剂的种类
为了解决氧还原反应过电势较高这一问题[48],通常在阴极表面覆盖一层催化剂以增加阴极与氧的结合力或降低ORR反应所需的活化能[49].GDEs常用的催化剂可分为以下几类:贵金属催化剂、碳基催化剂、杂原子掺杂碳材料催化剂和金属-氮-碳材料催化剂.表2总结了近年来报道的GDEs通过2e- ORR制备H2O2的催化剂[50-63].
3.1 贵金属基材料
贵金属材料由于催化效果和化学稳定性好被广泛应用于ORR催化剂.在纯贵金属中,Au(100)和Au(111)晶面在一定的pH值和电势范围下具有2e- ORR催化活性[64-65].而Pt和Pd由于和O2的紧密结合,O—O容易解离,以催化4e- ORR为主[66].因此,有学者将Pt、Pd与那些和O2结合较弱的金属(如Pd-Au[67-68]、Pt-Hg[69-70])组成合金,从而开发更高效2e- ORR催化剂.Jirkovsky等[71]制备了不同Pd掺杂比例的Pd-Au材料,结果显示当Pd掺杂为8%时,H2O2选择性可接近95%.但当掺杂量更高时,H2O2产量降低,反应逐渐向4e-过程转变.Siahrostami等[72]使用DFT考察了Pt、Pd、Rh、Ir、Au、Hg等30多种合金,通过计算得到PtHg4材料同时具有稳定性、较高ORR活性和二电子选择性.因此他们使用电沉积法制备出的PtHg4催化剂,在0.2~0.4 V的电位下获得了3 mA·cm-2的H2O2电流和96%的H2O2选择性.但是,Hg合金高毒性和贵金属合金的高成本限制了贵金属材料的实际使用.近年来,一个新兴的研究方向——单原子催化剂(SAC)为解决这两个问题提供了新的思路.
SAC指的是催化的活性金属仅以单原子形式分散[73-74].首先,SAC的构建为在原子水平上理解特定反应的催化机制和途径提供了一个简单的模型;其次,SAC作为催化剂,使原子利用效率最大化,从而提高了特定反应的反应活性,同时降低了原材料成本[75].如图5所示,在贵金属单原子材料中,活性位点分散,O2在活性位点表面以O—O键不容易断裂的Pauling模型吸附,有利于发生2e- ORR;而在非单原子材料中,由于活性位点的集中,O2可能以O—O键更容易断裂的Yeager模式吸附,更容易发生4e-过程[76-78].因此,通过将贵金属单原子负载到价格低廉、导电性好的碳基底材料上,合成了很多性能优异的2e- ORR催化剂.Chio等[76]制备了一种负载在硫掺杂沸石模板碳上的Pt单原子材料,选择性高达96%,在H池反应器中获得了97.5 μmol·h-1·cm-2的H2O2产量.Yang等[77]制备出的单原子分散的Pt/TiC选择性达到68.0%,显著高于未原子分散的Pt/TiC(24.8%).Wang等[50]制备出单原子分散的Pd-N-C材料在0.1~0.8 V的宽电位范围内均对H2O2生产具有较高的选择性(>87.5%).
3.2 碳基催化剂
碳材料由于其成本低、无毒、对环境友好,具有良好的热稳定性和化学稳定性,且具有较高的比表面积和优异的导电性能,能将O2通过2e- ORR还原为H2O2,而被广泛应用于气体扩散电极的阴极催化剂[79].此外,碳基催化剂作为阴极催化剂时析氢过电位高,对H2O2分解能力弱,有利于 H2O2生成[80].很多研究发现多种碳基材料均可作为GDE阴极产H2O2的高效催化材料,包括炭黑[44,81]、石墨烯[54,82]、碳纳米管[83-85]、碳毡及石墨毡[86-87]、碳基海绵[88-89]、网状玻璃碳(RVC)[90]和活性炭纤维(ACF)[91-92].这些碳质阴极也可以进行修饰和改性,促进H2O2生成[93-95].此外,碳基材料的性能也可以通过掺杂杂原子或单金属 [96-97],或是增加缺陷和氧官能团来进一步优化产H2O2效率[98].
据观察,GDEs碳基催化剂的电催化性能会受到多孔结构的影响,高度多孔结构能促进反应物和生成物的扩散从而改善GDEs的整体性能[99].Li等[100]根据2e- ORR过程研究了GDEs的多孔结构与H2O2活性之间的关系.他们用20%的硝酸预处理石墨阴极从而提高微孔表面积,并将H2O2产率提高至46.9%.值得注意的是,不仅比表面积和活性位点对H2O2的生成十分重要,O2的高效扩散对H2O2的生成也很重要[101].在另一项研究中,Zhang等[38]采用孔隙率高达90%的改性碳毡作为GDL,并在不使用气泵的情况下,直接利用空气中的氧气使其主动扩散至CL中,促进H2O2的生成,得到H2O2产出速率高达101.67 mg·h-1·cm-2.
3.3 杂原子掺杂碳材料
原始碳材料虽然表现出较高的2e- ORR选择性,但由于原始碳材料中的中性碳原子对于O2和ORR反应中间体的吸附/活化是惰性的,导致氧还原活性较低[2,102-103].如图6所示,适当引入杂原子(O、N、B、F等),利用杂原子和碳原子电负性差异来改变碳材料的电荷分布,从而提高碳材料氧还原反应活性.
