Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
2024-04-22DongMaQinhuiWang
Dong Ma,Qinhui Wang
State Key Laboratory of Clean Energy Utilization,Institute for Thermal Power Engineering,Zhejiang University,Hangzhou 310027,China
Keywords: Phosphogypsum Sulfur dioxide Copper slag Fluidized-bed Reduction Waste treatment
ABSTRACT The reduction of phosphogypsum(PG)to lime slag and SO2 using coke can effectively alleviate the environmental problems caused by PG.However,the PG decomposition temperature remains high and the product yield remains poor.By adding additives,the decomposition temperature can be further reduced and PG decomposition rate and product yield can be improved.However,the use of current additives such as Fe2O3 and SiO2 brings the problem of increasing economic cost.Therefore,it is proposed to use solid waste copper slag (CS) as a new additive to reduce PG to prepare SO2,which can reduce the cost and meet the environmental benefits at the same time.The effects of proportion,temperature and thermostatic time on PG decomposition are investigated by experimental and kinetic analysis combined with FactSage thermodynamic calculations to optimize the roasting conditions.Finally,the reaction mechanism is proposed.It is found that adding CS to the coke and PG system can increase the rate of PG decomposition and SO2 yield while lowering the PG decomposition temperature.For example,when the CS/PG mass ratio increases from 0 to 1,PG decomposition rate increases from 83.38% to 99.35%,SO2 yield increases from 78.62% to 96.81%,and PG decomposition temperature decreases from 992.4 °C to 949.6 °C.The optimal reaction parameters are CS/PG mass ratio of 1,Coke/PG mass ratio of 0.06 at 1100 °C for 20 min with 99.35% PG decomposition rate and 96.81% SO2 yield.The process proceeds according to the following reactions: 2CaSO4+0.7C+0.8Fe2SiO4→0.8Ca2SiO4+0.2Ca2Fe2O5+0.4Fe3O4+2SO2+0.7CO2 Finally,a process for decomposing PG with coke and CS is proposed.
1.Introduction
Phosphogypsum (PG) is a byproduct of phosphorus chemical firms when producing phosphoric acid.The production of the PG industry in China is 75.1 million tons in 2022,and the total usage is 36 million tons.Only 47.9% of the capacity is being utilized.CaSO4∙nH2O (n=0,0.5,1.5,2) is the primary constituent of PG[1].Additionally,it contains a variety of impurities like Si,Fe,Mg,Al,P,and F,as well as organic debris and trace metals like Cr and Pb[2].The large number of impurities and a certain degree of radiation severely limit the large-scale application of PG.Currently,PG is mainly treated by stacking.In addition to occupying a significant amount of area,piling of PG also readily results in the poisoning of the atmosphere and groundwater [3].As a result,PG treatment is urgently required.
It is feasible to reduce PG to SO2and lime slag with thermochemical methods.Lime slag can be used to make construction materials,while SO2can be used to make sulfuric acid [4,5].However,since direct decomposition temperature of PG is more than 1620 °C,direct heating is not used in industry to decompose PG,but the temperature of PG decomposition is reduced by adding reducing agents.Common reducing agents include carbon-based types,such as lignite [6],bituminous coal [7,8],anthracite [9],high-sulfur coal [10],coke [11],gangue [12],and petroleum coke[13].Sulfur-based species,such as sulfur [14],FeS2[15],thionite[16],and H2S [17,18].In addition,CO,H2[19,20] and other gases(CO-O2) [21] are also used as reducing agents.The inclusion of reducing chemicals reduces the decomposition temperature of PG considerably.Nonetheless,the temperature of PG decomposition remains high.Researchers have found that PG’s decomposition temperature may be further lowered by adding additives.Furthermore,the PG decomposition rate and product yield can be improved.The additives that have been studied include FeCl3[22],Fe2O3[23],iron phosphate slag [24],Al2O3[25],SiO2[26],potassium feldspar[27]and various transition group metal oxides,such as V2O5,MnO2,Cr2O3,CuO,CoCl3∙7H2O,Co2O3/Co3O4,etc.[11].Al2O3and SiO2can accelerate the reaction rate and reduce the initial decomposition temperature at the early stage of decomposition,but intermediate products such as calcium sulfosilicate and calcium sulfoaluminate are produced during the reaction,which inhibit the decomposition of PG [28].Therefore Si-based and Al-based additives are not perfect as PG decomposition additives.Currently,Fe-based additives are the most widely used in PG decomposition.However,the use of the above mentioned Fe-based additives brings the problem of increased economic costs.Therefore,it is necessary to find new iron-based additives to further reduce production costs.
