Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
2024-04-22HaixinSunJianleiQiJianfeiSunLinLiKunpengYuJintaoWuJianzhongYin
Haixin Sun,Jianlei Qi,Jianfei Sun,Lin Li,Kunpeng Yu,Jintao Wu,Jianzhong Yin,*
1 State Key Laboratory of Fine Chemicals,School of Chemical Engineering,Dalian University of Technology,Dalian 116024,China
2 College of Chemical Engineering,Inner Mongolia University of Technology,Hohhot 010051,China
Keywords: Iron(III) acetylacetonate Nickel(II) acetylacetonate Supercritical carbon dioxide Solubility measurement Correlation model Phase equilibrium
ABSTRACT As a common precursor for supercritical CO2 (scCO2) deposition techniques,solubility data of organometallic complexes in scCO2 is crucial for the preparation of nanocomposites.Recently,metal acetylacetonates have shown great potential for the preparation of single-atom catalytic materials.In this study,the solubilities of iron(III) acetylacetonate (Fe(acac)3) and nickel(II) acetylacetonate (Ni(acac)2)were measured at the temperature from 313.15 to 333.15 K and in the pressure range of 9.5–25.2 MPa to accumulate new solubility data.Solubility was measured using a static weight loss method.The semi-empirical models proposed by Chrastil and Sung et al.were used to correlate the solubility data of Fe(acac)3 and Ni(acac)2.The equations obtained can be used to predict the solubility of the same system in the experimental range.
1.Introduction
Carbon dioxide is the most widely used supercritical fluid(SCF)because of its non-toxicity,non-flammability,low cost,and moderate critical pressure and temperature [1].The solubility of metal complexes in supercritical carbon dioxide (scCO2) was initially investigated for applications in the development of supercritical fluid extraction processes for metals [2–5].It was then extended to applications such as materials processing and chemical reactions [6–10].Recently,metal acetylacetonate has been used as the precursor for scCO2deposition processes to prepare singleatom catalytic materials [11,12].
The solubility of organometallic complexes in scCO2is very important in practical applications and has been reported in some literature [13–16].Ashraf-Khorassaniet al.[17] described the dynamic and static solubility experimental techniques for measuring the solubility of compounds in SCF.In addition,four other conventional analytical techniques are frequently used to determine solubility in SCF,including: gravimetric [18],chromatographic[19,20],spectroscopic [21,22],and piezoelectric methods [23].Škergetet al.[24] and Radet al.[25] summarized the semiempirical models most popular used to correlate the solubility of solids in SCF,and solubility data for organometallic compounds can also be found directly in the literature [24,26,27].
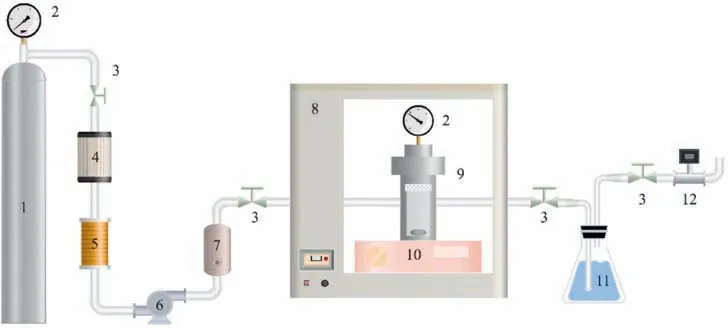
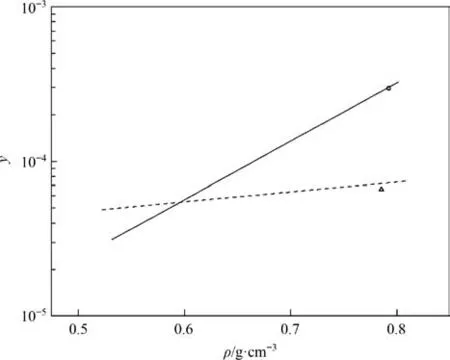
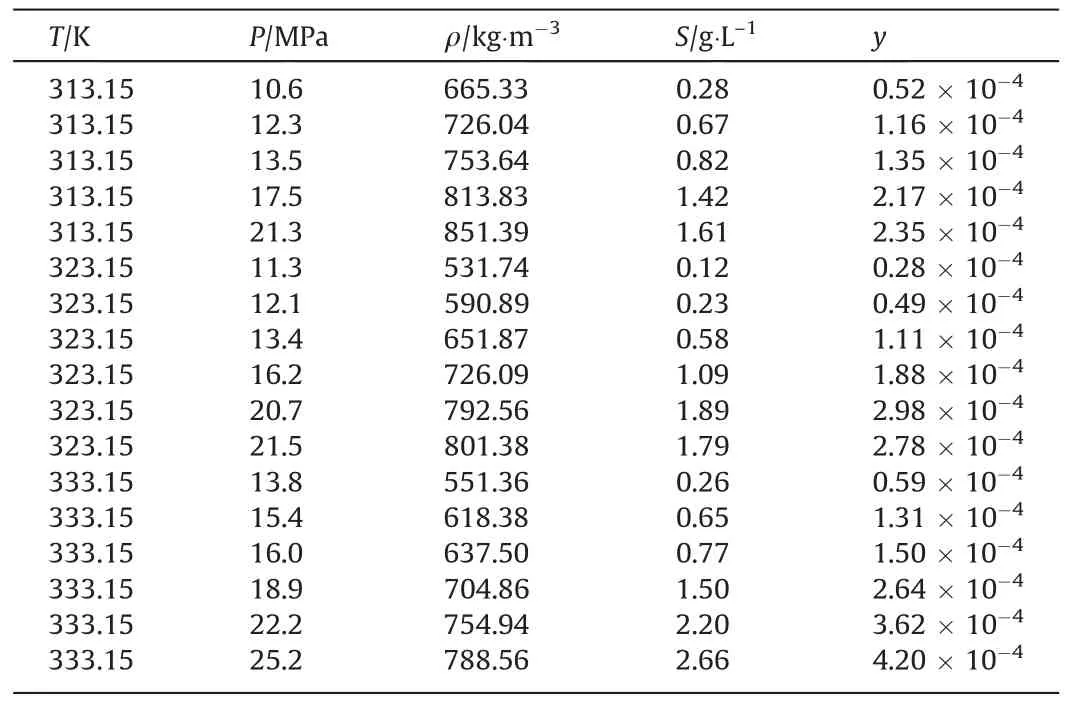
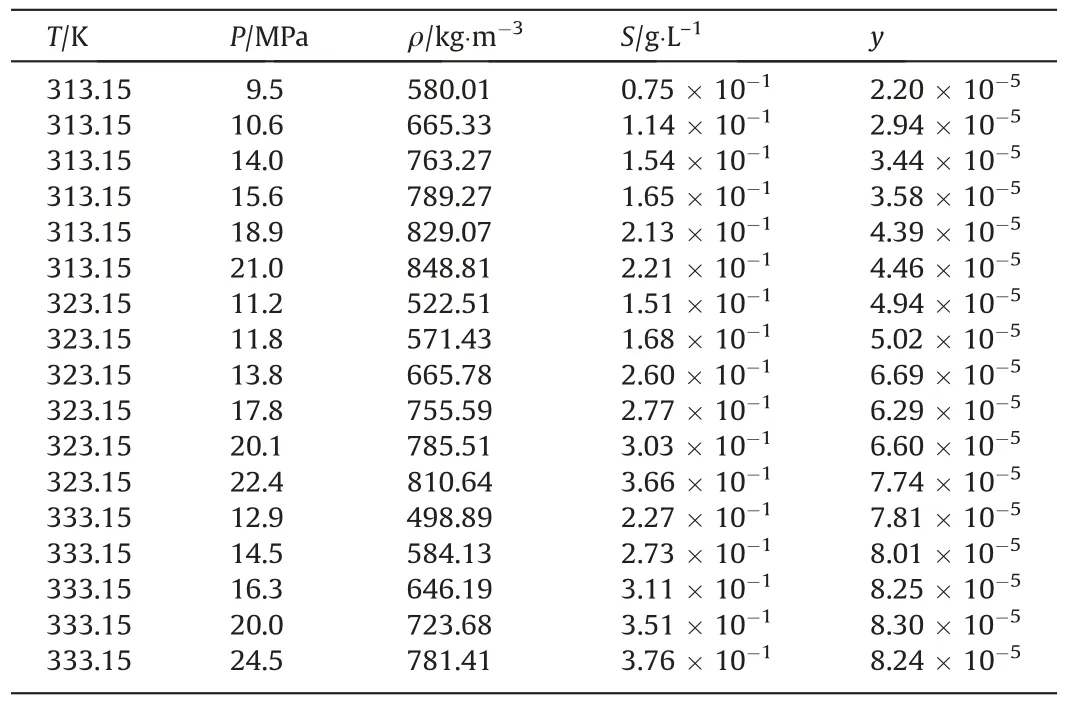
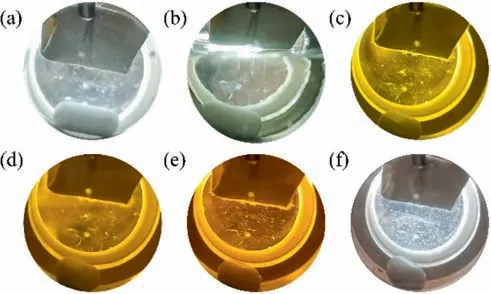