3.3.1 O掺杂
O掺杂是利用各种氧化手段将氧官能团如羟基(—OH)、醚键(C—O—C)、羰基(—CO)、羧基(—COOH)引入碳材料,包括化学氧化[56-57]、电化学氧化[104]、等离子体处理[105]等.许多研究应用O掺杂碳材料作为气体扩散阴极催化剂,获得了较高H2O2产量.He等[56]使用硝酸氧化石墨烯在气体扩散电极中生产H2O2,H2O2生成量是原始石墨烯的3倍.Zhang等[57]通过在马弗炉中加热来氧化炭黑,并使用搭载氧化炭黑的浮动空气阴极测试H2O2的生产性能,在-1.0 V vs.Ag/AgCl的电位下反应30 min,获得(517.7 ± 2.4) mg·L-1的H2O2,显著高于原始炭黑(65.3 ± 5.6) mg·L-1.他们指出,氧化炭黑的性能提升主要来自于氧官能团的贡献,一方面,在引入的—COOH上O2更倾向于发生2e-过程的Pauling吸附;另一方面,引入的C—O—C和—COOH由于和ORR反应中间体的吸附强度适中,二电子选择性较高.关于氧官能团对于2e- ORR的影响,Lu等[18]较为系统地审查了位于边缘/基面的氧官能团(—COOM、—OH、C—O—C)对于ORR反应中间体吸附强度的影响,结果显示边缘的—COOH和石墨烯基面上的C—O—C是具有较高ORR活性和二电子选择性的氧官能团.
3.3.2 N掺杂 N掺杂一般通过热解含N前驱体实现.Hu等[58]直接热解有聚多巴胺涂层聚苯乙烯球得到了具有丰富微孔的中空结构 N 掺杂碳球,获得了高达91.9%的H2O2选择性和85.1%的法拉第效率.Sun等[106]通过热解HNO3预氧化的有序介孔碳和富氮离子液体制备出N掺杂碳材料,发现在较低掺杂条件下,H2O2选择性提高;而随着氮含量进一步增加,选择性降低,可能是过量的N促进了H2O2分解.除了受N含量的影响,N的种类也影响着ORR活性和选择性.吡啶N一般被认为4e- ORR活性中心[107-109],吡咯N[110-112]和石墨N[109,113]被视为具有二电子活性.Lu等[114]通过热解三聚氰胺和CB的混合物来制备NCB材料和CNT一同作为气体扩散阴极的催化剂,结果显示气体扩散电极的疏水三相边界、NCB中的吡啶和吡咯N活性位点,以及CNT在电子转移中的桥接作用和高电活性表面积共同促进了H2O2的产生.此外,N官能团的选择性还受到pH值、N的位置、碳基底材料的影响.例如,Sun等[66]发现吡啶N在酸性环境中起重要作用.Duan等[115]发现吡啶N掺杂的碳边缘在碱性介质中对ORR具有高度活性.因此,需要优化制备方法,精确调控氮掺杂种类和含量,从而获得具有高活性和选择性的2e- ORR催化剂.
3.3.3 B掺杂 硼(B)只比碳少一个电子,并且它们的尺寸相似,B掺杂碳材料后将产生较小的晶格畸变,因此,B也是碳材料中的理想掺杂剂[116].2011年,Yang等[117]制备了含不同量B掺杂的碳纳米管作为ORR反应催化剂,发现ORR活性随着B的含量增加而提升.这主要是由于B掺杂后产生正电荷,带正电的B掺杂剂诱导O2在化学吸附;共轭体系中的一些π*电子在B掺杂碳上积累,然后转移到O2分子上,促进O2还原.Wu等[118]通过冷冻干燥法制备B掺杂水凝胶作为气体扩散阴极催化剂,发现B掺杂后H2O2产量从58.42 mg·L–1提高到75.03 mg·L–1.除此之外,B掺杂的碳材料在大电流密度下体现出更优越的性能.2021年,Xia等[59]制备的B掺杂碳材料在工业电流下(高达300 mA·cm–2)也能保持高H2O2生产的选择性(85%~90%).Ri等[119]通过制备热解B掺杂水凝胶来制备B掺杂的rGO气体扩散阴极催化剂,发现H2O2的累积量随着B含量增加而增高,在60 mA·cm–2的条件下电流效率增加了49.5%.这些研究进一步验证了B掺杂碳材料在气体扩散电极制备H2O2方面的巨大潜力.
3.3.4 F掺杂 F的高电负性可以诱导相邻的碳极化以产生活性位点.F掺杂碳材料制备可以通过和HF直接反应得到[120-121].Wang等[121]通过与HF反应制备的F-CNT材料作为气体扩散阴极,随着F的引入,H2O2选择性从69%~77%提高到82%~95%.Zhao等[60]通过热解MIL-53 (Al)与HF反应制备了F 掺杂的多孔碳材料,在-0.1 V vs. RHE和pH= 1的条件下反应3 h,得到了241.5 mmol·L-1的H2O2,是未掺杂F的碳材料在同等条件下的4倍.这些结果说明 F掺杂碳材料也是一种有效的H2O2催化剂[121].
