Highly selective extraction of aromatics from aliphatics by using metal chloride-based ionic liquids
2024-04-22HuiYuXiaojiaWuChuanqiGengXinyuLiChencanDuZhiyongZhouZhongqiRen
Hui Yu,Xiaojia Wu,Chuanqi Geng,Xinyu Li,Chencan Du,Zhiyong Zhou ,Zhongqi Ren
State Key Laboratory of Chemical Resource Engineering,Beijing University of Chemical Technology,Beijing 100029,China
Keywords: Ionic liquid Aromatic hydrocarbon Aliphatic hydrocarbon Extraction
ABSTRACT The separation of aromatics from aliphatics is essential for achieving maximum exploitation of oil resources in the petrochemical industry.In this study,a series of metal chloride-based ionic liquids were prepared and their performances in the separation of 1,2,3,4-tetrahydronaphthalene (tetralin)/dodecane and tetralin/decalin systems were studied.Among these ionic liquids,1-ethyl-3-methylimidazolium tetrachloroferrate ([EMIM][FeCl4]) with the highest selectivity was used as the extractant.Density functional theory calculations showed that [EMIM][FeCl4] interacted more strongly with tetralin than with dodecane and decalin.Energy decomposition analysis of [EMIM][FeCl4]-tetralin indicated that electrostatics and dispersion played essential roles,and induction cannot be neglected.The van der Waals forces was a main effect in [EMIM][FeCl4]-tetralin by independent gradient model analysis.The tetralin distribution coefficient and selectivity were 0.8 and 110,respectively,with 10% (mol) tetralin in the initial tetralin/dodecane system,and 0.67 and 19.5,respectively,with 10%(mol)tetralin in the initial tetralin/decalin system.The selectivity increased with decreasing alkyl chain length of the extractant.The influence of the extraction temperature,extractant dosage,and initial concentrations of the system components on the separation performance were studied.Recycling experiments showed that the regenerated [EMIM][FeCl4] could be used repeatedly.
1.Introduction
Diesel,which a mixture that includes polycyclic aromatic hydrocarbons [1],is an important raw material in the chemical industry.However,polycyclic aromatic hydrocarbons in vehicle diesel decrease the cetane number[2].They can also produce oxycarbide and particulate matter [3],which cause severe environmental pollution.Diesel is rich in paraffins and cycloalkanes,and these are ideal raw materials for ethylene cracking and catalytic reforming,respectively [4].It is untoward that the aromatics were removed from non-aromatics in diesel via traditional distillation owing to their fairly approximate boiling points [5].The separation of aromatics from non-aromatic compounds is therefore a knotty work for the petrochemical industry[6].The conventional methods used to separate aromatics from aliphatics are liquid-liquid extraction[7],extractive distillation [8],and azeotropic distillation [9].Liquid-liquid extraction with low energy consumption and mild operating conditions is most commonly adopted.Classical organic solvents such as sulfolane,N-methylpyrrolidone (NMP),N-formylmorpholine,dimethyl sulfoxide,and tetraethylene glycol are used in this process [10].Nevertheless,these solvents usually appearing with low selectivity are often evaporable [11].An extractant with high selectivity and fine recyclable feature is favored in industrial processes.