Copper slag (CS) is a waste slag containing iron,silicon,aluminum and calcium produced during the pyroprocess of copper refining.The Fe in CS is mainly in the form of ferrous vitrinite,iron olivine(Fe2SiO4)and magnetite[29].The present yearly emissions of CS are more than 14 million tons because thermal copper refining can result in the production of 2.2 tons of CS for every ton of copper metal produced [30].Since the economics of using CS to refine copper,iron and rare metals is still unreasonable,CS is currently mainly discarded directly or used as a construction alternative material for landfills,resulting in a large amount of wasted resources as well as environmental problems.Studies have demonstrated that Fe may considerably lower the decomposition temperature of PG and accelerate its decomposition process.This process mostly relies on zero-valent Fe and divalent Fe,which are abundant in CS [31].As a result,using Fe2SiO4in CS to promote the decomposition of PG can increase the resource use of CS.However,studies on the reduction of PG by CS as an additive have not been reported.As a result,coke is employed as a reducing agent and CS as an addition in this study to decompose PG and prepare SO2.In order to improve the conditions for this approach,the effects of proportion,thermostatic time and temperature on PG decomposition are inspected by experimental and kinetic analysis combined with FactSage thermodynamic calculations.Finally,the reaction mechanism and the flow of the process are proposed.
2.Experimental
2.1.Experimental raw materials
PG is taken from a phosphorus chemical enterprise in Yunnan,and CS is the slag of a non-ferrous metal company in the process of copper refining by fire.After sieving the PG and CS,any particles with a size less than 0.075 mm are dried at 105°C for 6 h.By using an X-ray fluorescence spectrometer (XRF,Panalytical Axios,Netherlands),the chemical composition of dried PG and CS can be determined,as indicated in Table 1 and Table 2,respectively.According to Table 1,the primary chemical components of PG are CaO and SO3,and it also includes 5.42% SiO2,0.15% Al2O3,0.61% P2O5,0.22% F,and trace quantities of organic materials.As shown in Table 2,the mass fractions of SiO2,Al2O3,Fe2O3and CaO in the copper slag are 21.63%,7.85%,45.66% and 5.07%,respectively,and also containes some heavy metals such as ZnO,CuO and PbO.The SO3content in the CS is 1.48% and the sulfur content is low.X-ray diffractometer (XRD,Rigaku Ultima IV,Japan) is used to perform the physical phase analysis of PG and CS,as illustrated in Fig.1S in Supplementary Material.It is clear that the main components of PG are SiO2,CaSO4∙2H2O,and CaSO4∙0.5H2O.The fact that the XRD peaks of CS are wrapped suggests that the ore sample contains a lot of amorphous minerals.The crystalline minerals are mainly Fe2SiO4as well as magnetite.The other minerals (CaO,Al2O3,MgO) are encapsulated by the iron olivine phase and have low content,so they are difficult to be detected by XRD.The mass fraction of Fe2SiO4in the CS is determined to be 51.64% by X-ray photoelectron spectroscopy (XPS,Thermo Escalab 250XI,United States) after split-peak fitting.
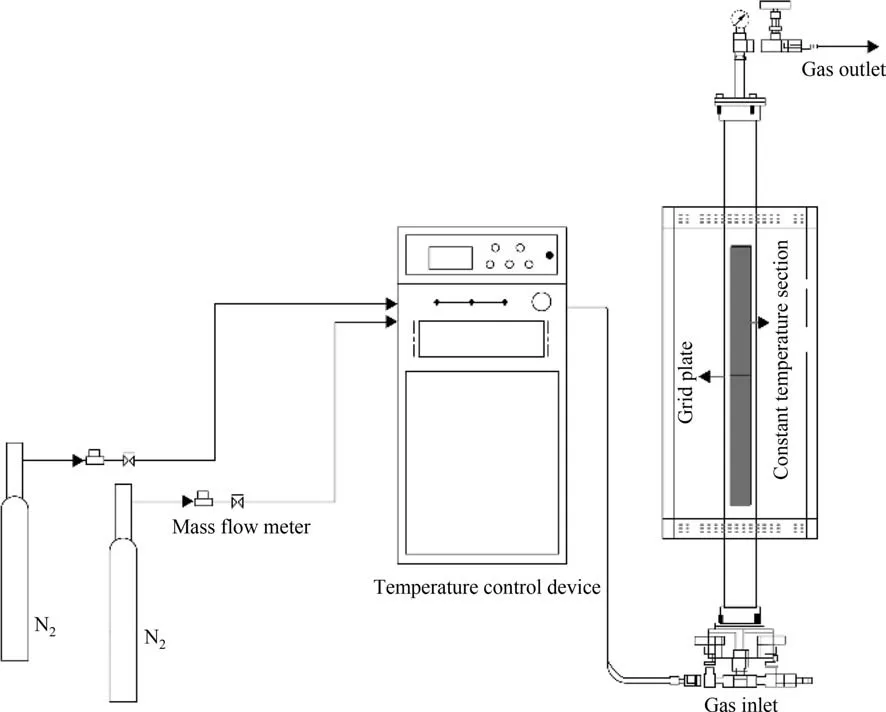
Fig.1.Flow chart of the small fluidized bed reactor.