3.3.5 共掺杂 多种杂原子的有效结合可能会产生协同效应,增加催化剂表面的活性位点和2e– ORR选择性,从而比单原子掺杂起到更好的产H2O2效果.目前研究人员已经开发出众多具有较高二电子催化活性的共掺杂碳基催化剂,例如:O/F[61];B/N[122-123]、N/P[124]催化剂.2022年,Gu等[61]成功通过硫酸、氢氟酸和CNT反应制备出O/F共掺杂的CNT作为气体扩散阴极,在pH值为1~9的条件下H2O2产量为196.5~232.2 mg·L–1,是未掺杂CNT产量的2倍.其催化性能的提升来自—COOH和CF2、CF3的协同作用,一方面—COOH能够促进O2的Pauling吸附,倾向于发生2e–ORR;另一方面F具有较高的电负性,可以促进O2的化学吸附,而CF2、CF3已经被证明具有合适的HOO*吸附能.两种元素协同作用,共同增强H2O2的产量[61].
3.4 金属-氮-碳材料(M-N-C)
多种氮掺杂碳材料已经被报道为2e– ORR催化剂,但它们的过电势通常较高,引入金属是一种解决该问题的有效策略[125].2013年Lanza团队[126]将5% CoPc和CB混合制成空气阴极,发现H2O2产量从176 mg·L–1增加到331 mg·L–1.次年,Lanza团队[127]发现5%的FePc和CB混合后,H2O2产量从175 mg·L–1增加到240 mg·L–1.该实验证明了过渡金属掺杂在气体扩散电极体现中产H2O2的可行性.
受到这类过渡金属大环配合物能提高H2O2产量的启发,M-N-C单原子材料被广泛研究.M-N-C材料是利用N将单原子分散的过渡金属原子锚定在碳材料中[128-130].目前已有多种方法合成的性能优异的M-N-C材料,如Jung等[14]通过在NH3气氛下热解Co2+浸渍过的碳材料合成Co-N-C,发现该材料在起始电位、ORR动力学、H2O2产量方面都显著优于N-C,在H池测试中表现出高达(420±20) mmol·g-1·h-1的产出速率.Zhang等[63]通过热解ZIF-67前驱体成功合成单原子Co-N-C,发现该材料在酸性介质中表现出>90%的H2O2选择性,并获得了2 500 mg·L-1·h-1的H2O2产出速率.Du等[62]使用炭黑吸附Co2+再热解的方法合成了负载在CB上的Co-N-C单原子催化剂,使用气体扩散电极在酸性条件下产生5.04 mol·h-1·g-1的高浓度H2O2产量.此外他们发现,若提高催化剂负载量,H2O2产量下降.这是因为当催化剂负载较高时,H2O2可能不能及时扩散到电解质中,而是在阴极表面继续被还原[131].除了负载量,M-N-C材料选择性还受到pH值的影响.一方面pH值可以通过改变电极表面基团的质子化程度、电解质和反应中间体形成相互作用来影响反应的选择性[62,132];另一方面,pH值影响着界面水排布的方式,从而影响着材料表面电荷分布,进一步影响了ORR活性[133].因此,在开发气体扩散电极产H2O2的M-N-C催化剂时,除了要考虑前驱体的选择,还要慎重确定负载量、介质pH值等因素.
4 总结与展望
近年来,H2O2作为典型的环境友好型氧化剂,被广泛应用于环境修复、化学合成、医疗消毒等多个领域.而电合成H2O2技术避免了传统制备H2O2工艺时复杂的流程,采用简易的电化学设备,通过2e- ORR过程原位制备H2O2,避免了H2O2的大规模运输,具有安全便携、绿色环保的特点.而将GDEs 作为电合成H2O2的阴极催化剂,不仅有利于H2O2的原位生成,还能降低能源消耗和提高氧气利用效率等.本文探讨了GDEs作为阴极时通过2e- ORR产H2O2的机制;详细讨论了GDEs的基本原理;分析了GDE催化剂的种类及优势,主要探究了贵金属催化剂、碳基及改性碳材料催化剂作为GDEs的性能和优势.总的来说,GDEs 阴极是电合成H2O2技术中非常有前途的电催化材料.
虽然GDEs作为阴极具有2e- ORR选择性,易于合成H2O2且成本低,但该方法合成的H2O2处于碱性条件下,而H2O2在碱性环境下易分解,这对系统的长期运行和H2O2的储存不利.因此,未来的研究应侧重于寻找提高催化材料在中性环境中活性的方法.其次,提高氧气的传质效率对GDEs生成H2O2是十分重要的.因此在优化催化层的同时也需要完善电极三相界面,优化电极疏水性储气层和亲水性催化剂层的结构.另外,还需要设计合理的电极构型和反应器构型,平衡性能与成本之间的关系,使电合成H2O2能运用到更大工程规模中.最后,还可以将电合成H2O2与可再生能源进行耦合联用,更环保高效地产H2O2.
参考文献
[1] 梁武洋,伏劲松,陈洪林,等. 活性炭负载Pd催化剂常温直接合成过氧化氢[J]. 四川师范大学学报(自然科学版),2022,45(2):232-239.
[2] AN J K, FENG Y J, ZHAO Q, et al. Electrosynthesis of H2O2 through a two-electron oxygen reduction reaction by carbon based catalysts: from mechanism, catalyst design to electrode fabrication[J]. Environmental Science and Ecotechnology,2022,11:100170.
