Rational surface charge engineering of haloalkane dehalogenase for boosting the enzymatic performance in organic solvent solutions
2024-04-22YinWuYanSun
Yin Wu,Yan Sun
Department of Biochemical Engineering,School of Chemical Engineering and Technology and Key Laboratory of Systems Bioengineering and Frontiers Science Center for Synthetic Biology (Ministry of Education),Tianjin University,Tianjin 300350,China
Keywords: Surface charge engineering Organic solvent resistance Molecular dynamics simulation Haloalkane dehalogenase
ABSTRACT Biocatalysis in organic solvents (OSs) has numerous important applications,but native enzymes in OSs often exhibit limited catalytic performance.Herein,we proposed a computation-aided surface charge engineering strategy to improve the catalytic performance of haloalkane dehalogenase DhaA in OSs based on the energetic analysis of substrate binding to the DhaA surface.Several variants with enhanced OS resistance were obtained by replacing negative charged residues on the surface with positive charged residue (Arg).Particularly,a four-substitution variant E16R/E93R/E121R/E257R exhibited the best catalytic performance (five-fold improvement in OS resistance and seven-fold half-life increase in 40% (vol)dimethylsulfoxide).As a result,the overall catalytic performance of the variant could be at least 26 times higher than the wild-type DhaA.Fluorescence spectroscopy and molecular dynamics simulation studies revealed that the residue substitution mainly enhanced OS resistance from four aspects:(a)improved the overall structural stability,(b) increased the hydrophobicity of the local microenvironment around the catalytic triad,(c)enriched the hydrophobic substrate around the enzyme molecule,and(d)lowered the contact frequency between OS molecules and the catalytic triad.Our findings validate that computationaided surface charge engineering is an effective and ingenious rational strategy for tailoring enzyme performance in OSs.
1.Introduction
The application of organic solvents (OSs) as reaction media for biocatalysts is indispensable for a broad range of applications in the chemical industries.Enzymatic catalysis in OSs has several advantages,including increased substrate solubility,enhanced selective process,and suppression of unwanted by-product formation [1].Therefore,non-aqueous biocatalysis in the presence of OSs dramatically lowers the cost of processes[2].However,the reduced activity,poor stability,and even deactivation of the enzymes by OSs largely limit biocatalytic reactions in neat OSs and aqueous OS mixtures.
Many techniques have been used to explore the interaction between enzymes and OSs from different aspects.For example,conformational changes and structural mobility of enzymes can be experimentally obtained from spectroscopies such as X-ray [3],circular dichroism(CD)[4],or nuclear magnetic resonance[5].The dynamics of the related solvent shell in aqueous media can be determined adequately by ultrafast fluorescence [6],nuclear Overhauser effect [7],and IR spectra [8].Furthermore,molecular dynamics(MD)simulations generate a complementary approach to explore the connection between protein dynamics and the stability of enzymes in OSs,which has been proven to exhibit high consistency with numerous experimental measurements [9].With the help of these techniques,OSs were found to deactivate enzymes mainly via five different mechanisms: (a) conformational changes[10],(b) loss of bound water molecules [11],(c) competitive inhibition[12],(d) interfacial inactivation[13],and(e)thermodynamic stabilization of the substrate ground state[14].
Numerous conventional methods,such as immobilization [15],chemical modification [16],chemical cross-linking [17],and additives[18],have been used to strengthen the stability of enzymes in OSs.In addition,protein-engineering strategies have succeeded in improving the properties of enzymes [18].As a strategy of protein engineering,protein surface design can not only significantly enhance the stability of enzymes [19] but also change the optimal conditions for enzyme catalysis [20].Besides,protein surface design has been validated to play an important role in boosting the OS resistance of enzymes [21].
Haloalkane dehalogenases (HLDs) catalyze the hydrolytic cleavage of carbon-halogen bonds in the halogenated compounds[22],the by-products and environmental pollutants commonly produced in the OS-based industries,like leather,paper pulp,printing,pharmaceutical,paint,and coating[23].As a result,HLDs have potential applications in the bioremediation of toxic environmental pollutants,as a biosensor for environmental pollutants,the decontamination of chemical warfare agents,and for protein labeling and cell imaging for protein analysis [24-26].However,HLDs (including DhaA,the HLD from Rhodococcus rhodochrous)destabilize and reduce the catalytic efficiency at high concentration of OSs,which greatly limits their broader applications [27].
In order to overcome the above problems,various traditional approaches have been used to strengthen the stability of DhaA in OSs [28,29].However,no research on enhancing the OS resistance of DhaA through in silico-guided protein surface design has been reported.Therefore,this work has proposed a computation-aided surface charge engineering strategy to improve the OS resistance of DhaA by manipulating computational MD simulations to analyze the contribution of residues for substrate binding.Based on the computations,we have found that replacing negative charged residues with positive charged residue (Arg) would result in favorable outcomes.The generated residue-substituted variants were then verified by computations with different programs,and their catalytic performance,including stability and kinetic characterization,in solutions of different OS concentrations were investigated to identify the multi-substitution variant with maximum improvement in OS resistance.The mechanism behind improvement in catalytic performance in OS solutions was studied by fluorescence spectroscopy and MD simulations.