Table 1 Chemical composition of PG/% (mass)

Table 2 Chemical composition of CS/% (mass)
To make coke,anning lignite is crushed and sieved,and particles between 0.125 and 0.25 mm in size are thoroughly pyrolyzed for 1 h at 1100°C(coke is prepared according to the industrial coking process).This particle size is chosen to increase the PG decomposition rate while facilitating product separation.Table 3 shows the industrial and elemental analyses of raw coal and coke conducted in accordance with GB/T 212–2008 and GB/T 31391–2015,respectively.
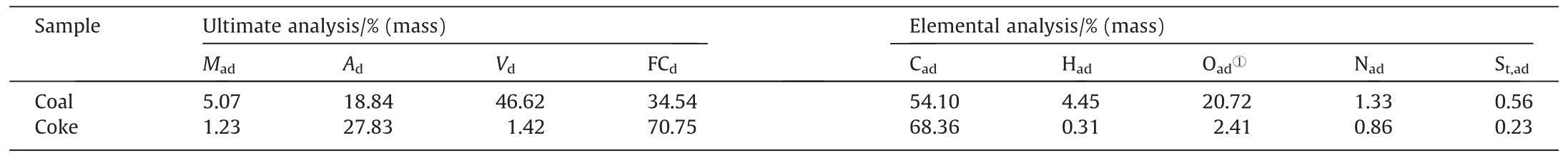
Table 3 Main characteristics of samples
2.2.Fluidized bed experiments
As shown in Fig.1,the tests are conducted in a little fluidized bed reactor.In the middle of the electrically heated furnace,the quartz tube is positioned vertically with the inner diameter of 50 mm.Furthermore,in the center of the quartz tube,a air cloth plate with the hole diameter of 20 μm has been inserted.At this aperture size,the material does not fall to the bottom of the reactor.To stop materials from leaving the reactor,a valve has been installed at the top of it.The reaction temperature is regulated using a temperature control system.A mass flow meter is used to precisely control the gas flow.Before starting the experiment,a mixture of CS,PG and coke is placed on the air distribution plate.To ensure that the material in the reactor could be equally fluidized,the N2flow rate is set to 2 L∙min-1.Previous studies have found negligible inter-particle drag when the fluidized air velocity is greater than 0.1 L∙min-1[21].The temperature is then increased to the specified temperature at a rate of 20°C∙min-1and kept constant for a period of time.The gas is collected with air bags for subsequent composition analysis.
2.3.Determination of product composition
The solid samples are collected and kept when the tests are done.The barium sulfate precipitation technique (GB/T 5484–2012)is employed to calculate the PG decomposition rate.A sulfur content measurement equipment (Shandong Huifen GCS-80,China) is used to determine the quantity of SO2.XRD is used to determine the composition of a product.The specific calculation process is shown in Eqs.(1)–(3).
2.4.Thermodynamic calculations
Based on the idea of the minimal Gibbs function,FactSage software calculates thermodynamic equilibrium and offers a vast database of chemical processes and thermodynamics.To compute the products and thermodynamic processes in this work,the equilib module and the reaction module are mostly used.The compound type is set to gas and solid,the beginning pressure is set to 0.1 MPa,and Fact-SLAGA is the solution type of choice.The reaction temperature is set at 1100 °C,the coke/PG mass ratio is 0 to 0.09,and the CS/PG mass ratio is 0 to 1.5.The impact of copper slag on the degradation of PG is examined through a set of 4 tests.The experimental conditions utilized in this work are listed in Table 4.