[3] 孙英涛,林业泓,蔡璇英,等. H2O2协助铜基催化剂激发新污染物表面裂解性能与供电子机制[J]. 能源环境保护,2023,37(2):187-195.
[4] 彭丹,孙芳芳,张明,等. Gd掺杂CeO2改性材料催化性能的理论研究[J]. 四川师范大学学报(自然科学版),2015,38(3):411-417.
[5] 陈家斌,姚广磊,纪睿成,等. 污水处理厂过氧乙酸消毒特性及应用进展[J]. 能源环境保护,2023,37(4):38-45.
[6] THIEL W R. New routes to hydrogen peroxide: alternatives for established processes?[J]. Angewandte Chemie International Edition,1999,38(21):3157-3158.
[7] YI Y H, WANG L, LI G, et al. A review on research progress in the direct synthesis of hydrogen peroxide from hydrogen and oxygen: noble-metal catalytic method, fuel-cell method and plasma method[J]. Catalysis Science & Technology,2016,6(6):1593-1610.
[8] YANG S, VERDAGUER-CASADEVALL A, ARNARSON L, et al. Toward the decentralized electrochemical production of H2O2: a focus on the catalysis[J]. ACS Catalysis,2018,8(5):4064-4081.
[9] 朱斯超,蒋若兰,王军,等. 电芬顿-膜蒸馏复合工艺同步脱盐及去除水中有机物研究[J]. 能源环境保护,2023,37(4):20-29.
[10] SHIN H, KIM H I, CHUNG D Y, et al. Scaffold-like titanium nitride nanotubes with a highly conductive porous architecture as a nanoparticle catalyst support for oxygen reduction[J]. ACS Catalysis,2016,6(6):3914-3920.
[11] ZHAO Q, AN J K, WANG S, et al. Superhydrophobic air-breathing cathode for efficient hydrogen peroxide generation through two-electron pathway oxygen reduction reaction[J]. ACS Applied Materials & Interfaces,2019,11(38):35410-35419.
[12] BERL E. A new cathodic process for the production of H2O2[J]. Transactions of the Electrochemical Society,1939,76(1):359.
[13] HAGE R, LIENKE A. Applications of transition-metal catalysts to textile and wood-pulp bleaching[J]. Angewandte Chemie International Edition,2006,45(2):206-222.
[14] JUNG E, SHIN H, LEE B H, et al. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production[J]. Nature Materials,2020,19(4):436-442.
[15] XIA C, BACK S, RINGE S, et al. Confined local oxygen gas promotes electrochemical water oxidation to hydrogen peroxide[J]. Nature Catalysis,2020,3:125-134.
[16] XIA C, XIA Y, ZHU P, et al. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte[J]. Science,2019,366(6462):226-231.
[17] KIM H W, ROSS M B, KORNIENKO N, et al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts[J]. Nature Catalysis,2018,1:282-290.
[18] LU Z, CHEN G, SIAHROSTAMI S, et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials[J]. Nature Catalysis,2018,1:156-162.
[19] SIAHROSTAMI S, VILLEGAS S J, BAGHERZADEH MOSTAGHIMI A H, et al. A review on challenges and successes in atomic-scale design of catalysts for electrochemical synthesis of hydrogen peroxide[J]. ACS Catalysis,2020,10(14):7495-7511.
[20] TAN X, TAHINI H A, SMITH S C. Understanding the high activity of mildly reduced graphene oxide electrocatalysts in oxygen reduction to hydrogen peroxide[J]. Materials Horizons,2019,6(7):1409-1415.
[21] ZHANG J Y, ZHANG H C, CHENG M J, et al. Tailoring the electrochemical production of H2O2: strategies for the rational design of high-performance electrocatalysts[J]. Small,2020,16(15):1902845.
[22] MONTEMORE M M, VAN SPRONSEN M A, MADIX R J, et al. O2 activation by metal surfaces: implications for bonding and reactivity on heterogeneous catalysts[J]. Chemical Reviews,2018,118(5):2816-2862.
[23] CHENG J, LIBISCH F, CARTER E A. Dissociative adsorption of O2 on Al(111): the role of orientational degrees of freedom[J]. The Journal of Physical Chemistry Letters,2015,6(9):1661-1665.
[24] YANG W L, HE W H, ZHANG F, et al. Single-step fabrication using a phase inversion method of poly(vinylidene fluoride) (PVDF) activated carbon air cathodes for microbial fuel cells[J]. Environmental Science & Technology Letters,2014,1(10):416-420.
[25] WANG J, LI C, RAUF M, et al. Gas diffusion electrodes for H2O2 production and their applications for electrochemical degradation of organic pollutants in water: a review[J]. Science of the Total Environment,2021,759:143459.
[26] SHARMA M, BAJRACHARYA S, GILDEMYN S, et al. A critical revisit of the key parameters used to describe microbial electrochemical systems[J]. Electrochimica Acta,2014,140:191-208.
[27] LI N, AN J K, ZHOU L A, et al. A novel carbon black graphite hybrid air-cathode for efficient hydrogen peroxide production in bioelectrochemical systems[J]. Journal of Power Sources,2016,306:495-502.