2.Materials and Methods
2.1.Computational section
2.1.1.Molecular dynamics simulation
The crystal structure of DhaA wild type(WT)was obtained from the Protein Data Bank(PDB ID 4HZG)[30].The DhaA WT structure was used as the template to generate the recombinants (E16R/E93R/E121R/E257R) by the FoldX software [31].The original conformation of DhaA and variant were used in the following MD simulations.
MD simulations and analysis were performed with the GROMACS 5.1.4 simulation software [32].The Amber99SB*-ILDN force field was used for the simulations of DhaA and variant in water and dimethylsulfoxide (DMSO) cosolvent [33].The restrained electrostatic potential charges of 4-bromomethyl-6,7-dimethoxycoumarin(COU-Br) and DMSO were assigned using the Multiwfn 3.7,and thereby the force fields were constructed for them[34].Structures were solvated into a dodecahedron box of SPC water molecules with a minimal distance of 1.4 nm from the edge of the box to the enzyme molecule.According to the experimental conditions (40%(vol)DMSO),the simulation systems were filled with water molecules in the water-only system,DMSO molecules and water molecules in the DMSO system,respectively.Besides,10 COU-Br molecules were randomly added into the systems when calculating the interactions between enzyme and substrate molecules.Counterions Na+and Cl-were used to neutralize the total net charge of the systems,and all the resulting systems have a net charge of zero.Prior to an MD simulation,the energy minimization of every system was accomplished by 50000 steps of steepest descent to remove atomic overlap as well as severely distorted bond lengths,bond angles,and torque.The systems were then heated to 30°C for 100 ps through velocity-rescale under the constant amount of substance (N),volume (V),and temperature (T) (NVT ensemble).Then,the systems were allowed to be equilibrated for 100 ps at a constant temperature (30°C) and constant pressure (1 atm,1 atm=101.325 kPa) using a Parrinello-Rahman pressure coupling(NPT ensemble).After proper minimizations and equilibrations,three independent MD simulations for each set of conditions were conducted in the NPT ensemble for 100 ns The electrostatic interaction was disposed of using a particle-mesh Ewald algorithm,which was also used to describe the long-range electrostatics.A 2.0 fs of timestep was used alongside the Verlet algorithm.The simulations were performed with a 1.4 nm cutoff of the neighboring atom list,Coulomb potential energies,and Lennard-Jones potential.VMD 1.9.3 was applied for visualization and analysis[35].
2.1.2.In silico generation of variants and stability analysis
The contribution of each residue for substrate(COU-Br)binding was calculated by the MM/PBSA software[36].The conservation of surface negative charged residues (Glu and Asp) was evaluated by ConSurf-DB [37].Residue sites with score >7 were regarded as highly conserved and removed from the candidate site list.Then,the remaining residue sites were replaced by positive charged residue(Arg),and variants were verified by calculation with mCSM[38],FoldX [31],and I-Mutant 3.0 [39].It should be noted that positive values computed by mCSM as well as I-Mutant 3.0 indicate stabilization,but negative values from FoldX indicate stabilization.
2.2.Experimental section
2.2.1.Enzyme preparation
DhaA WT was expressed in Escherichia coli(E.coli)and purified following a protocol in Ref.[27].Briefly,the expression strain E.coli BL21 (DE3) was plated on Luria-Bertani (LB) agar supplemented with 50 μg∙ml-1kanamycin.After overnight incubation,a single colony was inoculated into 200 ml of fresh LB medium and incubated at 37°C,220 r∙min-1until the optical density at 600 nm (OD600) reached 0.6-0.8.Then,protein overexpression was induced by adding 0.5 mmol∙L-1isopropyl β-D-1-thiogalactopyranoside,and the culture was continued for 18 h at 16°C.The cells were harvested by centrifugation(5000 g,30 min)at 4°C,and the cell pellet was stored at -80°C for further use.
The cell pellet was thawed and then disrupted by ultrasonication in lysis buffer containing 20 mmol∙L-1Na2HPO4-HCl,500 mmol∙L-1NaCl,and 10 mmol∙L-1imidazole (pH 7.5).Centrifugation of the cell homogenate was performed at 10000 g for 30 min at 4°C to expulse the cell debris,followed by filtration through a 0.45 μm syringe filter.Cleared lysates containing Histagged targeted proteins were purified by metal-chelate affinity chromatography on an AKTA purifier system equipped with Ni-NTA Sepharose resin (GE Healthcare,Germany),washed with the lysis buffer to wipe off the non-specific adsorption proteins,and finally eluted using elution buffer with 20 mmol∙L-1Na2HPO4-HCl,500 mmol∙L-1NaCl,and 300 mmol∙L-1imidazole(pH 7.5).Protein fractions were further purified by size-exclusion chromatography on an AKTA purifier system equipped with a Superdex200 Increase 10_300 GL column (GE Healthcare,Germany) against desalting buffer (50 mmol∙L-1Na2HPO4-NaH2PO4,pH 8.0).All purification processes were operated at 4°C.The purity of the obtained proteins was assayed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis,and the concentrations were determined with the BCA Protein Assay Kit (Solarbio,China).