Table 4 Experimental conditions
3.Results and Discussion
3.1.Thermogravimetric analysis
Fig.2 displays the TG-DTG-DSC findings for the three samples,including PG alone (coke/PG=0) (Fig.2(a)),PG-coke(coke/PG=0.06,CS/PG=0) (Fig.2(b)),and PG-coke-CS (coke/PG=0.06,CS/PG=1) (Fig.2(c)).Table 5 displays the mass loss of the three samples.For the PG alone sample,there are two separateperiods of weight reduction as shown in Fig.2(a).At 85.6–156.1°C,it mainly corresponds to the removal of water of crystallization in PG.The water mass loss is 7.31%.At 956.1–1200°C,the decomposition reaction of PG starts to occur as shown in reaction (4).

Fig.2.TG-DSC curves of the samples.

Table 5 Mass loss statistics for thermogravimetric curves
Fig.2(b) displays the TG-DTG-DSC finding for the PG-coke sample.At 86.2–151.9 °C,the mass loss is 7.14%,which mainly corresponds to the removal of water of crystallization in PG and coke.According to reactions(5)–(11),the reaction of PG with coke and CO account for the majority of the mass loss at 891.7–1008.7°C,which is 39.71%.According to reactions(12)–(14),the reaction of CaSO4with SiO2and CaS at 1052.4–1200°C results in a mass loss of 4.30%.
Fig.2(c) displays the TG-DTG-DSC finding for the PG-coke-CS sample.There are three basic phases to mass reduction.The initial mass loss peak,which corresponds to the removal of water of crystallization in PG,coke,and CS emerges at 85.6–146.8 °C with a mass loss of 4.52%.The second mass loss peak appears at 887.8–1002.8 °C with a mass loss of 28.74%,corresponding mainly to the reaction of reactions (5)–(11) and the reaction between CaSO4and C and Fe2SiO4.The third mass loss peak occurs at 1002.8–1200 °C with a mass loss of 3.23%,which mainly corresponds to the reactions (12)–(14).Compared to the PG-coke sample,the mass loss is smaller.This is primarily caused by the relative decrease in PG content of the sample and the rise in formation of complex oxides (such as Ca2SiO4and Ca2Fe2O5,among others)[27].It is worth mentioning that the presence of CS greatly decreases the decomposition temperature of PG.The peak temperature is lowered from 992.4°C to 949.6°C.In addition,the reaction temperature between CaSO4and CaS is also lowered with the peak temperature lowering from 1200 °C to 1148.4 °C.As a result,adding CS can lower the decomposition temperature of PG.
3.2.Thermodynamic calculations
As illustrated in Fig.3,the equilib module in FactSage software is employed to forecast the balanced composition of the PG-coke-CS system.From Fig.3(a),the mass of CaSO4progressively declines as the CS dose increases,whereas the mass of SO2gradually increases,and the products are mostly Ca2SiO4and Ca2Fe2O5.When the CS/PG mass ratio is 1,CaSO4has been entirely decomposed and the mass of SO2has reached its maximum.Continuing to increase the CS dosage,the SO2mass remains almost constant.As shown in Fig.3(b),Ca3Fe2Si3O12,Fe2O3and SO2appear in the product when coke is not added,which indicates that Fe2SiO4has been involved in the decomposition reaction of CaSO4and oxidized from+2 to+3 valence and SO2is produced.After the addition of coke,Ca3Fe2Si3O12gradually disappears and is converted to Fe3O4.Additionally,the inclusion of coke considerably speeds up CaSO4decomposition.When the coke/PG mass ratio reaches 0.06,CaSO4entirely breaks down,and the mass of SO2reaches its maximum.When the coke addition continues to increase,CaS appeares in the product and S2appears in the gas leading to the decrease of SO2mass.

Fig.3.(a) Effects of CS/PG mass ratio on product composition (1100 °C,coke/PG=0.06);(b) Effects of coke/PG mass ratio on product composition (1100 °C,CS/PG=1).
3.3.Effect of CS/PG mass ratio
Firstly,as shown in Fig.4,the impact of CS/PG on the rate of PG decomposition and the SO2yield is examined.Fig.4(a) shows that the SO2yield and PG decomposition rate can be greatly increased by adding CS to the coke and PG system.For instance,the SO2yield increases from 78.62% to 96.81% and PG decomposition rate increases from 83.38% to 99.35% when CS/PG mass ratio increases from 0 to 1.When the CS dose increases further,there are no significant changes in SO2yield and PG decomposition rate.Furthermore,the SO2yield is lower than PG decomposition rate due to the reaction between PG and coke at high temperature to form CO,and the reaction between SO2and CO to form COS and S2resulting in lower yield,as shown in reactions (15)–(16).It is demonstrated that COS and S2are produced by secondary reaction in the presence of CO,whereas SO2is produced by primary reaction[32,33].