[28] ZHOU W, MENG X X, GAO J H, et al. Hydrogen peroxide generation from O2 electroreduction for environmental remediation: a state-of-the-art review[J]. Chemosphere,2019,225:588-607.
[29] PREZ J F, LLANOS J, SEZ C, et al. The jet aerator as oxygen supplier for the electrochemical generation of H2O2[J]. Electrochimica Acta,2017,246:466-474.
[30] PREZ J, LLANOS J, SEZ C, et al. Electrochemical jet-cell for the in situ generation of hydrogen peroxide[J]. Electrochemistry Communications,2016,71:65-68.
[31] YANG W, HE W, ZHANG F, et al. Single-step fabrication using a phase inversion method of poly(vinylidene fluoride)(PVDF) activated carbon air cathodes for microbial fuel cells[J]. Environmental Science & Technology Letters,2014,1(10):416-420.
[32] DING P P, CUI L L, LI D, et al. Innovative dual-compartment flow reactor coupled with a gas diffusion electrode for in situ generation of H2O2[J]. Industrial & Engineering Chemistry Research,2019,58(16):6925-6932.
[33] YI K X, YANG W L, LOGAN B E. Defect free rolling phase inversion activated carbon air cathodes for scale-up electrochemical applications[J]. Chemical Engineering Journal,2023,454:140411.
[34] ZHAO Q, LI N, LIAO C M, et al. The UV/H2O2 process based on H2O2 in situ generation for water disinfection[J]. Journal of Hazardous Materials Letters,2021,2:100020.
[35] ZHANG H C, LI Y J, LI G H, et al. Scaling up floating air cathodes for energy-efficient H2O2 generation and electrochemical advanced oxidation processes[J]. Electrochimica Acta,2019,299:273-280.
[36] QIANG Z M, CHANG J H, HUANG C P. Electrochemical generation of hydrogen peroxide from dissolved oxygen in acidic solutions[J]. Water Research,2002,36(1):85-94.
[37] DA POZZO A, DI PALMA L, MERLI C, et al. An experimental comparison of a graphite electrode and a gas diffusion electrode for the cathodic production of hydrogen peroxide[J]. Journal of Applied Electrochemistry,2005,35(4):413-419.
[38] ZHANG Q Z, ZHOU M H, REN G B, et al. Highly efficient electrosynthesis of hydrogen peroxide on a superhydrophobic three-phase interface by natural air diffusion[J]. Nature Communications,2020,11(1):1731.
[39] BIDAULT F, BRETT D J L, MIDDLETON P H, et al. Review of gas diffusion cathodes for alkaline fuel cells[J]. Journal of Power Sources,2009,187(1):39-48.
[40] YANG K L, KAS R, SMITH W A, et al. Role of the carbon-based gas diffusion layer on flooding in a gas diffusion electrode cell for electrochemical CO2 reduction[J]. ACS Energy Letters,2021,6(1):33-40.
[41] ZHOU L, ZHOU M H, HU Z X, et al. Chemically modified graphite felt as an efficient cathode in electro-Fenton for p-nitrophenol degradation[J]. Electrochimica Acta,2014,140:376-383.
[42] YU X M, ZHOU M H, REN G B, et al. A novel dual gas diffusion electrodes system for efficient hydrogen peroxide generation used in electro-Fenton[J]. Chemical Engineering Journal,2015,263:92-100.
[43] MORAES A, ASSUMPO M H M T, PAPAI R, et al. Use of a vanadium nanostructured material for hydrogen peroxide electrogeneration[J]. Journal of Electroanalytical Chemistry,2014,719:127-132.
[44] LUO H J, LI C L, WU C Q, et al. In situ electrosynthesis of hydrogen peroxide with an improved gas diffusion cathode by rolling carbon black and PTFE[J]. RSC Advances,2015,5(80):65227-65235.
[45] BOYE B, DIENG M M, BRILLAS E. Degradation of herbicide 4-chlorophenoxyacetic acid by advanced electrochemical oxidation methods[J]. Environmental Science & Technology,2002,36(13):3030-3035.
[46] DE LAAT J, TRUONG LE G, LEGUBE B. A comparative study of the effects of chloride, sulfate and nitrate ions on the rates of decomposition of H2O2 and organic compounds by Fe(II)/H2O2 and Fe(III)/H2O2[J]. Chemosphere,2004,55(5):715-723.
[47] MOREIRA F C, BOAVENTURA R A R, BRILLAS E, et al. Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters[J]. Applied Catalysis B: Environmental,2017,202:217-261.
[48] FREGUIA S, RABAEY K, YUAN Z G, et al. Non-catalyzed cathodic oxygen reduction at graphite granules in microbial fuel cells[J]. Electrochimica Acta,2007,53(2):598-603.
[49] DUTEANU N, ERABLE B, SENTHIL KUMAR S M, et al. Effect of chemically modified Vulcan XC-72R on the performance of air-breathing cathode in a single-chamber microbial fuel cell[J]. Bioresource Technology,2010,101(14):5250-5255.
[50] WANG N, ZHAO X H, ZHANG R, et al. Highly selective oxygen reduction to hydrogen peroxide on a carbon-supported single-atom Pd electrocatalyst[J]. ACS Catalysis,2022,12(7):4156-4164.
[51] ZHANG C, SHEN W Q, GUO K, et al. A pentagonal defect-rich metal-free carbon electrocatalyst for boosting acidic O2 reduction to H2O2 production[J]. Journal of the American Chemical Society,2023,145(21):11589-11598.
