Facile synthesis of hierarchical NaX zeolite from natural kaolinite for efficient Knoevenagel condensation
2024-04-22WenXiaoPengDongChanWangJingdongXuTiesenLiHaiboZhuTinghaiWangRenweiXuYuanyuanYue
Wen Xiao,Peng Dong,Chan Wang,Jingdong Xu,Tiesen Li,Haibo Zhu,Tinghai Wang,Renwei Xu,*,Yuanyuan Yue,*
1 National Engineering Research Center of Chemical Fertilizer Catalyst,College of Chemical Engineering,Fuzhou University,Fuzhou 350108,China
2 Qingyuan Innovation Laboratory,Quanzhou 362801,China
3 Sinochem Quanzhou Energy Technology Co.,Ltd.,Quanzhou 362000,China
Keywords: Hierarchical NaX zeolite Template-free synthesis Natural kaolinite Knoevenagel condensation
ABSTRACT Zeolite catalysts have found extensive applications in the synthesis of various fine chemicals.However,the micropores of zeolites impose diffusion limitations on bulky molecules,greatly reducing the catalytic efficiency.Herein,we explore an economic and environmentally friendly method for synthesizing hierarchical NaX zeolite that exhibits improved catalytic performance in the Knoevenagel condensation reaction for producing the useful fine chemical 2-cyano-3-phenylacrylate.The synthesis was achieved via a low-temperature activation of kaolinite and subsequent in-situ transformation strategy without any template or seed.Systematic characterizations reveal that the synthesized NaX zeolite has both intercrystalline and intra-crystalline mesopores,smaller crystal size,and larger external specific surface area compared to commercial NaX zeolite.Detailed mechanism investigations show that the inter-crystalline mesopores are generated by stacking smaller crystals formed from in-situ crystallization of the depolymerized kaolinite,and the intra-crystalline mesopores are inherited from the pores in the depolymerized kaolinite.This synthesis strategy provides an energy-saving and effective way to construct hierarchical zeolites,which may gain wide applications in fine chemical manufacturing.
1.Introduction
Recently,zeolites have attracted great attentions in the synthesis of a wide range of fine chemicals,such as flavors,fragrances,pharmaceutical intermediates,detergents and pigments[1,2].Traditionally,fine chemicals are synthesizedviathe homogeneous catalysis system [3,4].However,the separation and recovery of the homogeneous catalysts are difficult and expensive,because they are typically dissolved in the reaction mixture [5].Moreover,some homogeneous catalysts,such as liquid acid and base,may generate hazardous waste or emit harmful byproducts,leading to environmental concerns [6,7].Zeolites are known to have several advantages in fine chemical synthesis as a heterogeneous catalyst[8,9].They are often able to be easily separated from the reaction mixture,allowing for their recovery and reuse,which can reduce costs and increase efficiency.Additionally,zeolites are commonly considered as environmentally friendly catalysts,as they are non-toxic and their production does not generate hazardous waste.Their porous structure and shape selectivity make them highly selective,resulting in high yields of desired products and reduced waste.Furthermore,zeolites are also stable under a wide range of reaction conditions,making them suitable for industrial processes.
In these years,there has been a significant interest in the zeolite-catalyzed Knoevenagel condensation reaction for the synthesis of fine chemicals such as pharmaceuticals,agrochemicals,and natural products [10,11].Knoevenagel condensation reaction is a versatile and efficient method for the formation of C-C bonds and the introduction of various functional groups into organic molecules [12].Therefore,the Knoevenagel condensation reaction is a valuable tool for the synthesis of complex organic molecules and has significant applications in the pharmaceutical and fine chemical industries [13–15].NaX zeolites with basic sites from the sodium cations in zeolite channel,can effectively catalyze the Knoevenagel condensation reaction under the mild conditions[16,17].However,the Knoevenagel condensation involves bulky molecules,and the micropore in conventional NaX zeolite brings about serious steric hindrance and diffusion limitation of bulky molecules,which greatly decrease the catalysis efficiency [18,19].
To tackle the issue above,scientists have been exploring the hierarchical zeolites with multi-modal pore structures to overcome the drawback of conventional zeolites.Hierarchical zeolites have a more open pore structure than conventional zeolites,enabling better accessibility of bulky reactants to active sites and reducing diffusion limitations[20–22].This makes them promising materials for heterogeneous catalysis applications.For instance,Verboekendet al.[19,23] reported a ten-fold increase in catalytic activity of hierarchical NaX zeolite compared to the microporous NaX zeolite in the Knoevenagel condensation of benzaldehyde with malononitrile.The enhanced activity was attributed to the improved accessibility of reactants to active sites in hierarchical zeolites.Grasset al.[18] demonstrated that the conversion rate over hierarchical NaX zeolite was higher than that over conventional NaX zeolite in the Knoevenagel condensation of benzaldehyde with ethyl cyanoacetate,indicating that hierarchical zeolites can enhance catalytic performance in this reaction as well.Therefore,many efforts have been devoted to the preparation and application of hierarchical NaX zeolite as a base catalyst in the Knoevenagel condensation reaction.