Fig.4.(a) Effects of CS/PG on product composition;(b) XRD patterns of the product;(c) variations of SO2 concentration with time (coke/PG=0.06,1100 °C,20 min).
It can be observed from the XRD patterns in Fig.4(b) that the intensity of the characteristic peak of CaSO4in the product is larger when no CS is added,and the products are mainly CaO,Ca(OH)2and small amounts of Ca2SiO4and CaSiO3.CaO is mainly formed through reactions (5)–(6) and reaction (12).Ca(OH)2is created as a result of the interaction of CaO with water vapor in air.Ca2SiO4and CaSiO3are mostly formed as a result of the reaction of CaO with SiO2in PG as shown in reactions (13)–(14).There is no obvious characteristic peak of Ca2Fe2O5in the product,and the intensity of the characteristic peaks of Ca2SiO4and CaSiO3is low,suggesting that SiO2and Fe2O3in the coke are not engaged in the PG decomposition.With the addition of CS,the characteristic peaks of Ca2Fe2O5and Fe3O4appear in the product,and the intensity of Ca2Fe2O5,Fe3O4and Ca2SiO4increase significantly with the increase of CS addition.At the same time,the intensity of the characteristic peak of CaSO4is decreasing significantly,which indicates that CS can dramatically accelerate the degradation of PG.
The trends of SO2concentration with time during the rise of three samples from room temperature to 1100 °C are shown in Fig.4(c).As can be observed,the concentration of SO2produced by PG alone decomposition is low.When coke is added,the peak SO2concentration increases from 233.7 mg∙m-3to 1435.6 mg∙m-3.When CS is added,the peak SO2concentration increases from from 1435.6 mg∙m-3to 2429.0 mg∙m-3.It can be seen that the addition of CS can significantly increase the SO2yield.
3.4.Effect of coke/PG mass ratio
Then the effect of coke/PG mass ratio on the PG decomposition rate and SO2yield is investigated as shown in Fig.5.As shown in Fig.5(a),increasing coke dosage can significantly increase the PG decomposition rate.For example,increasing the coke/PG mass ratio from 0 to 0.06 boosts the PG decomposition rate from 73.19% to 99.35%.Continuing to increase the amount of coke,the increase of PG decomposition rate is little.However,it is worth noting that the SO2yield increases and subsequently decreases as coke addition increases.For example,when the coke/PG mass ratio increases from 0 to 0.06,the SO2yield increases from 68.32% to 96.81%.And when the coke/PG mass ratio continues to increase to 0.09,the SO2yield decreases to 86.37%.
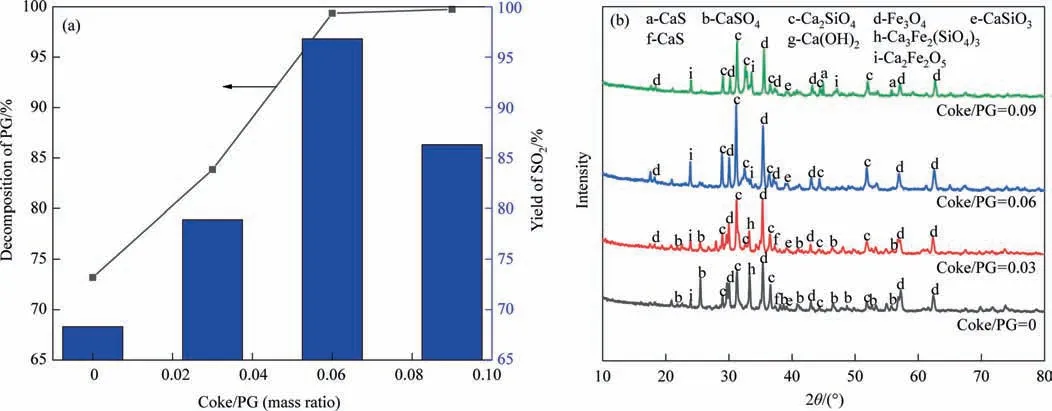
Fig.5.(a) Effects of coke/PG on product composition;(b) XRD patterns of the product (CS/PG=1,1100 °C,20 min).