The variants were constructed by stepwise site-directed mutagenesis with the plasmid pET28a(+)-DhaA purchased from GENEWIZ (China) as the template.The polymerase chain reaction primers are listed in Table S1 in Supplementary Material.E.coli JM109 and E.coli BL21(DE3)strains were used for gene cloning and expression,respectively.DhaA variants were expressed and purified following the same protocol as DhaA WT.
2.2.2.Enzymatic assays
The enzyme activity of DhaA or its variants was determined in microtiter plates using COU-Br as the substrate by a previously reported method with slight modification[40].In each well,freshly prepared substrate COU-Br solution (final concentration,100 μmol∙L-1) was added into PB buffer (50 mmol∙L-1,pH 8.0) or OS solution (40% (vol) DMSO) containing a purified enzyme (final concentration,4 μg∙ml-1)at 30°C,and fluorescence intensity was measured from the top using excitation/emission monochromators set to wavelengths 345/437 nm.Before the measurement,the microtiter plate was shaken for 2 s,and then the increase in fluorescence intensity was monitored at regular time intervals.Activity assays were performed at least in triplicate.
After 30 min incubation in PB buffer(50 mmol∙L-1,pH 8.0)or in OS solution(40%(vol)DMSO),the residual activity of DhaA(WT or variants)was measured.The OS resistance of DhaA(WT or variants)was termed as the residual activity ratio after 30 min incubation in the presence and absence of DMSO.
The enzymatic kinetics with DhaA WT or variants was determined by measuring the initial catalytic reaction rate at varying substrate concentrations.In the reaction,protein concentration of the enzyme sample was kept at 4 μg∙ml-1.The Michaelis constant(Km) and the maximum reaction rate (Vmax) were determined by fitting the measured reaction rates to the Michaelis-Menten equation (Eq.(1)) using Origin 2016 software.
where v is the reaction rate (μmol∙L-1∙s-1),[S] is the substrate concentration (μmol∙L-1),Vmaxis the maximum rate(μmol∙L-1∙s-1),and Kmis the Michaelis constant (μmol∙L-1).
Kinetics of OS inactivation was monitored by incubating the purified DhaA protein in the presence of 40% (vol) DMSO as cosolvent at 30°C.Half-life(min)was defined as the time required for an enzyme to reach the residual activity of one half of its initial value.
2.2.3.Fluorescence and CD spectroscopy
To investigate the influence of OSs on the conformational change,DhaA and variants in the absence or presence of different DMSO concentrations were incubated at 30°C for different times before measurement.Then enzymes were loaded into a quartz cuvette with path length 1.0 cm,which was excited at 270 nm for fluorescence spectroscopy experiments.The emission spectra were monitored in the range of 290-420 nm at a scanning speed of 200 nm∙min-1.The slit widths were 5.0 nm for both excitations and emissions.Emission baseline corrections were performed with a blank buffer scan.The reported spectra represented the average of three accumulations subtracted from the corresponding background fluorescence.
The far-UV CD spectra were conducted on the J-810 CD spectrometer (JASCO,Japan) in a quartz cell (1 mm) under nitrogen atmosphere.The continuous scanning was measured over the wavelength ranging from 190 to 260 nm in aqueous buffer and 40%(vol) DMSO with response time of 1 s and bandwidth of 2 nm at a scanning speed of 100 nm∙min-1,and the ellipticity data of the enzymes were collected.The average spectra of three scans were reported,from which the spectrum of a buffer blank was subtracted.
3.Results and Discussion
3.1.MD simulation suggested a surface charge engineering strategy
MD simulations were analyzed by the MM/PBSA software,resulting in the contribution of each residue for substrate binding,and residues with |ΔGbind|≥1.0 kcal∙mol-1are listed in Fig.S1.Interestingly,all the positive charged residues (Arg and Lys,especially Arg) exhibited negative ΔGbindvalues,reflecting the attraction between them and the substrate.By contrast,all the negative charged residues (Glu and Asp) possessed positive ΔGbindvalues,indicating their repulsion with the substrate.The greater the absolute value of ΔGbind,the stronger the interaction (attraction or repulsion).Based on the MD simulation results,a surface charge engineering strategy of replacing negative charged residues(Glu or Asp) on the surface with positive charged residue (Arg) was proposed(Fig.1(a)).Arginine was chosen as the mutated residue for its strong attraction with substrate molecules (Fig.S1) and the conducive effects on boosting OS resistance reported in literature[41].This strategy was expected to strengthen the interaction between substrate molecules and the enzyme and improve the stability of the enzyme in OSs.