The solubility behavior of metal complexes in scCO2is affected by the metals involved and their ligand side-chains [26].Aschenbrenneret al.[28] determined the solubility of various metal cyclopentadienyl (cp) complexes in scCO2with a dynamicgravimetric method at 333 K.The metal complexes were in increasing order of solubility as Cr(cp)2 Data on the solubility of metal acetylacetonates in scCO2is relatively scarce,especially considering their solubility in scCO2.The solubilities of Fe(acac)3in scCO2at 313 K and 333 K were determined by Roggemanet al.[31] and Harukiet al.[32] using spectroscopic and semi-intermittent flow methods,respectively.Andersenet al.[33] measured the solubilities of Fe(acac)3by near-infrared spectroscopy from 313 to 333 K with a highpressure photocell.Data on the solubility of Ni(acac)2in scCO2is more limited.Kerschet al.[34]measured the solubility of mixtures of Ni(acac)2with other metal acetylacetonates,such as Cu(acac)2,at 313 K,16 MPa.The usage of the mixture resulted in the experimental solubility of Cu(acac)2about one order of magnitude lower than that reported in the literature.Aschenbrenneret al.[28]determined the solubility of Ni(acac)2with a dynamicgravimetric method at 333 K. The application of metal complexes in scCO2is gradually expanding.In this study,the solubilities of Fe(acac)3and Ni(acac)2in scCO2were measured several times at 313.15,323.15 and 333.15 K with a static weight loss method to provide basic data for subsequent applications [35].Finally,the experimental data was correlated using the semi-empirical model proposed by Chrastil [36] and Sung and Shim [37] respectively. Fe(acac)3(analytically pure,>98%) and Ni(acac)2(analytically pure,>95%) were purchased from Shanghai Aladdin Biochemical Technology Co.,Ltd.Pure CO2(>99.99%) was purchased from Dalian Junfeng Gas Co.,Ltd.Deionized water was obtained by reverse osmosis and subsequent ion exchange.Ethanol(EtOH,analytically pure,>99.5%) was purchased from Tianjin Fuyu Fine Chemical Co.,Ltd.Deionized water and ethanol were only used for washing.All materials were used without further purification.Further,the chemical structures of the metal complexes are illustrated in Fig.1. The experimental equipment (Fig.2) consists of four main parts: CO2cylinder,CO2pressurization system comprising a cooling coil and a high pressure liquid chromatography (HPLC) pump,a system for measuring the solubility,and cold trap for residue collection.The system temperature was controlled by a thermostatic blast drying oven with an uncertainty of ±0.1 K.The system pressure was regulated using the HPLC pump with an accuracy of approximately ± 0.01 MPa,and measured by the pressure sensor connected to the digital indicator.During each measurement,the sample was weighed on a balance with a minimum scale of 10-4g and an accuracy of 10-3g.The volume of the stainless steel vessel was 7.3× 10-2L.Therefore,the lower limit of solubility measurement was 1.37 × 10-2g∙L–1. Fig.2.Experimental