Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
2024-04-22QiyaoYangXiaobinQiQinggangLyuZhipingZhu
Qiyao Yang ,Xiaobin Qi,Qinggang Lyu,Zhiping Zhu
1 North China Electric Power University,Beijing 102206,China
2 Institute of Engineering Thermophysics,Chinese Academy of Sciences,Beijing 100190,China
3 Shanxi Key Laboratory of Coal Flexible Combustion and Thermal Conversion,Datong 037000,China
4 University of Chinese Academy of Sciences,Beijing 100049,China
5 Shanxi Engineering Research Center of Coal Clean,Efficient Combustion and Gasification,Datong 037000,China
Keywords: Circulating fluidized bed Coal gasification fly ash Steam activation Pore structure evolution Fluidization activation
ABSTRACT Coal gasification fly ash (CGFA) is an industrial solid waste from the coal circulating fluidized bed (CFB)gasification process,and it needs to be effectively disposed to achieve sustainable development of the environment.To realize the application of CGFA as a precursor of porous carbon materials,the physicochemical properties of three kinds of CGFA from industrial CFB gasifiers are analyzed.Then,the activation potential of CGFA is acquired via steam activation experiments in a tube furnace reactor.Finally,the fluidization activation technology of CGFA is practiced in a bench-scale CFB test rig,and its advantages are highlighted.The results show that CGFA is characterized by a high carbon content in the range of 54.06%–74.09%,an ultrafine particle size (d50:16.3–26.1 μm),and a relatively developed pore structure (specific surface area SSA:139.29–551.97 m2∙g-1).The proportion of micropores in CGFA increases gradually with the coal rank.Steam activation experiments show that the pore development of CGFA mainly includes three stages: initial pore development,dynamic equilibrium between micropores and mesopores and pore collapse.The SSA of lignite fly ash (LFA),subbituminous fly ash (SBFA) and anthracite fly ash(AFA)is maximally increased by 105%,13% and 72% after steam activation;the order of the largest carbon reaction rate and decomposition ratio of steam among the three kinds of CGFA is SBFA>LFA>AFA.As the ratio of oxygen to carbon during the fluidization activation of LFA is from 0.09 to 0.19,the carbon conversion ratio increases from 14.4% to 26.8% and the cold gas efficiency increases from 6.8% to 10.2%.The SSA of LFA increases by up to 53.9% during the fluidization activation process,which is mainly due to the mesoporous development.Relative to steam activation in a tube furnace reactor,fluidization activation takes an extremely short time(seconds)to achieve the same activation effect.It is expected to further improve the activation effect of LFA by regulating the carbon conversion ratio range of 27%–35% to create pores in the initial development stage.
1.Introduction
Coal gasification,a process to convert coal into fuel gas or syngas,has the potential to allow efficient and clear utilization of coal.This thermal conversion process achieves a high-value utilization of lignite,subbituminous coal,and bituminous coal [1–3].However,with the popularization of gasification technology,the production of coal gasification fly ash (CGFA) increases accordingly.
Industrial coal gasifiers are typically categorized as fixed bed,fluidized bed and entrained flow [4–7].Circulating fluidized bed(CFB) coal gasification technology has the advantages of high gas–solid contact efficiency,strong adaptability to raw material types and stable operation;therefore,it occupies a large share in the market[8–10].For this gasification technology,the gasification agent is used as a fluidization medium to pass through the bed layer from the bottom to the top of the gas distribution plate so that the raw material is gasified with violent stirring and back mixing [11].When the generated gas leaves the fluidized bed layer,much of the CGFA and incompletely reacted carbon particles are entrained,leaving the gasifier from the top of the furnace;a small amount of slag particles are discharged from the bottom of the furnace [12].Methods for making good use of the residual carbon of CGFA are related to the economy of CFB gasification.