Up to now,the most common method for synthesizing hierarchical NaX zeolites involves the use of chemical reagents as raw materials and cationic surfactants as mesopore templates.For example,Liuet al.[24] explored the synthesis of hierarchical NaX zeolite by employing water glass and sodium aluminate as raw materials and cationic polymer or spirulina as organic template.Inayatet al.[25] synthesized hierarchical NaX zeolite with intracrystalline mesopores using sodium silicate and sodium aluminate as raw materials,along with 3-(trimethoxysilyl)propyl hexadecyl dimethyl ammonium chloride as a mesoporous template.Similarly,Gómezet al.[26] adopted NaAlO2and Na2SiO3as raw materials to synthesize hierarchical NaX zeolite in the presence of sodium dodecyl benzene sulfonate.Although the templating method using expensive organic templates and chemical reagents has been successful in synthesizing hierarchical NaX zeolites,it can increase the cost and environmental impact of the process which makes it less suitable for large-scale industrial applications [27–29].Therefore,there is growing interest in developing more sustainable and cost-effective approaches for synthesizing hierarchical NaX zeolites.
Considering the similar chemical composition between natural clay mineral and NaX zeolite,some literatures have reported the direct synthesis of NaX zeolites from kaolinite,as shown in Table 1.Nevertheless,there are several problems with these research works: (i) the activation temperature is usually above 500 °C,resulting in high energy consumption;(ii) the obtained NaX zeolites have low crystallinity and/or purity;(iii) most of the synthesized NaX zeolites only own microporous channels.Although the hierarchical NaX zeolite with mesopore size of 23 nm was directly synthesized by using the illite-kaolinite as raw material,but the specific surface area (SBET) of the obtained NaX zeolite was low,and a small amount of NaA impure phase was observed in the final product[30].Against the above-mentioned reports,we attempt to develop a facile and energy-saving method for synthesizing highly crystalline and pure hierarchical NaX zeolite from natural kaolinite without using any template or seed.The synthesis was achievedviathe low-temperature(250°C)activation of kaolinite and subsequentin-situtransformation strategy,and this synthesis strategy leads to the successful synthesis of highly crystalline and pure NaX zeolite with both inter-crystalline and intra-crystalline mesopores.The physicochemical properties of the resultant NaX zeolite were systematically investigated by various techniques,and the formation mechanism of hierarchical pores was discussed.In addi-tion,the catalytic performance of the synthesized hierarchical NaX zeolite was tested in the Knoevenagel condensation reaction between benzaldehyde and ethyl cyanoacetate.
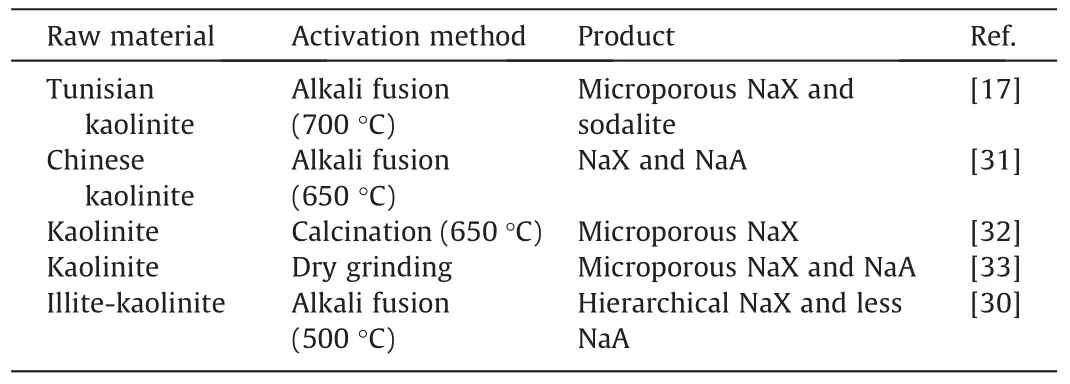
Table 1 Research progress in the synthesis of NaX zeolite from kaolinite
2.Experimental
2.1.Materials
The natural kaolinite was acquired from China Kaolinite Clay Co.,China.Solid silica (89.2% (mass) SiO2and 10.8% (mass) H2O)was purchased from Qingdao Gaomei Reagent Co.,China.Sodium hydroxide(96%(mass)) and ethanol (99.8%(mass))were obtained from Adamas Chemical Reagent Co.,China.Benzaldehyde (99%(mass))and ethyl cyanoacetate(99%(mass))were purchased from Macklin Chemical Reagent Co.,China.The commercial NaX zeolite was bought from Nankai University Catalyst Co.,Ltd,China.