The reason for this can be derived from the XRD patterns of the products in Fig.5(b).The strength of the CaSO4peak in the product diminishes dramatically as coke addition increases.When the coke/PG mass ratio increases to 0.06,the characteristic peaks of CaSO4in the product are already difficult to be detected,which indicates that the PG is close to complete decomposition at this time.However,at a coke/PG mass ratio of 0.09,the characteristic peak of CaS appears in the product,which is mainly due to reaction (7) and reaction (9).It is shown that there is a competitive reaction between CaO and CaS production,and CaSO4is more easily converted to CaS with increasing carbon content [4].It is also evident from the XRD patterns that the intensity of the characteristic peaks of Ca2Fe2O5and Ca2SiO4in the products increases significantly as the coke/PG mass ratio increases from 0 to 0.06.This suggests that increasing the carbon content enhances the chemical interaction between Fe2SiO4and CaSO4.When the coke/PG mass ratio increases to 0.09,the intensity of Ca2Fe2O5and Ca2SiO4characteristic peaks in the products decreases significantly,which indicates that the reaction between CaSO4and Fe2-SiO4occurrs to a lesser extent.The result is consistent with the thermodynamic simulation conclusions.In addition,PG decomposition alone and CS/PG (CS/PG=1 without coke) are compared as shown in Fig.2S.The addition of CS raises PG decomposition rate from 23.02% to 73.19% and the SO2yield from 21.38% to 68.32%.This indicates that CS significantly promotes the decomposition process of PG.Combined with Fig.5(b),it can be seen that there are obvious characteristic peaks of Ca3Fe2Si3O12,Fe3O4and Ca2-Fe2O5in the product,which shows that CS contributes to the PG decomposition and enhances the SO2yield.Furthermore,when coke concentration increases,Ca3Fe2Si3O12progressively decreases,while the strength of the distinctive peaks of Fe3O4and Ca2Fe2O5steadily increases,which indicates that Ca3Fe2Si3O12gradually decomposes to produce Fe3O4and Ca2Fe2O5.The result is consistent with the thermodynamic simulation results above.In summary,in order to obtain the target product SO2,the coke/PG mass ratio of 0.06 is fixed.
3.5.Effect of temperature
Then,as shown in Fig.6,the impact of temperature on PG decomposition rate and SO2yield is examined.As can be shown from Fig.6(a),warming increases greatly both the rate of PG decomposition and SO2yield.For instance,the rate of PG decomposition increases from 33.94% to 99.35% and SO2yield increases from 31.27% to 96.81% from 900 °C to 1100 °C.Furthermore,the rate of PG decomposition and SO2yield barely increase when the temperature reaches 1150 °C.The explanation for this might be that the PG decomposition process is mainly coupled gas–solid and solid–solid phase decomposition,and the reaction rate is influenced by the kinetics,so increasing the temperature is beneficial to speed up the reaction rate.
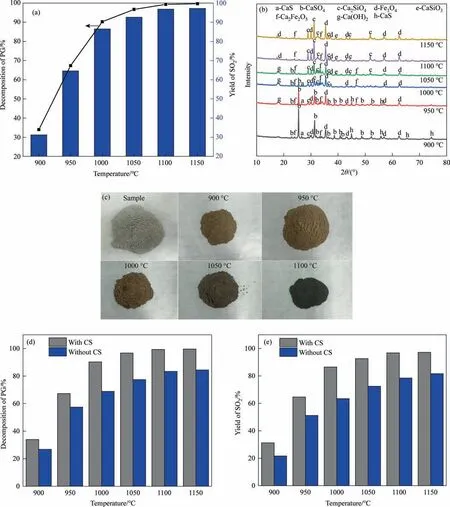
Fig.6.(a)Effects of temperature on product composition;(b)XRD patterns of the products.(c)Photographs of the products at different temperatures;effects of CS on(d)PG decomposition rate and (e) SO2 yield at different temperatures (CS/PG=1,coke/PG=0.06,20 min).
This outcome is also reflected in the XRD pattern of the product in Fig.6(b).At 900 °C,the reaction products are mainly CaS and unreacted CaSO4.The intensity of the characteristic peak of CaSO4significantly decreases and CaS also gradually disappears as temperature rises,which is mainly due to the reaction of reaction(12).The liquid-phase eutectic model previously put out by previous researches predicts that CaS can react with CaSO4at 850–900 °C [9].The distinctive peak of CaSO4is totally gone when the temperature approaches 1100 °C.In addition,the intensity of the characteristic peaks of Ca2SiO4and Ca2Fe2O5in the product is increasing from 900°C to 1000°C,which indicates that the chemical reaction between Fe2SiO4,C and CaSO4produces Ca2SiO4and Ca2Fe2O5.The strength of the characteristic peak of Ca2Fe2O5drops as the temperature rises to 1100 °C,whereas the intensity of the characteristic peak of Fe3O4dramatically increases.When combined with Fig.6(c),the raw material is off-white and the color changes to gray-red after the reaction at 900–950 °C.As the temperature continues to increase,the color of the product gradually deepens and becomes black.This suggests that when the temperature rises,the amount of Fe3O4steadily rises.The analysis may be due to the increase of Fe3O4content caused by the reduction of trivalent iron by coke at high temperature.