Fig.1.(a)The computation-aided surface charge engineering strategy,replacing specific Glu or Asp on the surface with Arg.(b)The specific activity of DhaA single substitutions in 40% (vol) DMSO relative to DhaA WT after 30 min incubation.(c) The specific activity of DhaA recombinants with 2-5 substitutions in 40% (vol) DMSO relative to DhaA WT after 30 min incubation.(d)The OS resistance of the DhaA variants relative to DhaA WT.The OS resistance of DhaA(WT or variants)was defined as the residual activity ratio after 30 min incubation in the presence and absence of DMSO.Error bars represent the standard deviations from three independent experiments.
The surface charge engineering strategy was implemented following the protocol described below.First,negative charged residues(Glu or Asp)on the surface of DhaA were chosen,and the highly conserved sites evaluated by ConSurf-DB were removed from the candidate site list.The rest sites were then substituted with Arg,and the ΔΔG values of the obtained variants were computed by mCSM,FoldX,and I-Mutant 3.0.It should be noted that mCSM and I-Mutant 3.0 return a positive value if substitution generates a stabilizing variant,and the greater the value of the positive figure,the stronger the stability.While the feedback result of FoldX is opposite to them.Nineteen stable variants were identified by mCSM (Table S2),and 12 stable variants were further verified from these 19 variants by using FoldX (Table S3).Next,Gibbs free energy changes of these 12 variants were further computed by the I-Mutant 3.0 web server.As a result,six stable variants (E16R,E93R,E121R,D139R,E257R,and D266R) predicted by all the three predictors were obtained(Table 1),and applied for the subsequent experimental verification.
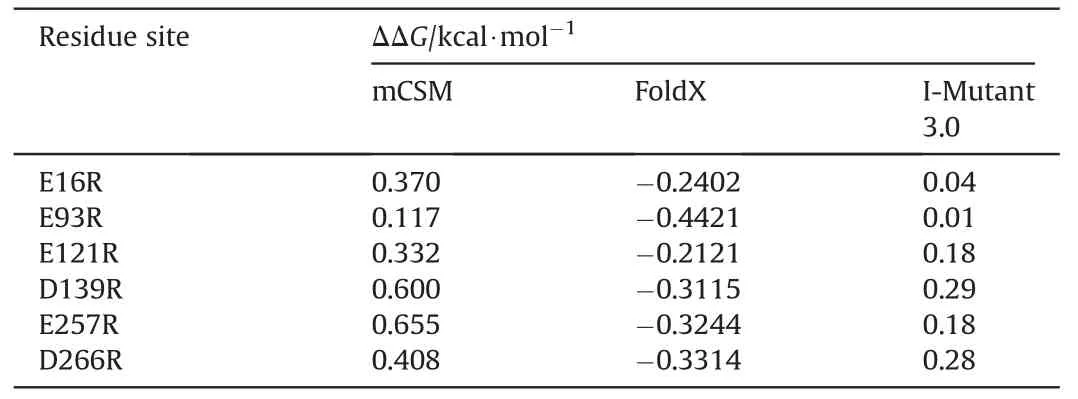
Table 1 The ΔΔG values of stable variants predicted by mCSM,FoldX,and I-Mutant 3.0
3.2.The surface charge engineering strategy yielded highly OS resistance DhaA variants
DhaA WT and six single-substitution variants were successfully constructed and expressed in E.coli BL21 (DE3).The purified enzymes were used to explore the catalytic activity in aqueous solutions of different DMSO concentrations.Most of the singlesubstitution variants had comparative specific activity to DhaA WT in buffer,except D139R and D266R (Fig.S2).Furthermore,the specific activity of the single-substitution variants (except D266R)was greatly improved in 40%(vol)DMSO,and the catalytic activity of the E257R variant was 1.76 times higher than that of DhaA WT(Fig.1(b)).The results proved that replacing specific Glu or Asp on the surface with Arg created the variants with enhanced catalytic activity of DhaA in DMSO.
Because of its highest catalytic activity in different reaction conditions,the variant E257R was chosen as the starting variant to combine the beneficial substitutions (E16R,E93R,E121R,and D139R,which showed enhanced activity in DMSO,Fig.1(b)) to design multi-substitution variants.Fifteen 2-to 5-substitution variants were successfully constructed and expressed,and the purified enzymes were used to study the catalytic activity in buffer(Fig.S3) and 40% (vol) DMSO (Fig.1(c)).As shown in Fig.1(c),the multi-substitution variants had similar to or significantly improved specific activity over DhaA WT in 40%(vol)DMSO.Particularly,the catalytic activity of E16R/E257R,E121R/E257R,E16R/E93R/E257R,E16R/E121R/E257R,E93R/E121R/E257R,and E16R/E93R/E121R/E257R variants was over 2.0 times higher than DhaA WT.
The OS resistance of the 21 single-and multi-substitution variants in 40% (vol) DMSO is given in Fig.S4.Among them,the six multi-substitution variants (E16R/E257R,E121R/E257R,E16R/E93R/E257R,E16R/E121R/E257R,E93R/E121R/E257R,and E16R/E93R/E121R/E257R) had dramatically enhanced OS resistance(2.5-5.0 times higher than DhaA WT),and E16R/E93R/E121R/E257R had the highest OS resistance,which was 5.0 times that of DhaA WT (Fig.1(d)).The results indicated that the combination of the beneficial substitutions resulted in the boosted catalytic performance of DhaA in DMSO as compared with the singlesubstitution variants.