CGFA is an industrial solid waste that has a high carbon content,it is different from circulating fluidized bed fly ash(CFBFA),usually used as building materials[13,14].In recent years,the combustion has been the main disposal method of CGFA[15].Ren and Bao[16]conducted the combustion experiment of CGFA on a 5 t∙d–1circulating CFB facility.The results showed that CGFA was stably burned in a specially designed CFB furnace without auxiliary fuel.However,during the combustion,there existed an issue of NOxand SO2emission.At the equivalent fuel–air-ratio of 2,the NOxemission was the lowest,195 mg∙m-3.At the equivalent fuel–air-ratio of 1.1,the SO2emission was the lowest,2100 mg∙m-3.Liet al.[17] used thermogravimetric to study the combustion process of CGFA in an oxygen-enriched atmosphere.The results showed that an oxygen-enriched combustion improved ignition and burnout performances and combustion stability.As the O2concentration increased from 30% to 70%,the activation energy of CGFA increased.Liuet al.[18] studied the flameless mode of CGFA in a 30 kW self-preheating combustion test rig.The results showed that a stable flameless combustion of CGFA was easily established,and in the combustion zone,the image luminosity was uniform and no obvious flame fronts was observed.The NOxemission was lowered down a lot and the general emissions were less than 100 mg∙m-3with the lowest value less than 50 mg∙m-3.Furthermore,the scholars also studied the co-combustion of CGFA and coal gangue (CG).Zhanget al.[19] studied their co-combustion characteristics and coupling mechanism.The results indicated that the combustion intensity of CGFA was more intense than that of CG.As the mass ratio of CGFA increased,the value of the combustion characteristic index gradually increased.The nitrogen-containing gaseous products mainly included NH3and NO.The coupling release of NH3was derived from the conversion of char-nitrogen.The mass ratio of CGFA and CG at 2:1 was suggested in actual co-combustion application.To maximize the utilization of CGFA,CGFA was also used for soil improvement,ecological fertilization and water restoration [20].
Concerning the special feature of developed porous structure,CGFA is suitable as a precursor for porous materials[21–24].There are two reported strategies for activating carbon: physical activation(using the activating agent of CO2or steam)and chemical activation (using the activating agent of KOH,H3PO4,NaOH,or ZnCl2)[25–28].Liet al.[29]studied the pore and chemical structure characteristics of CGFA.The results showed that CGFA had a welldeveloped pore structure,abundant active sites and carboncontaining functional groups.Yanget al.[30] analyzed the physicochemical properties of ten kinds of CGFA.It was found that the pore structure of CGFA was relatively developed,and part of the CGFA had the basic conditions to be used as porous carbon materials.The CGFA contained abundant amorphous carbon structure,thus the CGFA had a good reactivity and a potential to improve pore structure through further activation.Qiet al.[31] conducted the fluidization activation experiments of CGFA in a bench-scale CFB and established a theoretical model for the activation.The results showed that ultrafine particle size and developed pore structure greatly increased the diffusion rate of active components into CGFA particles.Under strong diffusion,increasing temperature is an important method for achieving the rapid and effective activation of CGFA in a limited time.Qiet al.[32]also studied the gasification of anthracite in a pilot-scale CFB gasifier and the pore structure evolution of CGFA during steam activation.After activation,theSSA(it is defined as the specific surface area)of CGFA maximally increased by 63%,reaching 452 m2∙g-1.Through the whole experiment,the periodic law of pore development was obtained,and the activation process was divided and quantified according to the amount of carbon loss to realize the optimal activation of CGFA.Nevertheless,research on the preparation of porous carbon materials using CGFA is still limited,especially that involving specific processes and equipment.
Against the aforementioned background,this study will first demonstrate the feasibility of the high-value utilization of CGFA as porous materialsviasteam activation experiments.Based on this,a fluidization activation technology for this utilization was experimentally studied in a bench-scale CFB test rig.
2.Methodology and Experiment
2.1.Materials and methodology
Three kinds of CGFA sampled in the fabric filter from different industrial CFB gasifiers were employed.The CGFA samples are originated from lignite,subbituminous coal,and anthracite,and they are named lignite fly ash(LFA),subbituminous fly ash(SBFA),and anthracite fly ash (AFA),respectively.
The proximate and ultimate analysis of the CGFA samples were performed according to the Chinese standards GB/T 212-2008,GB/T 214-2007,GB/T 476-2008 and GB/T 19227-2008.
The CGFA particle size distribution was measured by the wet method using a laser particle sizer (Analysette 22 NanoTec,FRITSCH,Germany).
The X-ray fluorescence (XRF) spectrometer of the ash was conducted in the experiment by using S8 TIGER (Bruker,Germany).The operating voltage was 20–60 kV,the operating current was 5–170 mA,the maximum output power was 4 kW,and the angle resolution was 0.001°.