2.2.Synthesis of hierarchical NaX zeolite
The hierarchical NaX zeolite was synthesized by employing the natural kaolinite as sole aluminum source and partial silicon source without using any template or seedviaanin-situtransformation approach.Firstly,the natural kaolinite was depolymerized in a submolten salt (SMS) medium as reported in our previous work [34].In a typical procedure,28.0 g of sodium hydroxide was appended into an open Teflon beaker containing 120.0 g of deionized water under strongly stirring.Then,20.0 g of natural kaolinite was added to the above sodium hydroxide solution.The resulting mixture was placed into an oven exposed to air at a preset temperature of 250 °C for 2 h to yield a SMS depolymerized kaolinite denoted as SMS-kaolinite.Secondly,SMS-kaolinite was mixed with deionized water,sodium hydroxide,and solid silica under vigorous stirring with the final molar composition of 9 Na2O:3.5 SiO2:1 Al2O3:385 H2O.After aging at 30 °C for 12 h,the mixture was transferred into a Teflon-lined stainless-steel autoclave and subjected to hydrothermal crystallization at 80 °C for 4 h without agitation.Finally,the solid product was filtered,washed thoroughly with deionized water,and dried overnight at 100 °C.
2.3.Characterizations
The powder X-ray diffraction (XRD) patterns of the samples were collected on a Rigaku Ultimate III diffractometer (40 kV,40 mA,Japan)using Cu Kα radiation from 5°to 40°with a scanning step of 0.02° at a scanning rate of 8(°)∙min-1.The relative crystallinity of the synthesized sample was calculated based on ASTM D3906-19 standard by comparing the area of their characteristic peaks (2θ=15.7° ± 0.2°,18.7° ± 0.2°,20.4° ± 0.3°,23.7° ± 0.4°,27.1°±0.5°,30.8°±0.5°,31.5°±0.5°,34.2°±0.5°)to that of a commercial NaX zeolite whose crystallinity is defined as 100%.Fourier transform infrared (FT-IR) analysis was used as a complementary tool for XRD to characterize the structure of zeolite.It was performed at room temperature on a Thermo Scientific Nicolet iS50 FT-IR spectrometer(USA)in the range of 4000–400 cm-1.The samples were ground with the dried KBr powder and pressed into small disc prior to FTIR characterization.The chemical composition of the solid samples was analyzed by X-ray fluorescence(XRF)conducted on a Bruker S4 Explorer instrument (Germany).The morphology and size of the samples were observed by field-emission scanning electron microscopy (FESEM) on a Hitachi S-4800 equipment(Japan).The transmission electron microscopy(TEM)images were taken using an FEI Tecnai F20 instrument(200 kV,USA)with the sample mounted on a C-flat TEM grid.The textural properties of the samples were studied by nitrogen adsorption–desorption measurements at -196 °C on an ASAP 2460 analyzer (USA),and the sample was dehydrated at 300°C for 6 h prior to the measurement.TheSBETwas calculated by the Brunauer-Emmett-Teller(BET) method,and the total pore volume (Vtotal) was estimated according to the adsorption capacities at the relative pressure (P/P0) of 0.95 [35].The external specific surface area (Sexter) and micropore volume(Vmicro)were calculated using thet-plot method[36,37].The Al and Si coordination environments in the zeolite were analyzed by using27Al and29Si magnetic angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy,respectively.The27Al MAS NMR spectra were collected on a JEOL ECA-600 spectrometer (Japan) at a resonance frequency of 156.4 MHz using a 4 mm sample rotor with a spinning rate of 15.0 kHz.The29Si MAS NMR spectra were recorded on a Bruker DSX 500 MHz spectrometer (Germany) at a resonance frequency of 79.5 MHz using a 6 mm sample rotor with a spinning rate of 5.5 kHz.The framework SiO2/Al2O3molar ratios of the samples were calculated from the29Si MAS NMR spectra using Eq.(1).
whereIrepresents integral intensity of a particular silicon resonance peak,ndenotes the number of framework Al atom connectedviaoxygen atom to one Si atom.
The variation of Si and Al concentration in the liquid phase of the synthesized samples under the different synthesis times were analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES) with a Perkin-Elmer 3300DV emission.The basicity of the zeolites was analyzed by temperatureprogrammed desorption of CO2(CO2-TPD) on Autochem 2920-Hiden HPR20.The sample was pretreated at 470 °C for 2 h in He flow and then cooled to 50 °C.After outgassing,10% CO2/He flow was introduced for 1 h in order to absorb the CO2,and then He flow passed through the sample for 15 min to desorb the weakly adsorbed CO2.Subsequently,the sample was heated under He flow from 50°C to 900°C with a rate of 10°C∙min-1.The concentration of desorbed CO2was measured with a TCD detector.