equipment:(1)high purity carbon dioxide cylinder;(2)pressure sensor;(3)high precision needle valve;(4)CO2 filter;(5)cooling coil;(6)high-pressure liquid chromatography(HPLC)pump;(7)buffer vessel;(8)thermostatic blast drying oven;(9)high-pressure stainless-steel vessel;(10)magnetic stirrer;(11)cold trap;(12)wet gas meter. The internal structure of the high-pressure stainless steel vessel and the procedure for solubility measurements have been described in detail in our previous studies [35].In a typical experiment,a certain amount of solute sealed in a filter-paper bag was placed in the porous cylindrical storage frame made of stainless steel,which was in turn installed in the upper part of the stainless steel vessel.The high-pressure vessel was then closed and placed in the thermostatic blast drying oven to preheat at the desired temperature for 2 h.Afterwards,the inlet and outlet needle valves were opened to replace the air in the reactor with CO2.CO2was then introduced into the vessel to reach the target pressure with a pressurized system.The vessel was kept at constant pressure and temperature for a specified time (about 24 h),during which electromagnetic stirring was used to improve mixing efficiency and reach dissolution equilibrium.Finally,the outlet valve was opened to quickly release the CO2from the vessel.Then,the filter-paper bag containing the undissolved salt was removed and weighed.The amount of dissolved solute was easily obtained as the mass difference of the filter-paper bag,and solubility was determined as the ratio of dissolved solute to the scCO2volume under the given pressure and temperature conditions. A longer equilibration time maybe required to ensure complete dissolution of the precursor in scCO2.Therefore,the dissolution time was extended to 48 h.The measured solubility data as shown in Fig.3 is in good agreement with the solubility isotherms,which proved that 24 h is sufficient to achieve dissolution equilibrium. Fig.3.Experimental solubilities of Fe(acac)3 and Ni(acac)2 in scCO2 for a dissolution time of 48 h.○,Fe(acac)3,at 323.15 K and 20.7 MPa;Δ,Ni(acac)2,at 323.15 K and 20.1 MPa;(—),solubility isotherm of Fe(acac)3 at 323.15 K for a dissolution time of 24 h;(---),solubility isotherm of Ni(acac)2 at 323.15 K for a dissolution time of 24 h. Experimental results for the solubilities of Fe(acac)3in scCO2at temperatures ranging from 313.15 to 333.15 K are listed in Table 1,and solvent densities were obtained from NIST (National Institute of Standards and Technology).The experimental results for Fe(acac)3depicted in Fig.4 were in general agreement with the solubility data reported in the literature.Considering the numerical magnitude of solubility,the present work was close to the measurements of Harukiet al.[32] and between those of Roggemanet al.[31] and Andersenet al.