The crystal phase composition was measured by an X-ray diffractometer (XRD,Bruker D2 PHASERX).The measurement was carried out in the range of the diffraction angle from 10° to 80°,with a scanning step size of 0.02° (tube voltage 40 kV,tube current 100 mA,Cu target and graphite monochromatic filter).
All samples were dried at 105°C for 12 h for further characterization.Then,the pore structure was measured by an automaticSSAand a pore size analyzer (ASAP2460,Micromeritics,USA).The working pressure was 0.1–0.15 MPa.TheSSAand pore size distribution were obtained by the Brunauer-Emmett-Teller (BET) method.
2.2.Steam activation experiments
Steam activation experiments of CGFA were conducted in a test rig of a vertical tube furnace,as illustrated in Fig.1.The test rig included a gas–water system,tube furnace reactor,and analyzer system.The reactor had an inner diameter of 26 mm and was designed with a porous plate with an aperture of less than 5 μm at the bottom for distributing the activating agent and loading the samples.Following the placement of samples into the reactor,a 50 ml∙min-1N2flow was introduced into the reactor to purge the exhaust air for 10 min.Afterward,the reactor was put into the furnace after the furnace increased to a target temperature(850,900,950,and 1000°C).At this time,a 25 ml∙h-1liquid water flow controlled by a double plunger pump was introduced.The liquid water was heated into superheated steam at the target temperature in the intake pipe.The apparent velocity of gas flow in the reactor exceeded the critical fluidization velocity of CGFA (approximately 0.026 m∙s-1),which placed CGFA particles in a fluidization state.The gas leaving the reactor was filtered and monitored online by a gas analysis.After a certain time,the experiment was terminated by turning off the double plunger pump.The reactor was removed from the furnace and purged continuously by N2until the samples were cooled to room temperature.

Fig.1.Schematic diagram of the vertical tube furnace test rig.
Due to the fine particle size of CGFA,the apparent gas flow rate in the reactor was lower than 0.083 m∙s-1;however,some CGFA particles still escaped.We assumed that the escaped CGFA had the same activation process as the residue in the reactor under these conditions;during steam activation experiments,the percentage of ash content of CGFA (wash,CGFA,%),the percentage of ash content of activated samples (wash,ACGFA,%),the percentage of carbon content of CGFA (wC,CGFA,%),the percentage of carbon content of activated samples (wC,ACGFA,%),the inlet flow rate of N2during the experiment (,L∙min–1),the actual yield of CGFA(YACGFA,%),the carbon conversion ratio (X,%),the generation rate of gaseous producti(ri,mol∙min-1),the carbon reaction rate of CGFA (rC,mol∙min-1) and the decomposition ratio of steam(,%) were estimated by Eqs.(1)–(5) [33].
2.3.Fluidization activation experiments
Fluidization activation experiments were conducted in a benchscale CFB test rig,as shown in Fig.2.The system had two main reaction units: a CFB unit and a downward gasifier.The CFB unit included a riser,cyclone,and seal loop.A detailed description of this test rig can be found in our previous study [31].

Fig.2.Schematic diagram of the CFB test rig(1:riser,2:cyclone,3:loop seal,4:downward gasifier,5:gas cooler,6:bag filter,7:stack,8:air compressor,9:oxygen cylinder,10: steamer,11: gas mixer I,12: gas mixer II,13: water pump,14: water tank,15: feeder,16: electric furnace I,and 17: electric furnace II).
The variable studied was the molar ratio of oxygen to carbon,and three ratios(0.09,0.15,and 0.19 mol∙mol-1)were investigated.Under stable conditions,the average feeding rate of CGFA was approximately 10 kg∙h-1;the main operating parameters were shown in Table 1.Each test was operated stably for more than 1 h.