3.3.Investigation of OS resistance profiles and kinetic characterization
The stability of DhaA WT and the six variants of dramatically enhanced activity relative to DhaA WT in 40%(vol)DMSO solution(E16R/E257R,E121R/E257R,E16R/E93R/E257R,E16R/E121R/E257R,E93R/E121R/E257R,and E16R/E93R/E121R/E257R,Fig.1(c)) was determined in 40%(vol)DMSO.As shown in Fig.S5,inactivation of all the enzymes occurred with the extension of incubation time,but the variants showed significantly higher stability than DhaA WT.The residual activity of DhaA WT reduced to only about 30% of the original activity after 90 min incubation,while the six variants retained more than 50% of the original activity under the same condition(Fig.S5a).It was noteworthy that the residual activity of E16R/E93R/E121R/E257R kept nearly 45% of the initial activity even after 480 min incubation.Moreover,as shown in Fig.S5b,the residual activity of the six variants was far higher than that of DhaA WT during the whole incubation.Particularly,the specific activity of E16R/E93R/E121R/E257R at 270 min was still comparable to the initial activity of DhaA WT.Based on the data shown in Fig.S5b,the overall catalytic performance of E16R/E93R/E121R/E257R was calculated to be at least 26 times higher than DhaA WT.
The one-step inactivation dynamics model [29] was used to fit the inactivation process of DhaA WT and the six variants,and the half-life (T1/2,min),the parameter of kinetic stability representing the time required to halve residual activity,was calculated according to the following equation:
where kdrepresents the deactivation rate constant (min-1),corresponding to the negative slope of the dependence of the natural logarithm of relative activity on time (Eq.(3)).
where α(t)is the residual specific activity(μmol∙min-1∙mg-1),α(0)is the initial specific activity (μmol∙min-1∙mg-1),and t is the incubation time (min).
The half-life values in 40% (vol) DMSO of DhaA WT and the six variants predicted from the fitting are listed in Table 2.As shown in the table,the half-life of the six variants in 40% (vol) DMSO was much longer than that of DhaA WT and extended with the increase of the quantity of substituted sites.E16R/E93R/E121R/E257R had the longest half-life in 40% (vol) DMSO (385 min),which was 7.0 times that of DhaA WT.The results illustrated that replacing negative charged residues on the surface with positive charged residue (Arg) created the DhaA variants of significantly strengthened stability in DMSO solution,and the combination of beneficial substitutions further enhanced the positive effect of residue substitution.Furthermore,owing to the dramatically boosted enzymatic performance as compared with other relevant works(Table S4),the four-substitution variant was proven to be an efficient and suitable enzyme for application in OS-containing aqueous solutions.

Table 2 Comparison of half-life in 40% (vol)DMSO for DhaA WT and variants
The intrinsic fluorescence spectra of proteins are principally derived from two aromatic amino acid residues,tyrosine and tryptophan,and the change in the intrinsic fluorescence emissions can be used to monitor the change in tertiary structures.Therefore,the conformational changes of DhaA WT and the six variants in different DMSO concentrations were examined by fluorescence spectroscopy.As shown in Fig.2,the emission maxima (λmax) of DhaA WT and the variants showed red shifts with increasing DMSO concentration,representing that the overall structures of enzymes became unstable under the influence of DMSO[42].However,the λmaxof the variants showed blue shifts compared with DhaA WT at 40% (vol) or 80% (vol) DMSO(Fig.2(b) and (c)),indicating that the overall structure of the six variants was more compact than DhaA WT in the co-solvent solutions,verifying the enhanced structural stability of the variants[42].The blue shifts of λmaxalso suggested that the enzyme molecules after residue substitution were less aggregation-prone in DMSO solutions than DhaA WT [43].

Fig.2.Fluorescence spectra of DhaA WT and variants in different DMSO concentrations.The black lines represented the emission maxima difference of DhaA WT and variants;AU,adsorbance unit.
In order to further explore the influence of DMSO on the tertiary structure of enzymes,the fluorescence spectroscopy of DhaA WT and the best variant E16R/E93R/E121R/E257R was examined with increasing incubation time up to 20 h in 40% (vol) DMSO (Fig.3).The λmaxof DhaA WT was red shifts with increasing incubation time,indicating that the overall structure of DhaA WT gradually became loose,consequently resulting in the weakened structural stability[42].In the meantime,the relative fluorescence intensity of DhaA WT decreased with increasing incubation time,which may suggest the microenvironment alteration of DhaA WT.The resulting conformational change may not be conducive to the enzymatic catalysis,so the residual catalytic activity of DhaA WT reduced with increasing incubation time (Fig.S5).By contrast,the λmaxand the relative fluorescence intensity of E16R/E93R/E121R/E257R kept almost unchanged with increasing incubation time,indicating that the tertiary structure of E16R/E93R/E121R/E257R retained stable even after incubation in 40%(vol)DMSO for 20 h.The fluorescence results showed that the surface charge engineering strategy used in this work weakened the adverse effect of DMSO on the tertiary structure of DhaA and strengthened the structural stability of the enzyme molecule in the DMSO solution.