2.4.Catalytic test
The catalytic performance of the zeolites was evaluated by using the Knoevenagel condensation of benzaldehyde with ethyl cyanoacetate to produce 2-cyano-3-phenylacrylate as a model reaction,as shown in Fig.1.In a typical test,20 cm3of reaction mixture was prepared by mixing benzaldehyde (0.56 mol∙L-1)and ethyl cyanoacetate (0.56 mol∙L-1) in a molar ratio of 1:1,and ethanol (85% (mass)) as solvent.The mixture was kept at 70 °C under continuous stirring (400 r∙min-1,0–4 h) and the reaction was initiated by the addition of 3% (mass) of catalyst.Aliquots were periodically collected,filtered,and analyzed by gas chromatography using a Shimadzu GC-2014 chromatograph equipped with flame ionization detector and RTX-1 capillary column.The chosen injection volume of this solution into the gas chromatograph was 0.5 l with an injector temperature of 250 °C.The column was heated with 5°C∙min-1from 80°C to 190°C and kept for 3 min at 190 °C.Then,a heating ramp of 5 °C∙min-1to 250 °C was applied followed by an isothermal step of 2 min at 250°C.Benzaldehyde conversions were determined from the respective areas under the GC curves.Quantification for benzaldehyde conversion calculations was performed using Eq.(2),based on analytical curves of 2-cyano-3-phenylacrylate.
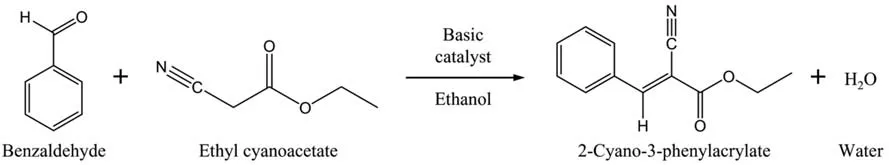
Fig.1.Model of the Knoevenagel condensation reaction used in this study.
whereCbenzaldehyde,0andCbenzaldehyde,1represent the concentration of benzaldehyde in the solution before and after the reaction,respectively.
3.Results and Discussion
3.1.Chemical composition and phase structure of kaolinite and SMSkaolinite
The kaolinite used in this synthesis is composed of approximately 95% (mass) Al2O3and SiO2,as well as small amounts of impurities (Table S1 in Supplementary Material).The XRD pattern of the kaolinite displays two prominent peaks at 2θ=12.3° and 24.9°,which correspond to the kaolinite phase (Fig.S1) [38].After depolymerization,the SMS-kaolinite maintains the same SiO2/Al2O3molar ratio as the original kaolinite,but the content of Na2O increases significantly owing to the use of a large quantity of sodium hydroxide.The XRD pattern of the SMS-kaolinite shows that the characteristic peaks of the kaolinite become invisible,and new strong peaks at 2θ=30.1°,31.4°,35.2°,and 36.0°corresponding to the NaAlSiO4phase appear.This indicates that the kaolinite mineral has been transformed into highly active aluminosilicate speciesviaSMS activation [39].Additionally,a weak peak at 2θ=38.5°,attributed to sodium hydroxide,is detected due to the excessive addition of sodium hydroxide.
3.2.Physicochemical properties of hierarchical NaX zeolite
The structures of the synthesized and commercial NaX zeolite were determined by XRD and FT-IR,as shown in Fig.2.The XRD pattern of the synthesized NaX is identical to that of the commercial NaX zeolite (Fig.2(a)),and both exhibit characteristic diffraction peaks attributed to FAU structure without other unidentified peaks.The relative crystallinity of the synthesized sample is 87%.Additionally,the FT-IR spectra in Fig.2(b) display the peaks at 970,752 and 672 cm-1attributed to the symmetric and asymmetric stretching modes of SiO4tetrahedron,as well as peaks at 563 and 464 cm-1assigned to the vibration of double six-membered rings and the bending mode of T-O bonds (T=Si or Al),respectively [40,41].Besides the above characteristic bands belonging to FAU zeolite,no other peak is observed,indicating that there are no impurities or other phases present in the synthesized product.It is worth noting that the bulk SiO2/Al2O3molar ratios in the commercial and synthesized zeolites are quite close,with values of 2.33 and 2.23,respectively(Table 2).Based on the characterization results presented above,it can be concluded that the highly crystalline and pure NaX zeolite is successfully synthesized from SMS-kaolinitevia in-situtemplate-free transformation strategy.

Fig.2.XRD patterns (a) and FT-IR spectra (b) of the different NaX zeolites.