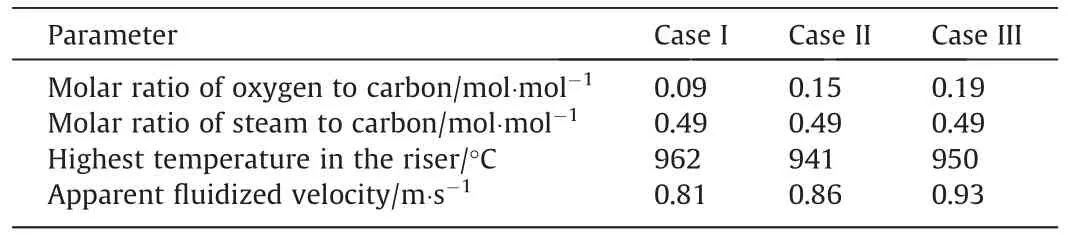
Table 1 Main operating parameters during the experimental process
During fluidization activation experiments,the dry gas yield(m3∙kg–1),the carbon conversion ratio (XC,%) and cold gas efficiency (ηgas,%) were estimated by Eqs.(6)–(8),in Eq.(8),the HHV represented high heating value [32].
whereFCGFAwas the feeding quantity of CGFA,kg∙h–1;wherewC,CGFAwas the percentage of carbon content of CGFA,%;wherewas the volume concentration of CO/CO2/CH4,%;where HHVgas/HHVCGFAwas the high heating value of gas/CGFA,kcal∙m–3.
3.Results and Discussion
3.1.Physicochemical property
The proximate and ultimate analysis results of the three kinds of CGFA are shown in Table 2.The moisture content of CGFA is extremely low,and it is near zero for LFA and SBFA.The volatile content is as low as 2.10%–3.67%.The fixed carbon content is in the range of 54.06%–74.09%.Therefore,CGFA is characterized by low moisture,low volatile matter and high carbon content,indicating that CGFA can be used as a potential raw material to prepare porous carbon materials [34,35].

Table 2 Proximate and ultimate analysis of samples
The particle size distribution is shown in Fig.3.The particle size of CGFA is extremely fine,mostly below 100 μm.The cumulative volume fractions of 10%,50%,and 90% (d10,d50andd90) are 3.3–11.6 μm,16.3–26.1 μm,and 51.0–74.7 μm,respectively.Most of the particles are concentrated below 100 μm.According to Geldart[37],the particle classification method shows that some samples are C-type particles.
The results of composition of CGFA are shown in Fig.4.Among the three kinds of CGFA in Fig.4(a),the ash composition is very different.SBFA contains more alkali metal and alkaline earth metal components that will catalyze the gasification reaction [38–40],including CaO,MgO and Na2O;its total amount reaches 46.4%.The AFA contains more inert components,such as SiO2and Al2O3,and the sum of the two is more than 78%.From Fig.4(b),it is found that the mineral composition of CGFA is also different among the three samples.The LFA contains oldhamite,the SBFA contains nepheline and lime,the main phases in the AFA samples are quartz and mullite.The difference in ash composition is mainly related to raw coal [27].
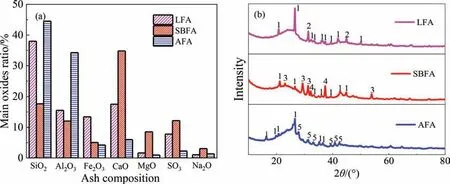
Fig.4.Results of (a) ash composition and (b) XRD patterns of samples (1: quartz,2: oldhamite,3: nepheline,4: lime,5: mullite).
The N2adsorption and desorption isotherms of the samples are shown in Fig.5(a).According to the definition of the International Union of Pure and Applied Chemistry (IUPAC) [41],it is inferred that the isotherm of AFA is type I with a large slope at lowP/P0;the isotherms of LFA and SBFA are type IV (i.e.,typical mesopore structure),without an obvious plateau period.The overlap presented in the isotherms of LFA and AFA at lowP/P0values indicates that there are semi-open micropores[42,43].The two types of pore structures mentioned above are indirectly described by the corresponding pore size distributions,as indicated by Fig.5(b).

Fig.5.Results of N2 adsorption test of samples: (a) N2 adsorption and desorption isotherms and (b) pore size distribution.
The characteristic parameters of pore structure,which are calculated according to the isotherms,are shown in Table 3.CGFA has a developed pore structure with a specific surface area (SSA)above 139.29 m2∙g-1,a pore volume (Vtot) above 0.16 cm3∙g-1and an average pore diameter(dave)of 2.38–5.59 nm.In particular,SBFA has the most developed pore structure (SSA: 551.97 m2∙g-1andVtot: 0.60 cm3∙g-1).This CGFA basically meets the conditions for use as porous carbon [44].