Fig.3.Fluorescence spectra of DhaA WT and variant E16R/E93R/E121R/E257R in 40%(vol)DMSO as a function of time.The black lines represented the emission maxima difference at different times.
Far-UV CD spectroscopy can provide the secondary structures of a protein.The secondary structure changes of DhaA WT and the best variant E16R/E93R/E121R/E257R in aqueous buffer and in 40%(vol)DMSO were thus explored by examining the CD spectroscopy(Fig.S6).The far-UV CD spectroscopy of DhaA WT in aqueous buffer was similar to that of E16R/E93R/E121R/E257R,verifying that the secondary structure did not significantly change after the residue substitutions.However,the presence of DMSO strongly interfered with the protein signal because of its high absorbance in the far-UV spectral region [44,45],so it was impossible to monitor the secondary structures using CD spectroscopy with adequate accuracy in the DMSO solution.Despite the interference of DMSO,the spectra above 220 nm of DhaA WT showed more significant change than that of the four-substitution variant,indicating that the presence of DMSO caused less negative influence on the secondary structure of the four-substitution variant.In other words,the CD data demonstrated that the surface charge engineering improved the stability of enzyme's secondary structures in the OS solution.
The enzymatic kinetics of DhaA WT and the best variant E16R/E93R/E121R/E257R in 40%(vol)DMSO are shown in Fig.S7,and the kinetic parameters are listed in Table 3.The smaller Kmvalue and significantly higher kcatvalue were observed for the variant E16R/E93R/E121R/E257R than DhaA WT,generating a 2.0-fold increase in the catalytic efficiency (kcat/Km).The results represented the significant improvement of the enzyme-substrate affinity and catalytic activity of the variant E16R/E93R/E121R/E257R.The high catalytic efficiency may be attributed to the enhanced interaction between enzyme and substrate after residue substitution,which may enrich the hydrophobic substrate around the enzyme molecule.Besides,the change in the conformation of enzyme molecules by high-concentration OS solution was reported to cause the loss of catalytic activity [46],so another important cause for the high catalytic efficiency may be the boosted structural stability after residue substitution,which reduced the negative impact of DMSO on the catalytic activity of DhaA.

Table 3 Kinetic parameters of DhaA WT and variant E16R/E93R/E121R/E257R in 40% (vol)DMSO
3.4.Computational analysis revealed the molecular mechanism of improved OS resistance of the DhaA variant
MD simulations of DhaA WT and the best variant E16R/E93R/E121R/E257R in the absence or presence of 40% (vol) DMSO were utilized to reveal the molecular mechanism behind the improved OS resistance of DhaA variants.The structure of variant E16R/E93R/E121R/E257R was generated by the FoldX software using the DhaA WT structure as the template,and the overall structure of them is shown in Fig.S8.MD simulation trajectories describe the conformational changes in protein during simulation and were used to evaluate the structural stability of DhaA WT and the variant.As can be seen in Figs.S9(a),S10(a),S11(a),there was no obvious difference in root mean square deviation(RMSD)of the Cα atoms,radius of gyration (Rg)value,and root mean square fluctuation(RMSF) of the Cα atoms per residue between DhaA WT and variant in the absence of 40% (vol) DMSO.There were also similar Cα-RMSD values in the presence of 40%(vol)DMSO(Fig.S9(b));however,the variant kept low Rgvalue during the whole simulation(Fig.S10(b)).In contrast,DhaA WT exhibited higher mean value of Rgwithin the simulated time,representing that the structure of DhaA WT in the high-DMSO solution fluctuated more violently than that of the variant.This indicated that replacing negative charged residues on the surface with the positive charged residue(Arg)benefited in the stabilization of the overall structure of DhaA.As shown in Fig.S11(b),the average RMSF values of DhaA WT were higher than those of the variant in several regions,implying a significant change in the overall structure of DhaA WT,which should be responsible for its poor OS resistance.As for the variant,the RMSFs in the corresponding regions kept relatively lower than those in DhaA WT,reflecting its structural robustness.