[52] ZHAO J J, FU C H, YE K, et al. Manipulating the oxygen reduction reaction pathway on Pt-coordinated motifs[J]. Nature Communications,2022,13(1):685.
[53] LI H, QUISPE-CARDENAS E, YANG S, et al. Electrosynthesis of >20 g/L H2O2 from Air[J]. ACS ES T Eng,2022,2(2):242-250.
[54] DONG H, DONG B B, SUN L, et al. Electro-UV/H2O2 system with RGO-modified air diffusion cathode for simulative antibiotic-manufacture effluent treatment[J]. Chemical Engineering Journal,2020,390:124650.
[55] ZHANG Y Y, DANIEL G, LANZALACO S, et al. H2O2 production at gas-diffusion cathodes made from agarose-derived carbons with different textural properties for acebutolol degradation in chloride media[J]. Journal of Hazardous Materials,2022,423:127005.
[56] HE H H, JIANG B, YUAN J J, et al. Cost-effective electrogeneration of H2O2 utilizing HNO3 modified graphite/polytetrafluoroethylene cathode with exterior hydrophobic film[J]. Journal of Colloid and Interface Science,2019,533:471-480.
[57] ZHANG H C, LI Y J, ZHAO Y S, et al. Carbon black oxidized by air calcination for enhanced H2O2 generation and effective organics degradation[J]. ACS Applied Materials & Interfaces,2019,11(31):27846-27853.
[58] HU Y Z, ZHANG J J, SHEN T, et al. Efficient electrochemical production of H2O2 on hollow N-doped carbon nanospheres with abundant micropores[J]. ACS Applied Materials & Interfaces,2021,13(25):29551-29557.
[59] XIA Y, ZHAO X H, XIA C, et al. Highly active and selective oxygen reduction to H2O2 on boron-doped carbon for high production rates[J]. Nature Communications,2021,12(1):4225.
[60] ZHAO K, SU Y, QUAN X, et al. Enhanced H2O2 production by selective electrochemical reduction of O2 on fluorine-doped hierarchically porous carbon[J]. Journal of Catalysis,2018,357:118-126.
[61] GU Y Y, FU H J, HUANG Z W, et al. O/F Co-doped CNTs promoted graphite felt gas diffusion cathode for highly efficient and durable H2O2 evolution without aeration[J]. Journal of Cleaner Production,2022,341:130799.
[62] DU Y X, YANG Q, LU W T, et al. Carbon black-supported single-atom Co-N-C as an efficient oxygen reduction electrocatalyst for H2O2 production in acidic media and microbial fuel cell in neutral media[J]. Advanced Functional Materials,2023,33(27):2300895.
[63] ZHANG J, LIU W, HE F, et al. Highly dispersed Co atoms anchored in porous nitrogen-doped carbon for acidic H2O2 electrosynthesis[J]. Chemical Engineering Journal,2022,438:135619.
[64] MEI D, HE Z D, ZHENG Y L, et al. Mechanistic and kinetic implications on the ORR on a Au(100) electrode: pH, temperature and H-D kinetic isotope effects[J]. Physical Chemistry Chemical Physics:PCCP,2014,16(27):13762-13773.
[65] LU F, ZHANG Y, LIU S Z, et al. Surface proton transfer promotes four-electron oxygen reduction on gold nanocrystal surfaces in alkaline solution[J]. Journal of the American Chemical Society,2017,139(21):7310-7317.
[66] SUN Y Y, LI S, JOVANOV Z P, et al. Structure, activity, and faradaic efficiency of nitrogen-doped porous carbon catalysts for direct electrochemical hydrogen peroxide production[J]. ChemSusChem,2018,11(19):3388-3395.
[67] JIRKOVSK J S, PANAS I, AHLBERG E, et al. Single atom hot-spots at Au-Pd nanoalloys for electrocatalytic H2O2 production[J]. Journal of the American Chemical Society,2011,133(48):19432-19441.
[68] ZHAO X, YANG H, XU J, et al. Bimetallic PdAu nanoframes for electrochemical H2O2 production in acids[J]. ACS Materials Letters,2021,3(7):996-1002.
[69] SIAHROSTAMI S, VERDAGUER-CASADEVALL A, KARAMAD M, et al. Enabling direct H2O2 production through rational electrocatalyst design[J]. Nature Materials,2013,12(12):1137-1143.
[70] VERDAGUER-CASADEVALL A, DEIANA D, KARAMAD M, et al. Trends in the electrochemical synthesis of H2O2: enhancing activity and selectivity by electrocatalytic site engineering[J]. Nano Letters,2014,14(3):1603-1608.
[71] JIRKOVSK J S, PANAS I, AHLBERG E, et al. Single atom hot-spots at Au-Pd nanoalloys for electrocatalytic H2O2 production[J]. Journal of the American Chemical Society,2011,133(48):19432-19441.
[72] SIAHROSTAMI S, VERDAGUER-CASADEVALL A, KARAMAD M, et al. Enabling direct H2O2 production through rational electrocatalyst design[J]. Nature Materials,2013,12(12):1137-1143.
[73] ZHU C Z, FU S F, SHI Q R, et al. Single-atom electrocatalysts[J]. Angewandte Chemie International Edition,2017,56(45):13944-13960.