Table 3 Characteristic parameters of pore structure according to nitrogen adsorption test
3.2.Steam activation characteristics
Fig.6 shows the variations in the volume concentration of the gas component with time during the activation of CGFA.The main gas components during the activation include H2,CO,CO2,and CH4.These concentrations follow a trend of first rapidly increasing and then slowly decreasing.With an increase in temperature,the trend is accelerated.
According to the gas components,it is inferred that the reactions listed in Table 4 occur during the activation and that R1 dominates the activation process.Since R1 is endothermic,increasing the temperature promotes the forward reaction.The presence of CO2may be attributed to the water gas shift reaction R2,which occurs under the catalysis of alkali and alkaline earth metal(AAEM)in coal ash[31].For the three CGFA cases,the CO2concentration in the gas is only positively correlated with the AAEM content inside CGFA (Fig.4(a)) in the order of SBFA,LFA,and AFA.

Table 4 Main reactions involved during the experimental process [12,45]
Fig.7 shows the variations in the carbon reaction rate and decomposition ratio of steam.The two parameters partly reflect the activation intensity.Clearly,the activation process is enhanced with an increase in temperature,and the three CGFA cases are in the order of SBFA,LFA,and AFA (from strong to weak).At 1000 °C,the maximum carbon reaction rates for SBFA,LFA,andAFA are 10.8 × 10-3,8.3 × 10-3and 6.5 × 10-3mol∙min-1,corresponding to maximum steam decomposition rates of 60.9%,51.5%,and 31.9%,respectively.The strongest activation for SBFA may be due to the enrichment of AAEM inside,especially alkali metals(Na2O content in the SBFA ash reaches 3%,as shown in Fig.4(a)),acting as an activation catalyst [39].In addition,the parabola-like trends of the carbon conversion ratio and steam decomposition ratio are related to the evolution of the internal pore structure[46,47].
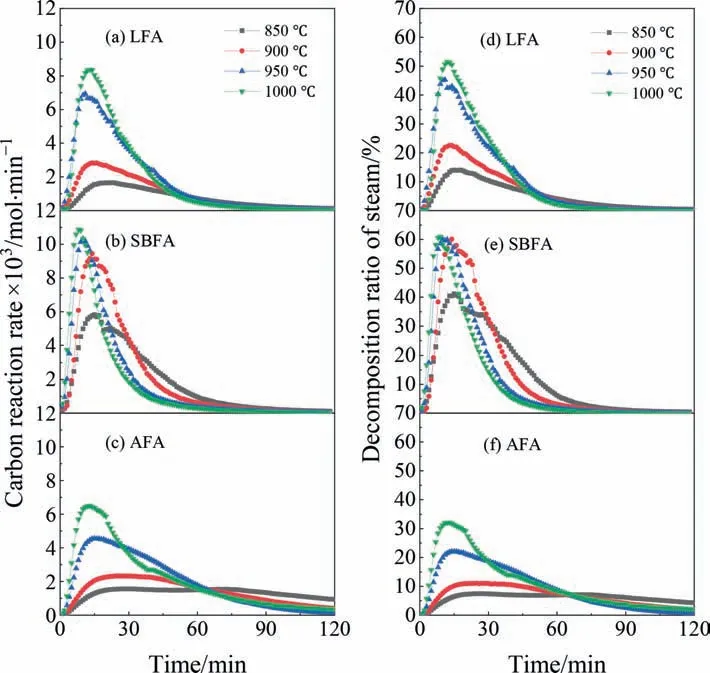
Fig.7.Variations in (a)–(c) carbon reaction rate and (d)–(f) steam decomposition ratio with time during the steam activation of CGFA.
3.3.Pore structure evolution during steam activation
Fig.8(a)–(c) shows the variations in theSSAof activated CGFA with time.For the three CGFA samples,the overall variations inSSAare similar,first increasing,then basically remaining constant,and finally decreasing.After activation,theSSAis maximally increased by 105% for LFA (285 m2∙g-1),by 13% for SBFA(623 m2∙g-1),and by 72% for AFA (452 m2∙g-1).This finding indicates that CGFA has the potential to develop pore structures through steam activation.