[33].The average absolute relative deviations (AARD) between the solubility isotherm of this work at 313.15 K and literature data were 2.63%,6.71%,and 15.66%,respectively,when pressure exceeded 10.6 MPa.The AARD were 16.12%,14.41%,and 25.40% at 333.15 K and pressure of more than 13.8 MPa,respectively.In addition,the isotherm of the present work at 323.15 K was significantly higher than the solubility data of Andersenet al.[33] with AARD of 69.37%. Table 1 Experimental solubility data for Fe(acac)3 in scCO2 Fig.4.Experimental solubilities of Fe(acac)3 in scCO2.■,●,▲,this work at 313.15,323.15,and 333.15 K;×,+,Haruki et al.[32]at 313 and 333 K;,◇,Roggeman et al.[31] at 313.15 and 333.15 K;□,○,Δ,Andersen et al.[33] at 313,323,and 333 K. The effects of temperature and pressure on the solubility of Fe(acac)3in scCO2were analyzed.For a given temperature,the solubility increases with pressure due to the higher density of the solvent.A crossover region of solubility isotherms with pressure is observed at the pressure range studied.The range of this region calculated from a semi-empirical model associated with experimental data is 16.34–19.08 MPa.Below this range,the solubility decreases with temperature due to the decrease in solvent density.When the pressure exceeds 19.08 MPa,solubility increases with temperature due to the increase in the solute vapor pressure. Experimental results for the solubilities of Ni(acac)2in scCO2at temperatures ranging from 313.15 to 333.15 K are listed in Table 2.The experimental results of Ni(acac)2are also described in Fig.5 along with the solubilities reported in the literature.The solubility isotherm of the present work at 333.15 K deviated significantly from the experimental solubility of Aschenbrenneret al.[28] with AARD of 47.4%at 30 MPa.Furthermore,the solubility measured at 313 K and 16 MPa by Kerschet al.[34] was approximately two orders of magnitude lower than the experimental results of the present work. Table 2 Experimental solubility data for Ni(acac)2 in scCO2 Fig.5.Experimental solubilities of Ni(acac)2 in scCO2.■,●,▲,this work at 313.15,323.15,and 333.15 K;×,Aschenbrenner et al.[28] at 333 K. The effects of temperature and pressure on the solubility of Ni(acac)2were discussed.The solubility of Ni(acac)2presents a logarithmic relationship with pressure at the studied temperatures.The slope of the solubility isotherm decreases with increasing pressure.For a given pressure,the solubility increases with temperature. Solubility data for both systems were fitted to the semiempirical equations proposed by Chrastil [36] and by Sung and Shim [37].Chrastil model provides a linear relationship between the natural logarithms of the solubility,yand the solvent density,ρ (kg∙m-3),and is given by Eq.(1): wherea,bandkare temperature independent adjustable parameters.In the original modelais related to the molecular mass of solute and solvent,bconsiders the heats of solvation and vaporization andkis an association number. The semi-empirical model presented by Sung and Shim whose slope of the solubility isotherms decreases with increasing temperature provides a linear relationship between the natural logarithm of solubility and the solvent density given by Eq.