The effects of the presence or absence of CS on PG decomposition rate and SO2yield at different temperatures are also compared as shown in Fig.6(c)and(d).As can be seen that the addition of CS can obviously increase the PG decomposition rate and SO2yield at the same temperature.Furthermore,the influence of adding CS on PG decomposition rate and SO2yield is substantially smaller at low temperatures than at high temperatures.For example,the SO2yield and PG decomposition rate are 21.66% and 26.78%,respectively at 900°C when CS is not added.The SO2yield and PG decomposition rate are 31.20% and 33.94%,respectively when CS is added.The difference between the two is little.However,when the temperature reaches 1100°C,the SO2yield and PG decomposition rate are 78.45% and 83.72%,respectively without the addition of CS.After the addition of CS,the SO2yield and PG decomposition rate are 96.81% and 99.35%,respectively.The difference between the two widens considerably.The main reason for this is a rise in the degree of redox reaction between CS and coke and PG at high temperature,resulting in the increase of PG decomposition rate and SO2yield.Therefore,in order to obtain the target product SO2,the reaction temperature should be controlled at 1100 °C.
3.6.The effect of thermostatic time
Finally,as shown in Fig.7,the impact of thermostatic time on the rate of PG decomposition and SO2yield is examined.Increasing the thermostatic time has a favorable effect on the rate of PG decomposition and SO2yield,as can be shown in Fig.7(a).For instance,the PG decomposition rate increases from 87.95% to 99.35% and SO2yield increases from 84.63% to 96.81% from 0 min to 20 min.Continuing to extend the thermostatic time to 30 min,the increase of both is little.This indicates that the PG is close to complete decomposition at 20 min.
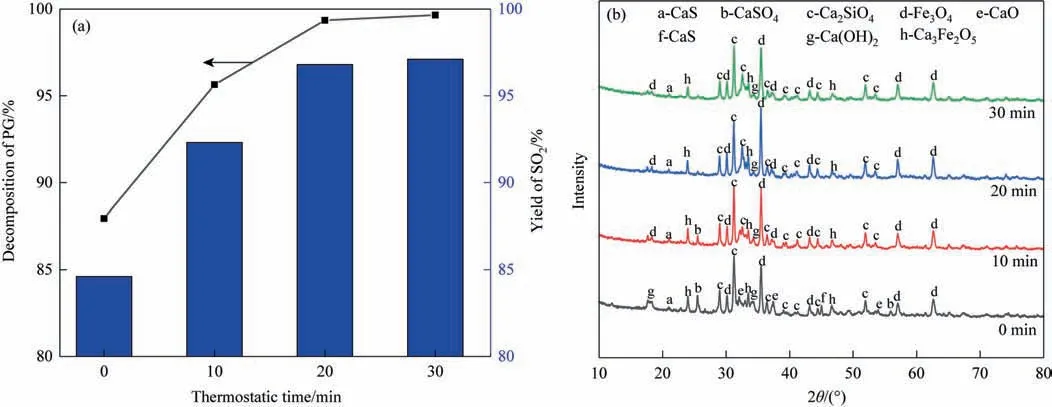
Fig.7.(a) Effects of thermostatic time on product composition;(b) XRD patterns of the product (CS/PG=1,Coke/PG=0.06,1100 °C).
The XRD pattern in Fig.7(b) shows that the typical CaSO4and CaS peaks in the product progressively drop and vanish as the time increases from 0 to 20 min which is mainly due to the reaction of reaction (12).In addition,the characteristic peaks of CaO also disappears completely at 10 min,probably due to the reaction of reactions(13)–(14).The XRD patterns of the products at 30 min are not much different from those at 20 min,showing that the PG is almost completely decomposed at 20 min.Therefore,in order to obtain the target product SO2,the thermostatic time is controlled at 20 min.
3.7.Reaction mechanism and process flow
Based on thermodynamic simulations and experimental conclusions,It is determined that CS-assisted coke reduction PG may greatly enhance PG decomposition rate and SO2yield,and the reaction mechanism is shown in reactions (17)–(21).Firstly,part of CaSO4is reduced by Fe2SiO4to produce SO2.Fe2SiO4is oxidized to Ca3Fe2Si3O12and Fe2O3.Subsequently,Ca3Fe2Si3O12and Fe2O3are reduced to Fe3O4in the presence of coke,and CaSiO3is produced.CaSO4,CaS and CaSiO3reacted at high temperature to form Ca2SiO4.Meanwhile,CaSO4is reduced to CaS by coke.The liquid phase eutectic model predicts that Fe2O3considerably facilitates the interaction of CaS with CaSO4to form Ca2Fe2O5,while releasing SO2.The total reaction equation is shown in reactions(22)–(24).