[74] LIU J Y. Catalysis by supported single metal atoms[J]. ACS Catalysis,2017,7(1):34-59.
[75] YIN P Q, YAO T, WU Y E, et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts[J]. Angewandte Chemie International Edition,2016,55(36):10800-10805.
[76] CHOI C H, KIM M, KWON H C, et al. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst[J]. Nature Communications,2016,7:10922.
[77] YANG S, TAK Y J, KIM J, et al. Support effects in single-atom platinum catalysts for electrochemical oxygen reduction[J]. ACS Catalysis,2017,7(2):1301-1307.
[78] SONG M, LIU W, ZHANG J J, et al. Single-atom catalysts for H2O2 electrosynthesis via two-electron oxygen reduction reaction[J]. Advanced Functional Materials,2023,33(15):2212087.
[79] ASSUMPO M H M T, DE SOUZA R F B, RASCIO D C, et al. A comparative study of the electrogeneration of hydrogen peroxide using Vulcan and Printex carbon supports[J]. Carbon,2011,49(8):2842-2851.
[80] BRILLAS E, SIRS I, OTURAN M A. Electro-Fenton process and related electrochemical technologies based on Fentons reaction chemistry[J]. Chemical Reviews,2009,109(12):6570-6631.
[81] ASSUMPO M H M T, DE SOUZA R F B, REIS R M, et al. Low tungsten content of nanostructured material supported on carbon for the degradation of phenol[J]. Applied Catalysis B: Environmental,2013,142/143:479-486.
[82] GARCIA-RODRIGUEZ O, LEE Y Y, OLVERA-VARGAS H, et al. Mineralization of electronic wastewater by electro-Fenton with an enhanced graphene-based gas diffusion cathode[J]. Electrochimica Acta,2018,276:12-20.
[83] YANG H J, ZHOU M H, YANG W L, et al. Rolling-made gas diffusion electrode with carbon nanotube for electro-Fenton degradation of acetylsalicylic acid[J]. Chemosphere,2018,206:439-446.
[84] ZHANG X, FU J, ZHANG Y, et al. A nitrogen functionalized carbon nanotube cathode for highly efficient electrocatalytic generation of H2O2 in Electro-Fenton system[J]. Separation and Purification Technology,2008,64(1):116-123.
[85] ZAREI M, NIAEI A, SALARI D, et al. Application of response surface methodology for optimization of peroxi-coagulation of textile dye solution using carbon nanotube-PTFE cathode[J]. Journal of Hazardous Materials,2010,173(1/2/3):544-551.
[86] OTURAN M A, OTURAN N, EDELAHI M C, et al. Oxidative degradation of herbicide diuron in aqueous medium by Fentons reaction based advanced oxidation processes[J]. Chemical Engineering Journal,2011,171(1):127-135.
[87] LI D, ZHENG T, LIU Y L, et al. A novel Electro-Fenton process characterized by aeration from inside a graphite felt electrode with enhanced electrogeneration of H2O2 and cycle of Fe3+/Fe2+[J]. Journal of Hazardous Materials,2020,396:122591.
[88] WU Y, WANG Y, LIN Z Q, et al. Three-dimensional α-Fe2O3/amino-functionalization carbon nanotube sponge for adsorption and oxidative removal of tetrabromobisphenol A[J]. Separation and Purification Technology,2019,211:359-367.
[89] ZCAN A,瘙塁AHIN Y, SAVA瘙塁 KOPARAL A, et al. Carbon sponge as a new cathode material for the electro-Fenton process: comparison with carbon felt cathode and application to degradation of synthetic dye basic blue 3 in aqueous medium[J]. Journal of Electroanalytical Chemistry,2008,616(1/2):71-78.
[90] TSYGANOK A I, YAMANAKA I, OTSUKA K. Dechlorination of chloroaromatics by electrocatalytic reduction over palladium-loaded carbon felt at room temperature[J]. Chemosphere,1999,39(11):1819-1831.
[91] NI X Y, LIU H, WANG C, et al. Comparison of carbonized and graphitized carbon fiber electrodes under flow-through electrode system (FES) for high-efficiency bacterial inactivation[J]. Water Research,2020,168:115150.
[92] LIU H, NI X Y, HUO Z Y, et al. Carbon fiber-based flow-through electrode system (FES) for water disinfection via direct oxidation mechanism with a sequential reduction-oxidation process[J]. Environmental Science & Technology,2019,53(6):3238-3249.
[93] LAI W K, XIE G Y, DAI R Z, et al. Kinetics and mechanisms of oxytetracycline degradation in an electro-Fenton system with a modified graphite felt cathode[J]. Journal of Environmental Management,2020,257:109968.
[94] XIA G S, LU Y H, XU H B. Electrogeneration of hydrogen peroxide for electro-Fenton via oxygen reduction using polyacrylonitrile-based carbon fiber brush cathode[J]. Electrochimica Acta,2015,158:390-396.
[95] WANG W, LI Y C, LI Y W, et al. Electro-Fenton and photoelectro-Fenton degradation of sulfamethazine using an active gas diffusion electrode without aeration[J]. Chemosphere,2020,250:126177.