(2): wherea,b,canddare all adjustable parameters independent of temperature. The relationship between the molar fraction solubility of Fe(acac)3and the density of scCO2is illustrated on the double logarithmic chart in Fig.6.Approximately linear relationships were obtained for the solubility isotherms,and the slopes of the isotherms were nearly identical.The correlation results for the solubilities of Fe(acac)3using Eqs.(1) and (2) are listed in Table 3.Reasonable correlation results were obtained using the empirical models proposed by Chrastil and Sung and Shim. Table 3 Correlated results for the solubilities of Fe(acac)3 using Eqs.(1) and (2) Fig.6.Relationship between the logarithm of the molar fraction solubility of Fe(acac)3 and the logarithm of the density of scCO2. ■,●,▲,this work at 313.15,323.15,and 333.15 K;(—),Eq.(1);(---),Eq.(2). The relationship between the molar fraction solubility of Ni(acac)2and the density of scCO2is illustrated on the double logarithmic chart in Fig.7.Nearly linear behaviors were observed for the solubility isotherms,and the slope of the isotherms decreased with increasing temperature.The correlation results for the solubilities of Ni(acac)2using Eqs.(1) and (2) are listed in Table 4.The solubility data for Ni(acac)2correlated better with the semiempirical model proposed by Sung and Shim [37].Reproducibility of the experimental isothermal solubility was 9.20% at its maximum and 15.02% as a whole. Table 4 Correlated results for the solubilities of Ni(acac)2 using Eqs.(1) and (2) Fig.7.Relationship between the logarithm of the molar fraction solubility of Ni(acac)2 and the logarithm of the density of scCO2.■,●,▲,this work at 313.15,323.15,and 333.15 K;(—),Eq.(1);(---),Eq.(2). In the solubility determination experiment,a large amount of brick-red Fe(acac)3could be clearly observed inside the highpressure reactor after CO2removal,but green Ni(acac)2was only observed on the surface of the filter paper bag with a small amount,due to the difference in color and solubility of the two compounds.In order to visualized the dissolution process of metal complexes in scCO2,the solubility experiment of Fe(acac)3was carried out at 313.15 K and 13 MPa using a phase equilibrium experimental apparatus.The experimental set-up has been described in our previous study [11].In a typical experiment,the Fe(acac)3wrapped by filter paper was loaded into the reactor.A constant flow of high purity CO2was then slowly filled into the phase equilibrium cell with a plunger pump at 313.15 K until its pressure increased to 13 MPa. The dissolution process of Fe(acac)3in scCO2is illustrated in Fig.8.The initial state of the high-pressure reactor containing the sight glass without scCO2filling is shown in Fig.8(a).After filling with scCO2,the liquid level gradually raised (Fig.8(b)) until it filled the whole viewport.Under the action of magnetic stirring,the