The time-averaged solvent accessible surface area (SASA) was calculated for further exploration about the molecular mechanism of the enhanced OS resistance of the variant(Fig.S12).The variant E16R/E93R/E121R/E257R exhibited similar values of time-averaged total,hydrophobic,and hydrophilic SASA with DhaA WT in the absence or presence of DMSO,indicating that the residue substitution did not appreciably affect the overall exposure degree of the enzyme molecule.Next,the time-averaged SASA of specific residues,i.e.,the substituted sites and the catalytic triad (Asp106,Glu130,and His272),was computed to evaluate the influence of residue substitution on the local microenvironment of the enzyme molecule(Fig.S13).The substituted sites of the variant E16R/E93R/E121R/E257R exhibited higher time-averaged SASA values in the absence or presence of DMSO(Fig.S13(a)and(b)),illustrating that the substituted sites were more exposed after residue substitution.The higher exposure degree after residue substitution may be attributed to the longer side chain of Arg compared to Glu(Fig.S8).As for the catalytic triad,there was no obvious correlation between the time-averaged SASA value and the residue substitution in the absence of DMSO (Fig.S13(c)).Nevertheless,the time-averaged SASA values of the catalytic triad in the presence of DMSO reduced greatly after the residue substitution (Fig.S13(d)).This may be due to the structural change caused by the“remote effect”that occurred frequently in protein engineering [47],although the catalytic triad inside the enzyme molecule had no direct relationship with the substituted sites.The lower exposure degree of the catalytic triad resulted in the more hydrophobic microenvironment,which was favorable to the binding of hydrophobic substrate and finally reflected in the reduced Kmvalue (increased substrate affinity) of the variant (Table 3).
The solvation properties were then examined in the presence of DMSO,including the hydration level around the substituted sites,the OS solvation level around the substituted sites,the hydration level around the catalytic triad,and the OS solvation level around the catalytic triad.The hydration level was defined as the number of water molecules,whose O atom was within 0.35 nm distance cutoff of any non-hydrogen atom of specific residues of DhaA,and the OS solvation level was defined as the number of DMSO molecules,whose S2 atom was within 6.8 Å(1 Å=0.1 nm)distance cutoff of any non-hydrogen atom of specific residues of DhaA.The hydration level around the substituted sites did not exhibit appreciable changes (Fig.4(a)).However,the local OS solvent level around the substituted sites of the variant was higher than that found in DhaA WT (Fig.4(b)).At the same time,the calculated result of the time-averaged frequency of contacts between the substituted sites and OS molecules indicated that the substituted sites of the variant exhibited higher OS contact frequency (Fig.5).The higher OS solvation level and contact frequency with OS molecules implied that the local microenvironment around the substituted sites became more hydrophobic after residue substitution,leading to the strengthened hydrophobic interactions between DhaA and hydrophobic substrate,and thus enriching the hydrophobic substrate around enzyme molecule.The radial distribution function (RDF) of substrate molecules relative to the DhaA surface was calculated to explore the distribution of substrate around enzyme molecule(Fig.6).For DhaA WT,the substrate tended to distribute homogeneously throughout the system.However,the substrate molecules were more likely to be distributed around the variant molecule,resulting in the higher substrate concentration around enzyme molecule.This would be one of the causes for the high catalytic efficiency of the variant (Table 3).
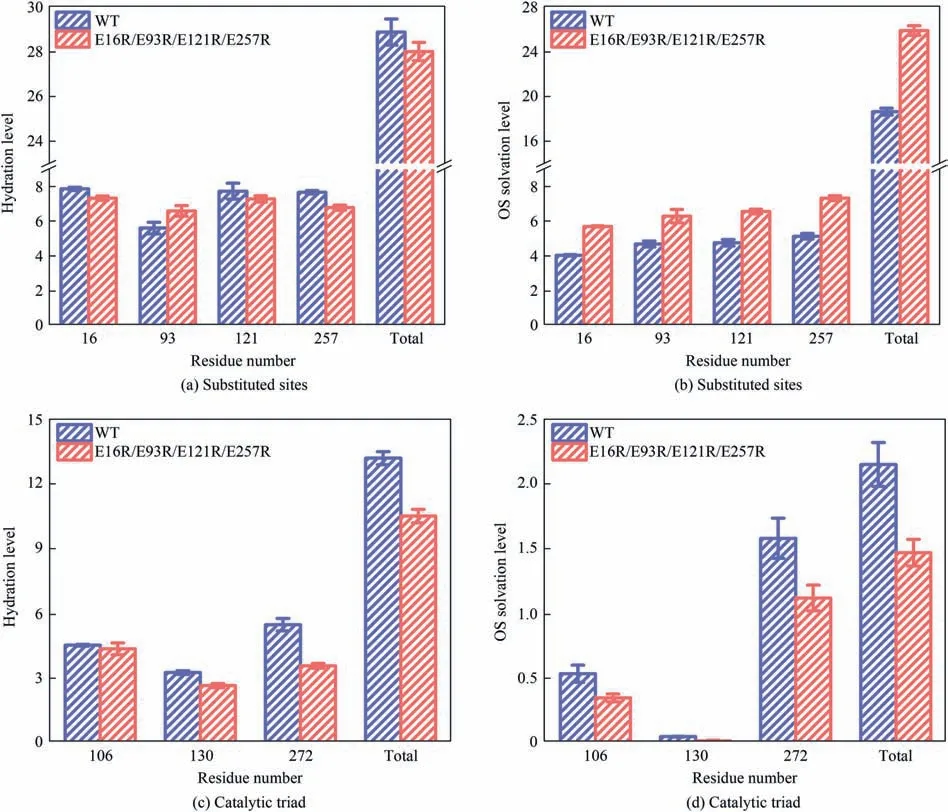
Fig.4.Hydration level around(a)substituted sites and(c)catalytic triad of DhaA WT and the variant E16R/E93R/E121R/E257R in 40%(vol)DMSO averaged over the last 40 ns of MD trajectories.Water molecules whose O atom was within 3.5 Å distance cutoff of any non-hydrogen atom of DhaA were described as the first hydration shell and the number of water as hydration level.OS solvation level around(b)substituted sites and(d)catalytic triad of DhaA WT and variant E16R/E93R/E121R/E257R in 40%(vol)DMSO averaged over the last 40 ns of MD trajectories.DMSO molecules whose S2 atom was within 0.68 nm distance cutoff of any non-hydrogen atom of DhaA were described as the OS layer and the number of DMSO as OS solvation level.Error bars represent the standard deviations from three independent MD simulation runs.