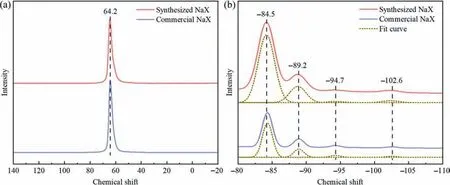
Fig.3.27Al (a) and 29Si (b) MAS NMR spectra of the different NaX zeolites.
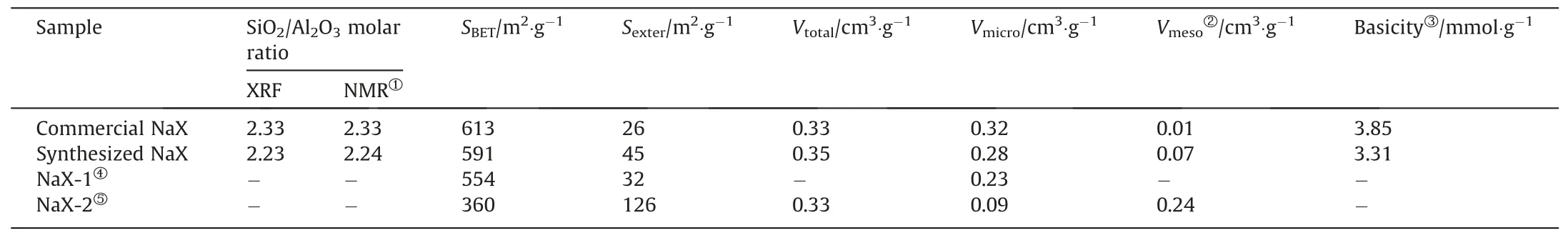
Table 2 Physicochemical parameters of the different NaX zeolites
The Al and Si coordination environments in the different zeolites were investigated by using the NMR characterization.As shown in Fig.3(a),the27Al MAS NMR spectra of the commercial and synthesized NaX zeolites feature a single peak at 64.2 attributed to tetrahedral coordination Al species in zeolite framework[42].This suggests that all of the Al species originating from SMS-kaolinite are incorporated into the tetrahedrally coordinated Si-O-Si network [24].Fig.3(b) displays that the29Si MAS NMR spectra have four peaks at -102.6,-94.7,-89.2 and -84.5 assigned to the coordination of Si(OAl)(OSi)3,Si(OAl)2(OSi)2,Si(OAl)3(OSi)and Si(OAl)4,respectively[43].Additionally,the framework SiO2/Al2O3molar ratios of the commercial and synthesized samples are 2.33 and 2.24(Table 2),respectively,which are in good agreement with their bulk SiO2/Al2O3molar ratios.
The morphologies of the different NaX zeolites are shown in Fig.4.The particles of the commercial NaX zeolite are in a highly dispersed state,and display a typical octahedral morphology with uniform size ofca.4 μm(Fig.4(a)and(b)).Fig.4(c)and(d)displays that the particles of the synthesized NaX zeolite are in a stacked state,and exhibit the irregular aggregate-like octahedral shapes with smaller crystal size(ca.1.5 μm).Lots of inter-crystalline cavities are visibly observed in the synthesized NaX zeolite,as seen from the TEM image in Fig.4(e),indicating that there are a certain number of inter-crystalline mesopores in the synthesized NaX zeolite which come from the interstitial spaces among the crystals.Furthermore,the TEM images in Figs.4(f)and S2 reveal that there are some intra-crystalline mesopores in the synthesized NaX crystals.Especially,Fig.S2(c) and (d) exhibits some channel-like light regions extending from the crystals surface to its interior,signifying the presence of intra-crystalline mesopores open to the outside in the synthesized NaX zeolite.As reported elsewhere [44],the generation of intra-crystalline mesopores in hierarchical zeolite is due to the duplication of mesopores from the depolymerized mineral into the zeolite structure.In this work,the intra-crystalline mesopores in the synthesized NaX zeolite may also derives from SMS-kaolinite.The formation mechanism of inter-crystalline and intra-crystalline mesopores in the synthesized NaX zeolite will be deeply discussed in Section 3.3.

Fig.4.FESEM images of the commercial (a,b) and synthesized (c,d) NaX zeolites,and TEM images of the synthesized NaX zeolite: inter-(e) and intra-(f) crystalline mesopores.