[121] WANG W, LU X Y, SU P, et al. Enhancement of hydrogen peroxide production by electrochemical reduction of oxygen on carbon nanotubes modified with fluorine[J]. Chemosphere,2020,259:127423.
[122] LI X Y, WANG X P, XIAO G Z, et al. Identifying active sites of boron, nitrogen Co-doped carbon materials for the oxygen reduction reaction to hydrogen peroxide[J]. Journal of Colloid and Interface Science,2021,602:799-809.
[123] LIU Z M, GAO D Z, HU L N, et al. Metal-free boron-rich borocarbonitride catalysts for high-efficient oxygen reduction to produce hydrogen peroxide[J]. ChemistrySelect,2022,7(5):e202104203.
[124] LI K Q, LIU J M, LI J, et al. Effects of N mono- and N/P dual-doping on H2O2, OH generation, and MB electrochemical degradation efficiency of activated carbon fiber electrodes[J]. Chemosphere,2018,193:800-810.
[125] SUN Y Y, SILVIOLI L, SAHRAIE N R, et al. Activity-selectivity trends in the electrochemical production of hydrogen peroxide over single-site metal-nitrogen-carbon catalysts[J]. Journal of the American Chemical Society,2019,141(31):12372-12381.
[126] BARROS W R P, REIS R M, ROCHA R S, et al. Electrogeneration of hydrogen peroxide in acidic medium using gas diffusion electrodes modified with cobalt (II) phthalocyanine[J]. Electrochimica Acta,2013,104:12-18.
[127] SILVA F L, REIS R M, BARROS W R P, et al. Electrogeneration of hydrogen peroxide in gas diffusion electrodes: application of iron (II) phthalocyanine as a modifier of carbon black[J]. Journal of Electroanalytical Chemistry,2014,722/723:32-37.
[128] ZHAO C X, LI B Q, LIU J N, et al. Intrinsic electrocatalytic activity regulation of M-N-C single-atom catalysts for the oxygen reduction reaction[J]. Angewandte Chemie International Edition,2021,60(9):4448-4463.
[129] LIU J L, XIAO J X, LUO B C, et al. Central metal and ligand effects on oxygen electrocatalysis over 3d transition metal single-atom catalysts: a theoretical investigation[J]. Chemical Engineering Journal,2022,427:132038.
[130] SINGH K, RAZMJOOEI F, YU J S. Active sites and factors influencing them for efficient oxygen reduction reaction in metal-N coordinated pyrolyzed and non-pyrolyzed catalysts: a review[J]. Journal of Materials Chemistry A,2017,5(38):20095-20119.
[131] BONAKDARPOUR A, LEFEVRE M, YANG R Z, et al. Impact of loading in RRDE experiments on Fe-N-C catalysts: two- or four-electron oxygen reduction?[J]. Electrochemical and Solid-State Letters,2008,11(6):B105.
[132] ROJAS-CARBONELL S, ARTYUSHKOVA K, SEROV A, et al. Effect of pH on the activity of platinum group metal-free catalysts in oxygen reduction reaction[J]. ACS Catalysis,2018,8(4):3041-3053.
[133] LI P, JIAO Y Z, RUAN Y E, et al. Revealing the role of double-layer microenvironments in pH-dependent oxygen reduction activity over metal-nitrogen-carbon catalysts[J]. Nature Communications,2023,14(1):6936.
Research Progress in Electrosynthesis of Hydrogen Peroxide with Gas Diffusion Electrodes
YANG Wulin1, YI Kexin1, YUAN Xiayu1, HUANG Xingjun2,NI Jinyuan2, HU Junjie2, WANG Lijun2, LIU Sheng3
(1. School of Environmental Science and Engineering, Peking University, Beijing 100871;2. Chengdu Sotec Technology Co. Ltd., Chengdu 610000, Sichuan;3. Tianfu Yongxing Laboratory, Chengdu 610213, Sichuan)
Abstract:Hydrogen peroxide (H2O2), a highly effective oxidant, is widely used in chemical synthesis, sterilization and wastewater treatment. Traditional anthraquinone process was replaced by H2O2 electrosynthesis via two-electron oxygen reduction (2e- ORR), because of its high performance and environmentally friendly property. As a high-efficiency H2O2 production cathode, gas diffusion electrodes (GDEs) exhibit lower cost, lower energy consumption and higher oxygen utilization efficiency. In this paper, we investigate the mechanism of H2O2 production by 2e- ORR with GDEs. Besides, the configuration, principle and optimization method of GDEs are discussed. The GDEs catalysts were also analyzed. Finally, some future directions are pointed out, which is beneficial to promote the H2O2 electrosynthesis towards marketization.
Keywords:gas diffusion electrode(GDEs); hydrogen peroxide; carbon catalysts; two-electron oxygen reduction reaction(2e- ORR)(编辑 陶志宁)
基金项目:国家自然科学基金(52100021)和2023年中央引导地方科技发展项目(2023ZYD0278)
第一作者简介:杨武霖(1989—),男,研究员,博士生导师,主要从事微生物电化学、电化学膜分离、电化学高级氧化等及其在能源-资源-环境问题中的应用等方面的研究,E-mail:wulin.yang@pku.edu.cn
引用格式:杨武霖,易可欣,袁夏雨,等. 气体扩散电极电合成过氧化氢技术研究进展[J]. 四川师范大学学报(自然科学版),2024,47(5):596-610.