Fig.8.Variations in SSA of activated CGFA with time during the steam activation: (a) LFA,(b) SBFA,and (c) AFA.
Due to the greatest physical activation potential,the case of LFA is focused on to reveal the evolution of the pore structure during activation.Fig.9 shows the variations inSmicandSextof the activated LFA with time.As activation proceeds,bothSmicandSextbasically follow the dynamic law of developing and collapsing.Relative toSext,Smiccontributes more to the increase inSSA.Before the collapse of the pore structure,there is a stage in which the micropores and mesopores fluctuate dynamically;however,the totalSSAremains basically unchanged.Overall,increasing the temperature significantly accelerates the activation progress of CGFA without weakening the activation potential of CGFA.This result provides an important theoretical basis for the rapid and effective activation of CGFA.From the perspective of the whole life cycle of the pore structure,the evolution of the pore structure is divided into three stages [32]: initial development,dynamic equilibrium,and final collapse.
Fig.9.Variations in Smic and Sext of activated LFA with time during the steam activation: (a) 850 °C,900 °C,(c) 950 °C,and (d) 1000 °C.
More detailed pore distribution characteristics are obtained by calculating the proportions ofSmicandSextinSSA,as shown in Fig.10.As shown in Fig.10(a),the pore structure in the LFA is dominated by mesopores with a proportion up to 90.64%.Within the first 15 min during the activation,the ratio of micropores gradually develops from 9.36% to 57.43%.For the SBFA in Fig.10(b),however,as the activation time increases,the ratio of mesopores gradually increases from 49.09% to 72.66%,indicating that the increase inSSAis mainly due to the contribution of mesopores.For Fig.10(a)and (b),the proportion of micropores re-increases at 30 and 45 min,maybe due to excessive activation resulting in the formation of new fragmented micropores after pore fragmentation.For AFA in Fig.10(c),as the activation time increases,the number of micropores gradually increase and then decrease,the number of mesopores begin to increase.It means that both of micropores and mesopores development contribute to the improvement inSSA.Regarding the overall activation process,the pore development of LFA occurs mainly due to the contribution of micropore development,the pore development of SBFA occurs mainly due to the contribution of mesopore development,and the pore development of AFA contributes to both.It is concluded that the kind of CGFA greatly impacts the evolution of pore structure during activation,which may be related to coal rank and gasification conditions[30,48].
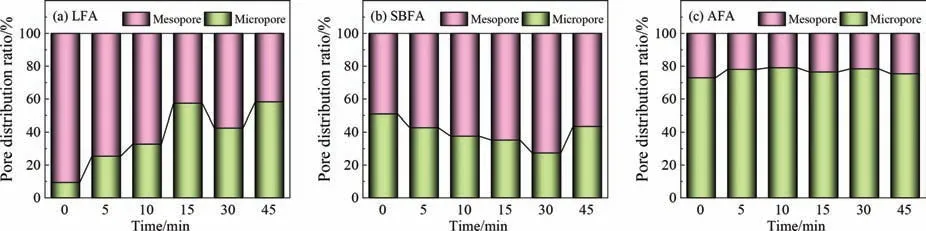
Fig.10.Pore structure distribution ratios of samples at 850 °C: (a) LFA,(b) SBFA,and (c) AFA.
Fig.11 shows the relationships betweenSSAand the carbon conversion ratio for all cases.Under different activation temperature conditions,the changes inSSAwith carbon conversion ratio are regular: first increase,then remain unchanged briefly,and finally decrease.This regularity corresponds well to the three-stage evolution of the pore structure,it is indicated that the activation process is quantified into three stages according to the carbon conversion ratio [32].For LFA,the carbon conversion ratios corresponding to the three stages are 0–35%,35%–52%,and >52%,respectively.For SBFA,the ratios are 0–22%,22%–56%,and >56%,respectively.For AFA,the ratios are 0–15%,15%–65% and >65%,respectively.To achieve the pore development of CGFA,it is presumed that the carbon conversion ratio of LFA,SBFA and AFA shall be separately controlled within 35%,22%,and 15%.
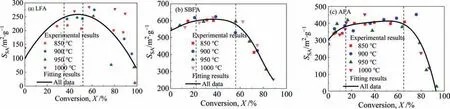
Fig.11.Variations in the SSA values of activated CGFA with carbon conversion ratio for all cases of samples: (a) LFA,(b) SBFA and (c) AFA.