Fig.5.Time-averaged frequency of contacts between the DhaA specific amino acids and DMSO molecule in 40%(vol)DMSO.Residue-DMSO contact was defined as residue-DMSO distance being 0.25 nm or less.Contact frequency was calculated over the last 40 ns from three independent MD simulation runs.The ramp was colored from white to red to indicate the change of residue-DMSO contact frequency from low to high.

Fig.6.The RDF of substrate on the DhaA surface in 40% (vol) DMSO was evaluated as the COU-Br bromine atom around the DhaA residues.RDF was calculated over the last 40 ns from three independent MD simulation runs.
Lower hydration level around the catalytic triad was observed after the residue substitution (Fig.4(c)),indicating the increase in the hydrophobicity of the local microenvironment,which was consistent with the simulation result of time-averaged SASA(Fig.S13(d)).It was noteworthy that the residue substitution also reduced the OS solvation level around the catalytic triad(Fig.4(d)),and the OS contact frequency of His272 was less than half that before the residue substitution(Fig.5).The lower OS solvation level and OS contact frequency weakened the adverse interactions between OS molecules and the catalytic triad of DhaA.In addition,the internal-atomic distance between the catalytic triad of the variant was more stable than that of DhaA WT (Fig.S14),which may be attributed to the“remote effect”caused by the residue substitution.The improved structural stability of the catalytic triad also reduced the negative impact of OS molecules.
4.Conclusions
Based on the energetic analysis of substrate binding to DhaA,we have proposed a surface charge engineering strategy to enhance DhaA performance in OS solutions.It was implemented by replacing negative charged residues (Glu or Asp) on the surface with positive charged residue (Arg).System computational and experimental studies obtained six single-substitution and 15 multisubstitution variants.It was found that most of the residuesubstitution variants exhibited boosted catalytic activity and OS stability as compared to DhaA WT.In particular,the variant E16R/E93R/E121R/E257R showed the best OS resistance and half-life in 40%(vol)DMSO solution,which were five-fold and seven-fold that of DhaA WT,respectively.Fluorescent spectroscopy confirmed the improved structural stability of residue-substitution variants,in consistence with the MD simulation results in the presence of DMSO.Moreover,computational studies on DhaA WT and the best variant E16R/E93R/E121R/E257R indicated that the residuesubstitution of DhaA increased the hydrophobicity of the local environment around the catalytic triad,which could improve the substrate affinity,consequently enhancing the enzyme activity and catalytic efficiency.The enriched hydrophobic substrate around enzyme molecule and lower OS contact frequency of the catalytic triad after the residue substitution were two other important factors for the enhanced catalytic efficiency and enzyme stability in DMSO solutions.On the other hand,as a negative-charged enzyme at the reaction condition,the decreased negative charge and increased positive charge on the surface of DhaA variants enlarged the possibility of protein aggregation at high temperature,which limited the applications of DhaA variants at a very high temperature.Nonetheless,the surface charge engineering strategy was demonstrated effective for protein stabilization and provided a sturdy enzyme variant toward biocatalysis in OSs at mild reaction temperature.It is anticipated that this surface charge engineering strategy can be extended to improve the catalytic performance of other α/β-hydrolases in OSs.
CRediT Authorship Contribution Statement
Yan Sun:Investigation,Project administration,Resources,Supervision,Writing-original draft,Writing-review&editing.Yin Wu:Data curation,Formal analysis,Investigation,Methodology,Software,Visualization,Writing -original draft.
Declaration of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Data will be made available on request.
Acknowledgements
This work was funded by the National Key Research and Development Program of China (2018YFA0900702).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.08.007.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of PrFexCo1-xO3/Mt catalyst and study on degradation of 2-hydroxybenzoic acid wastewater by catalytic wet peroxide oxidation
- Active MoS2-based electrode for green ammonia synthesis
- Long-term operation optimization of circulating cooling water systems under fouling conditions
- Effect of bubble morphology and behavior on power consumption in non-Newtonian fluids’ aeration process
- Preparation and properties of high-energy-density aluminum/boroncontaining gelled fuels
- Highly selective extraction of aromatics from aliphatics by using metal chloride-based ionic liquids