The results of ΔGand lgKof reaction(5)and reaction(17)–(24)are displayed in Fig.8.Both reaction (17)–(21) continue spontaneously at 1100 °C,as shown in Fig.8(a) and (b).The addition of Fe2SiO4can greatly lower the decomposition temperature of PG as seen in Fig.8(c) and (d).Compared to reaction (5),which can only happen spontaneously at 849 °C,the spontaneous reaction temperatures of reactions (22)–(24) are substantially lower.
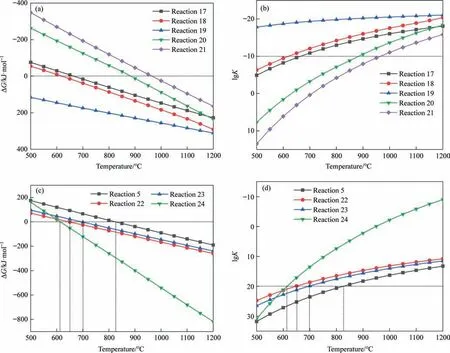
Fig.8.ΔG and lgK versus temperature for each reaction: (a)-(b) reactions (17)–(21);(c)-(d) reactions (22)–(24).
Based on this,a process flow for decomposing PG with coke and CS is proposed.The process can alleviate the environmental pollution caused by PG and produce chemical products such as SO2,Fe3O4,Ca2SiO4and Ca2Fe2O5.Fig.9 depicts the method’s flow chart.The method comprises four steps including mixing of raw materials,roasting in an inert atmosphere,separation of gaseous products and separation of solid products.The following conclusions are drawn after optimizing the reaction conditions.The CS/PG mass ratio is 1,the coke/PG mass ratio is 0.06 at 1100°C.The decomposition rate of PG under these conditions is 99.35% and the yield of SO2is 96.81%.In addition,Fe2SiO4is converted to Fe3O4,Ca2Fe2O5and Ca2SiO4.For the separation of Fe3O4,it can be carried out by magnetic separation method.The remaining Ca2Fe2O5and Ca2SiO4can be used as construction materials.For the separation of CO2and SO2,a catalyst such as V2O5can be used to oxidize SO2to SO3to prepare sulfuric acid.In this process,CO2does not undergo any reaction[34].CO2can be used as a refrigerant,etc.The gas mixture is then separated by passing it through concentrated sulfuric acid to prepare fuming sulfuric acid.

Fig.9.Schematic diagram of PG-coke-CS roasting process.
4.Conclusions
A new process for SO2preparation from solid waste copper slag(CS) assisted coke reduction of phosphogypsum (PG) is proposed.The effect of process conditions on PG decomposition is investigated by experimental and kinetic analysis combined with Fact-Sage thermodynamic calculations.It is found that adding CS to coke and PG system can increase PG decomposition rate and SO2yield while lowering the PG decomposition temperature.For example,when the CS/PG mass ratio increases from 0 to 1,PG decomposition rate increases from 83.38% to 99.35%,SO2yield increases from 78.62% to 96.81%,and PG decomposition temperature decreases from 992.4 °C to 949.6 °C.The optimal reaction parameters are CS/PG (mass ratio) of 1,coke/PG of 0.06 at 1100 °C for 20 min with 99.35% PG decomposition rate and 96.81% SO2yield.Finally,reaction mechanism and a process flow for decomposing PG with coke and CS is proposed.Fe2SiO4in CS react with CaSO4and C to form Ca2SiO4,the 2-valent iron is oxidised to Fe3O4and Ca2Fe2O5,and the+6-valent sulphur in CaSO4is reduced to SO2.
CRediT Authorship Contribution Statement
Dong Ma:Formal analysis,Data curation,Investigation,Writing– original draft.Qinhui Wang:Supervision.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Qinhui Wang reports financial support was provided by The school-enterprise cooperation projects.
Acknowledgements
Thanks for the financial support from the school-enterprise cooperation projects (2019-KYY-508101-0078).
Supplementary Material
XRD patterns of PG and CS(Fig.1S);PG decomposition rate and SO2yield for PG alone and CS/PG (CS/PG=1,no coke added)(Fig.2S).Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.07.008.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- The Al2O3 and Mn/Al2O3 sorbents highly utilized in destructive sorption of NF3