3.4.Fluidization activation characteristics
Due to the largest physical activation potential,LFA is selected for the fluidization activation experiment.Fig.12 illustrates the volume concentration of the gas component and the HHV of thegas during activation.During the activation process,CO2,CO,and H2are dominant in the gas;the volume concentration of CH4is low,below 0.3%.According to experience,at such a low ratio of oxygen to carbon,the concentration of combustible components and HHV of the gas either increase with increasing ratio,or first increase to the peak and then decrease.If not considering the influences of other factors,the regularity presented in Fig.12(a)is obviously unreasonable.By considering the actual operating conditions listed in Table 1,this irrationality is caused by the difference in operating temperature among the three cases.At a ratio of 0.15(Case II),the operating temperature is the lowest among the three cases,approximately 941 °C.In this case,the exothermic reaction C-O2shifts toward the direction of combustion with generating more CO2,and the endothermic reaction C-H2O is suppressed[31].This influence of lower temperature can be reflected by the final volume concentration of the gas component and HHV,as shown in Fig.12(a).
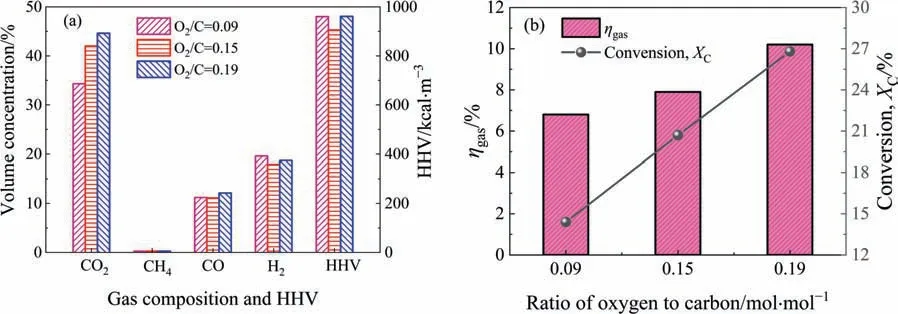
Fig.12.Gasification characteristics of the fluidization activation of LFA: (a) volume concentration of gas component and high heating value of gas (HHV) and (b) cold gas efficiency (ηgas) and carbon conversion ratio (XC).
However,the carbon conversion ratio and cold gas efficiency increase with increasing ratio of oxygen to carbon,as shown in Fig.12(b).As the ratio of oxygen to carbon increases from 0.09 to 0.19,the carbon conversion ratio increases from 14.4% to 26.8%,and the cold gas efficiency increases from 6.8%to 10.2%.This result shows that the fluidization activation process of LFA obtains partial gasification benefits.
Fig.13 illustrates the results of the pore structure of activated LFA during fluidization activation.Relative to raw ash,the pore structure of the activated LFA becomes more developed after fluidization activation.When evaluating the activation effect according to theSSA,there seems to be an optimal ratio of oxygen to carbon.At a ratio of 0.15 mol∙mol-1,theSSAof activated LFA is the highest,reaching 214 m2∙g-1,which is 53.9% higher than that of raw ash.This finding indicates that CGFA is effectively activated in this complex fluidization environment.
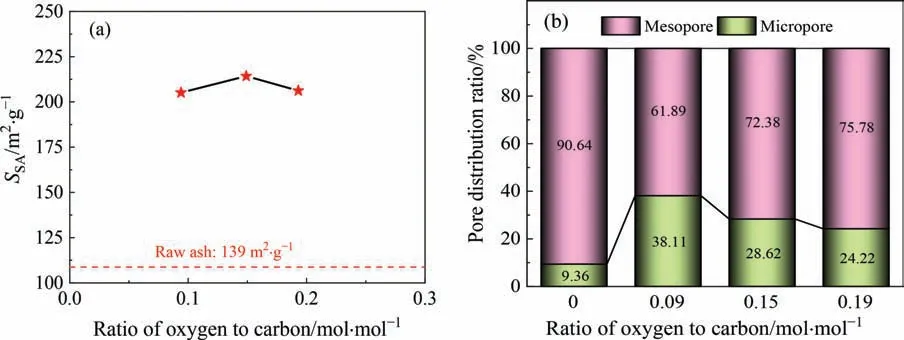
Fig.13.Pore structures of activated LFA during fluidization activation:(a)variations in SSA with ratio of oxygen to carbon and(b)variations in pore distribution ratios with ratio of oxygen to carbon.