Fig.5(a) shows that the N2adsorption–desorption isotherms of the commercial NaX zeolite belong to typical type I,indicating the microporous nature of this sample [45].In contrast,the isotherms of the synthesized NaX zeolite present a H4-type hysteresis loop atP/P0ranging from 0.4 to 1.0,suggesting the existence of mesoporous structure [46].The pore size distribution estimated by the BJH method reveals that the mesopore size in the synthesized NaX zeolite is narrowly centered atca.12 nm (Fig.5(b)).From Table 2,it can be seen that theSBETandVtotalof the synthesized NaX zeolite are comparable to those of the commercial NaX zeolite.However,it is noteworthy that theSexterandVmesoof the synthesized NaX zeolite are 1.7 and 7.0 times those of the commercial NaX zeolite,respectively,which is attributed to the presence of inter-crystalline and intra-crystalline mesopores in the former.According to previously reported literatures,Table 2 also shows the texture parameters of two NaX zeolites synthesized from kaolinite.The values ofSBETandVmicroof NaX-1 sample are lower than those of the synthesized NaX zeolite,and it only owns the sole microporous channel.In terms of NaX-2 sample,it exhibits abundant external surface and trimodal macro-meso-microporous structure,while the values of itsSBETandVmicroare unsatisfactory,meaning that NaX-2 has the low crystallinity.Obviously,the synthesized NaX zeolite in this study possesses excellent texture parameters and high crystallinity.

Fig.5.N2 adsorption–desorption isotherms (a) and pore size distribution curves (b) of the different NaX zeolites.
According to the above results,it can be inferred that the synthesized and commercial NaX zeolites have similar SiO2/Al2O3ratios and silicon and aluminum coordination environments,but the synthesized sample is a hierarchical NaX zeolite with both microporous and mesoporous structures,and it is distinctly different from the commercial NaX zeolite which only has a single microporous structure.
3.3.Formation mechanism of hierarchical pores
To reveal the formation mechanism of hierarchical pores in the synthesized NaX zeolite,the evolution of the starting materials during crystallization was carefully investigated.Fig.6(a) displays the XRD patterns of the solid products obtained at different crystallization times.It is apparent that the weak diffraction peaks corresponding to the FAU-type zeolite emerge when the crystallization time is 2.0 h,indicating that NaX zeolite can be obtained from SMSkaolinite without any seed or structure directing agent.As crystallization proceeds,the intensity of these diffraction peaks increases gradually,which suggests a progressive growth of NaX crystallites.These diffraction peaks no longer change after crystallization for 3.5 h,indicating that the crystallization of NaX zeolite basically completes during this period.The crystallization process of the synthesized NaX zeolite can be concisely reflected from a kinetic curve obtained from XRD results(Fig.6(b)),which shows a typical S-shaped crystallization growth curve consisting of three stages:induction period(0–2.0 h),fast growth(2.0–3.5 h)and stable stage(3.5–4.5 h),in a good agreement with previous report [47,48].
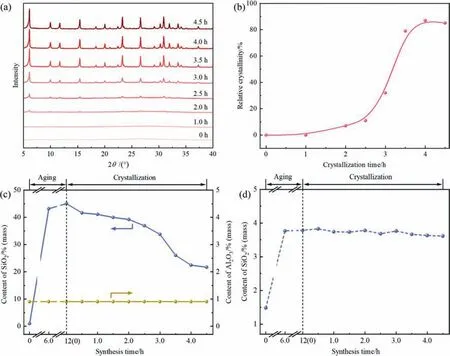
Fig.6.XRD patterns of the solid sample obtained at different crystallization times(a),the crystallization kinetic curve of the synthesized NaX zeolite(b),the content of the dissolvable SiO2 and Al2O3 in the synthesis system at different synthesis times during the synthesis of the synthesized NaX zeolite(c),and the content of the dissolvable SiO2 in SMS-kaolinite under the same conditions as the synthesized NaX zeolite except the addition of solid silica at different synthesis times (d).
Fig.6(c) shows the content of the dissolvable SiO2and Al2O3in the synthesis system at different synthesis times during the synthesis of the synthesized NaX zeolite.As can be seen,the content of the dissolvable Al2O3in the synthesis system remains extremely low during both aging and crystallization processes.Differently,the content of the dissolvable SiO2in the synthesis system increases significantly during aging.For comparison,the content of the dissolvable SiO2in SMS-kaolinite under the same conditions as the synthesized NaX zeolite except the addition of solid silica was tracked (Fig.6(d)).It is observed that when solid silica is not added in the synthesis system,the content of the dissolvable SiO2in SMS-kaolinite remains at a very low level(ca.3.7%(mass)).This signifies that more than 96% (mass) of the SiO2in SMSkaolinite are not dissolved throughout the synthesis,which conforms to our previous results[44,49].By comparing the concentration (0.239 and 0.010 mol∙L-1,respectively) of SiO2in the liquid phase with and without the addition of solid silica at the end of aging,it is found that the difference in SiO2concentration in the liquid phase under these two conditions is close to the additive amount of solid silica.Simultaneously,Fig.7(a)and(b)shows that massive solid silica is clearly observed at aging time of 0 h,and these solid silica completely disappear at the end of aging;while the amorphous particles with abundant pores of SMS-kaolinite keep unchanged during aging.Hence,it can be deduced that almost all solid silica as active silicon species are dissolved in the synthetic solution during aging,whereas the aluminum and silicon species in SMS-kaolinite are almost insoluble in the synthesis system.