unsaturated scCO2diffused into the filter paper bag,dissolving the Fe(acac)3inside and bringing it out.Due to the high solubility,the scCO2with dissolved Fe(acac)3showed a significant color change(from colorless to light yellow,Fig.8(c))after 15 min of dissolution.By extending the dissolution time,scCO2gradually changed from light yellow to dark yellow (Fig.8(d)).Eventually,the scCO2with dissolved Fe(acac)3exhibited the original red color of Fe(acac)3(Fig.8(e)).Rapidly removing scCO2from the system,a large amount of brick-red Fe(acac)3precipitated and dispersed in the bottom of the cavity (Fig.8(f)). Fig.8.Changes of Fe(acac)3 in scCO2 with dissolution time:(a)initial state without scCO2 filling;(b) state during scCO2 filling;(c) state after 15 min of Fe(acac)3 dissolution in scCO2;(d) state after 1 h of dissolution;(e) state after 4 h of dissolution;(f) state after pressure removal. In this work,the solubilities of Fe(acac)3and Ni(acac)2in scCO2were measured using a static mass loss method.The molar fraction solubility values ranged from 0.28 × 10–4to 4.20 × 10–4and 2.20×10–5to 8.30×10–5,respectively,in wide ranges of pressure(9.5–25.2 MPa)and temperature(313.15–333.15 K).The solubility data obtained can be used to guide the materials preparation by supercritical fluid deposition method and is also the basic data for supercritical fluid chemical engineering.In double logarithmic plots,approximately linear relationships were observed for the solubility isotherms of both Fe(acac)3and Ni(acac)2.The slope of the former was almost equal and independent of temperature,while the slope of the latter decreased with increasing temperature.The experimental solubility data were successfully correlated using the empirical models proposed by Chrastil and Sung and Shim.In the solubility determination experiments,a large amount of brick-red Fe(acac)3was clearly observed in the high-pressure reactor after the removal of CO2.In order to visualize the dissolution process of the metal complexes in scCO2,a high-pressure reactor with a viewable window was again used to perform the experiment. Data Availability Data will be made available on request. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgements The authors are appreciative of financial support from the National Key Research and Development Program of China(2020YFA0710202),the National Natural Science Foundation of China (21978043,U1662130),Inner Mongolia University of Technology Scientific Research Initial Funding (DC2300001240),Talent Introduction Support Project of Inner Mongolia (DC2300001426).2.Materials and Methods
2.1.Materials
2.2.Apparatus and procedure
3.Results and Discussion
3.1.Solubility of Fe(acac)3

3.2.Solubility of Ni(acac)2

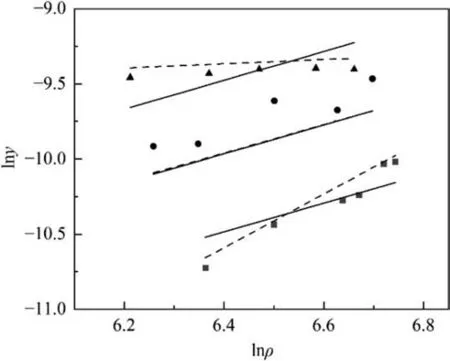
3.3.Solubility data correlation


3.4.Phase equilibrium experiment
4.Conclusions
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
- The Al2O3 and Mn/Al2O3 sorbents highly utilized in destructive sorption of NF3