As illustrated in Fig.13(b),with an increase in the ratio of oxygen to carbon,the proportion of micropores decreases from 38.11%to 24.22%;this phenomenon indicates that increasing the ratio is not conducive to the formation of micropores but conductive to the formation of mesopores.Hence,the evolution of the pore structure during fluidization activation obviously differs from that during steam activation.The intervention of oxygen is mainly responsible for this phenomenon because of its extremely rapid reaction with carbon.
3.5.Comparison between fluidization and steam activation
The actual fluidization activation of CGFA is extremely complex since it involves the effectiveness of the activation reaction under the action of various active media,including O2and H2O,and issues relevant to mass transfer and flow between the gas and solid phases.By comparison,steam activation in the tube furnace is simpler and more effective.The latter provides theoretical reference for the analysis of the former.
Fig.14(a) shows the variations in theSSAof the activated LFA with time during fluidization and steam activation.In the fluidization activation process,the activation time of LFA is on the order of seconds;in the steam activation process,the activation time is controlled on the order of minutes or hours.Under the same activation effect,the time required for fluidization activation is approximately two orders of magnitude shorter than that of steam activation.Clearly,the presence of O2significantly accelerates the activation progress of CGFA.
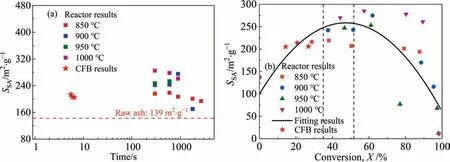
Fig.14.Comparison of SSA of activated LFA between fluidization and steam activation:(a) variations in SSA with time and(b) variations in SSA with carbon conversion ratio.
Fig.14(b) shows the variations in theSSAof the activated LFA with the carbon conversion ratio during fluidization and steam activation.It is observed that there are few differences between the fluidization activation results and theoretical activation curves fitted according to the results of steam activation.This phenomenon indicates that under experimental conditions,the activation potential is not obviously weakened by the violent reaction of C-O2.In addition,according to the three-stage division standard,the fluidization activation process of LFA is still in the development stage of the pore structure.It is expected to further improve the activation effect of LFA by regulating the carbon conversion ratio range of 27%–35% to generate pores in the initial development stage.
4.Conclusions
In this paper,it is aimed to demonstrate the feasibility of the high-value utilization of CGFA.Through the study of physicochemical properties,steam activation and fluidization activation,the main conclusions are as follows:
(1) The three kinds of CGFA are characterized by a high carbon content in the range of 54.06%–74.09%,an ultrafine particle size (d50: 16.3–26.1 μm),and a relatively developed pore structure (SSA: 139.29–551.97 m2∙g-1).The proportion of micropores in CGFA increases gradually with increasing coal rank.
(2) The kind of raw coal affects the development of pores during the activation.TheSSAvalues of LFA,SBFA and AFA are maximally increased by 105%,13% and 72% after steam activation.From the perspective of pore development,the activation of CGFA mainly includes three stages:initial pore development stage,dynamic equilibrium stage and pore collapse stage.The carbon reaction rate and decomposition ratio of steam of SBFA is the highest among the three kinds of CGFA.
(3) The fluidization activation process of LFA obtains partial gasification benefits.As the ratio of oxygen to carbon increases from 0.09 to 0.19,the carbon conversion ratio increases from 14.4% to 26.8%,and the cold gas efficiency increases from 6.8% to 10.2%.TheSSAof LFA increases to 53.9% during the fluidization activation process,it is mainly caused by mesoporous development.Relative to steam activation,fluidization activation takes an extremely short time on the order of seconds to achieve the same activation effect.It is expected to further improve the activation effect of LFA by regulating the carbon conversion ratio range of 27%–35% to generate pores in the initial development stage.
Data Availability
The authors do not have permission to share data.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the Special Research Assistant Fund Project of Chinese Academy of Sciences.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
- The Al2O3 and Mn/Al2O3 sorbents highly utilized in destructive sorption of NF3