Fig.7.FESEM images of solid sample obtained at the beginning(a)and end(b)of aging and at different crystallization time of 0 h(c),1.0(d),2.0(e),2.5(f),3.0(g),3.5(h)and 4.0 (i) h.
During crystallization stage,the content of the dissolvable SiO2in the synthesis system of the synthesized NaX zeolite gradually decreases (Fig.6(c)).Combined with the results of XRD (Fig.6(a)),it can be seen that a few NaX zeolite crystals emerges at the surface of amorphous particles at crystallization time of 2.0 h(Fig.7(e)).These results suggest that partial silicon species from the dissolved solid silica crystallize into embryonic crystals.These tiny crystals serve as the nutrients and interact with SMS-kaolinite functioning as nucleation centers[44],which greatly promotes the transformation of amorphous particles into NaX zeolite crystals.As shown in Fig.7(f),more and more NaX zeolite crystals are generated and starts to stack at the surface of amorphous particles.Subsequently,the amorphous particles continue to provide silicon and aluminum nutrients for the crystal growth,and these zeolite crystals have aggregated into particles with their growth (Fig.7(g)).After crystallization for 3.5 h(Fig.7(h)and(i)),the amorphous particles disappear,and only NaX zeolite crystals are observed in the sample,and thus it indicates that the amorphous particles are completely transformed into NaX zeolite.Meanwhile,these stacked NaX zeolite crystals grow into aggregate-like particles.Combined with the ICP-OES results(Fig.6(c)and(d)),it can be concluded that SMS-kaolinite is not dissolved,and its morphology is maintained throughout the process,while solid silica dissolves in the synthetic solution and interacts with SMS-kaolinite to provide raw materials for crystal growth during the induction period.During the fast growth stage,the NaX zeolite crystals grow and gradually aggregate at the surface of SMS-kaolinite,which largely boosts the crystallization of SMS-kaolinite into zeolite.Finally,SMS-kaolinite undergoes in-situ transformation into hierarchical NaX zeolite with inter-crystalline mesopores,while preserving its original morphology.
To understand the formation mechanism of intra-crystalline mesopores in the synthesized zeolite,the texture properties of the initial sample obtained at aging time of 0 h were studied by N2adsorption–desorption,and the results are shown in Fig.8(a).The isotherms of the initial product exhibit the characteristics of type I and IV ones along with an evident H4-type hysteresis.Apart from the slight adsorption at the low pressure,a substantial uptake is detected in theP/P0range from 0.6 to 1.0.These adsorption behaviors clearly confirm the existence of a certain amount of mesopores in the initial sample [50].These mesopores are determined to originate from SMS-kaolinite,based on the result that the initial mixture mainly contains smooth solid silica and porous SMS-kaolinite (Fig.7(a)).The pore size distribution presented in Fig.8(b)reveals that the mesopore size of the initial sample is centered at 15 nm,similar to that of the synthesized hierarchical NaX zeolite.Therefore,it suggests that the intra-crystalline mesopores of the hierarchical NaX zeolite are inherited from SMS-kaolinite acting as nucleation centers.Compared with the previous studies(Table 1),our work not only achieves the synthesis of pure NaX zeolite with high crystallinity,but also prepares hierarchical NaX zeolite with high porosity based onin-situtransformation of SMS-kaolinite obtained by an energy-saving activation treatment.A possible formation process for thein-situtransformation of SMS-kaolinite into the hierarchical NaX zeolite with both intercrystalline and intra-crystalline mesopores is schematically illustrated in Fig.9.
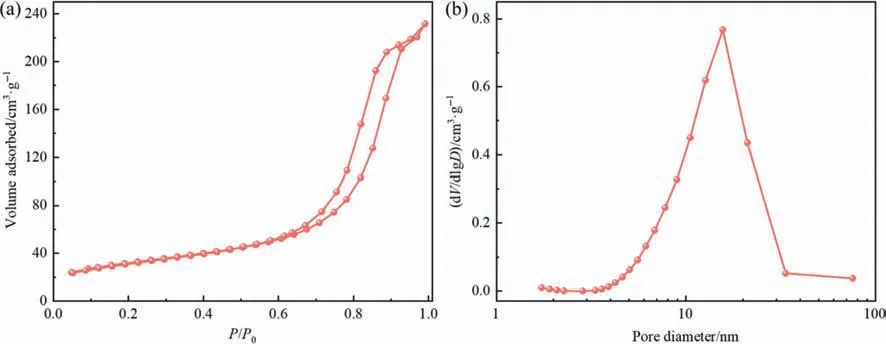
Fig.8.N2 adsorption–desorption isotherms (a) and pore size distribution curve (b) of the initial sample obtained at aging time of 0 h.
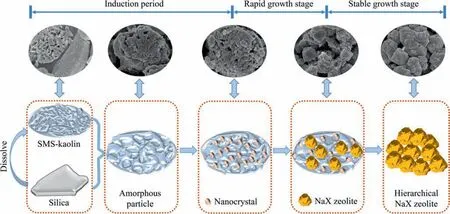
Fig.9.Schematic illustration of a possible formation process for in-situ template-free transformation of the SMS-kaolinite into the hierarchical NaX zeolite.
3.4.Catalytic tests of NaX zeolites in Knoevenagel condensation reaction
The catalytic performance of the synthesized hierarchical NaX along with commercial NaX was evaluated in the Knoevenagel condensation of benzaldehyde with ethyl cyanoacetate.Knoevenagel condensation is a base-catalyzed reaction,and thus the basicity of two zeolite catalysts was investigated.As seen from Fig.10(a),the CO2-TPD profiles of the zeolites show three desorption peaks at 80–220,220–450 and 480–700 °C,which are ascribed to the weak,medium-strong,and strong basic sites,respectively[51,52].The total basic sites data can be quantified from CO2-TPD measurement(Table 2),and the total basic sites in the synthesized NaX zeolite(3.31 mmol∙g-1)is slightly lower than that in commercial NaX (3.85 mmol∙g-1).However,the synthesized NaX zeolite has evident strong basic sites that is not detectable in commercial NaX,and these strong basic sites could be beneficial for the Knoevenagel condensation reaction.

Fig.10.CO2-TPD profiles of the different NaX zeolites(a)and conversion of benzaldehyde over the different NaX zeolites in the Knoevenagel condensation reaction at 70°C(b).
The catalytic test results in Fig.10(b)show that only 2-cyano-3-phenylacrylate and water are formed during the reaction process,with a constant selectivity of 100% for 2-cyano-3-phenylacrylate[53–55].The conversion of benzaldehyde over both hierarchical NaX and commercial NaX increases evidently in the initial 2.0 h,and then remains basically unchanged.Notably,the hierarchical NaX zeolite consistently shows higher benzaldehyde conversion throughout the entire reaction time.In particular,the conversion of benzaldehyde over the hierarchical NaX zeolite achieves 90.6% after 2 h,but that over the commercial NaX zeolite is only 83.7%.Therefore,it can be concluded that the synthesized hierarchical NaX exhibits superior catalytic performance in the Knoevenagel condensation reaction.
The improved catalytic performance of the synthesized hierarchical NaX zeolite is mainly attributed to its possession of both inter-crystalline and intra-crystalline mesopores.This pore structure substantially accelerates the diffusion of bulky molecules within the zeolite channel,thereby enhancing the accessibility of reactants to basic sites and facilitating their conversion.Moreover,the synthesized hierarchical NaX zeolite has a certain level of strong basic sites that is not observed in the conventional NaX zeolite,and these strong basic sites are in favor of the Knoevenagel condensation reaction.Therefore,hierarchical NaX zeolite has significant potential as a base catalyst for various chemical reactions involving bulky molecules.
4.Conclusions
In summary,a highly crystalline and pure hierarchical NaX zeolite has been successfully synthesized from natural kaolinite without using any template or seed.Systematic characterization reveals that the synthesized NaX zeolite possesses both inter-crystalline and intra-crystalline mesopores,as well as strong basic sites which is not detected in conventional NaX zeolite.Detailed investigation shows that inter-crystalline mesopores in the synthesized NaX zeolite originate from the agglomeration of small crystals,while the intra-crystalline mesopores are inherited from SMS-kaolinite.The unique pore structure and base property enable hierarchical NaX zeolite to be an efficient catalyst for the Knoevenagel condensation reaction between benzaldehyde and ethyl cyanoacetate.After only 2 h,the conversion of benzaldehyde over the hierarchical NaX zeolite reaches 90%,greatly outperforming that over commercial NaX zeolite.This provides an economic and green protocol for preparing hierarchical NaX zeolites with promising applications in fine chemical synthesis.These results not only contribute to the development of more efficient hierarchical zeolite but also provide fundamental insights into the formation mechanism of hierarchical zeolites.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The financial supports from the National Natural Science Foundation of China (22178059,22208054 and 22072019),Natural Science Foundation of Fujian Province,China (2020J01513),Sinochem Quanzhou Energy Technology Co.,Ltd.(ZHQZKJ-19-F-ZS-0076) and Qingyuan Innovation Laboratory (00121002) are genuinely acknowledged.
Supplementary Material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.cjche.2023.07.007.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