Ionic liquids (ILs),which are usually regarded as organic salts comprising cations and organic or inorganic anions[12],have been receiving increasing attention in the recent decades [13] due to their strong dissolving capacities,low vapor pressures,and high thermal and chemical stabilities [14-16].They are used in many fields by tuning the chemical structures of the cation and anion to prepare targeted ILs with specified properties [17];therefore,ILs have potential applications in liquid-liquid extraction.Li et al.[18]studied the use of NMP,sulfolane,and N-formylmorpholine for separating 1-methylnaphthalene from dodecane,which suggested that 1-methylnaphthalene had the highest solubility in NMP and that sulfolane had the highest selectivity for 1-methylnaphthalene.Furthermore,it was indicated that the extractant-possessing planar construction was inclined to have higher solubility for 1-methylnaphthalene because of the smaller steric hindrance and the group with more electronegativity had higher selectivity for 1-methylnaphthalene.Finally,it was also found that the van der Waals (VDW) force made a major contribution between the three solvents and 1-methylnaphthalene by reduced density gradient and atoms in molecules analyses.Ge et al.[19]used a deep eutectic solvent containing tetrabutylphosphonium bromide and levulinic acid to separate 1,2,3,4-tetrahydronaphthalene (tetralin) from dodecane,indicating that the tetrabutylphosphonium cation could interact with tetralin through CH-π interaction by spatial distribution function with molecular dynamic simulation.It was suggested that π-π interaction was found between C=O bond of levulinic acid and tetralin,and no hydrogen bonds were detected between deep eutectic solvent(DES)and tetralin.Li et al.[20]used[Bz-DBU][NTf2]to separate tetralin from dodecane,and the results showed that the selectivity could reach 54.2 in the initial system with 50%(mol)tetralin,which suggested that this extractant had a certain degree of separation effect.Nevertheless,the anion of extractant contained fluorine,which was not friendly to the environment.Thus,it is necessary to explore a more suitable extractant.Previous research showed that because they can participate in π-π interactions,aromatics are more soluble than alkanes in NMP-[21]or imidazolium-based [22] ILs.In addition,the distribution coefficient and selectivity can be increased by using a metal chloride as the IL anion [23].
In this work,a series of metal chloride-based ILs were synthesized and screened for separating tetralin/dodecane and tetralin/decalin systems.The screening results showed that the best extractant was 1-ethyl-3-methylimidazolium tetrachloroferrate([EMIM][FeCl4]).The interactions between [EMIM][FeCl4] and tetralin,dodecane,and decalin were calculated by using the Gaussian 09 software.The results indicated that the interactions between[EMIM][FeCl4] and tetralin were stronger than those between[EMIM][FeCl4]and dodecane or decalin.The PSI4 program was used to determine which type of interaction predominated in [EMIM][FeCl4]-tetralin.It was inferred from the results that electrostatics and dispersion played primary roles,and the effect of induction was not negligible.Intermediate gradient model(IGM)analysis showed that VDW interactions were predominant in [EMIM][FeCl4]-tetralin.The alkyl chain length of the extractant,extraction temperature,extractant dosage,initial concentrations of system components,and regeneration times were researched.The results suggest that[EMIM][FeCl4]will be applied in the future in industry.
2.Experimental
2.1.Materials
NMP(CAS 872-50-4,>99%)and decane(CAS 124-18-5,>98.5%)were obtained from J&K Scientific(Beijing,China).Ethanol(CAS 64-17-5,>99.7%) was purchased from Sinopharm Chemical Reagents(Shanghai,China).Tetralin (CAS 119-64-2,>97%),dodecane (CAS 112-40-3,>98%),anhydrous tin(II)chloride(CAS 7772-99-8,>99%),and decalin(CAS 91-17-8,>99%)were acquired from the Shanghai Macklin Biochemical Co.,Ltd.(Shanghai,China).Anhydrous iron(III)chloride (CAS 7705-08-0,>98%) was obtained from the Shanghai Aladdin Biochemical Technology Co.,Ltd.(Shanghai,China).Zinc chloride (CAS 7646-85-7,>98%) was purchased from Energy Chemicals (Shanghai,China).1-Hexyl-3-methylimidazolium chloride ([HMIM][Cl],CAS 171058-17-6,>98%),1-ethyl-3-methyl imidazolium tetrachloroferrate ([EMIM][FeCl4],CAS 850331-04-3,>98%),1-butyl-3-methylimidazolium tetrachloroferrate ([BMIM][FeCl4],CAS 359845-21-9,>98%),1-hexyl-3-methylimidazolium tetrachloroferrate ([HMIM][FeCl4],CAS 887305-67-1,>98%),and 1-octyl-3-methylimidazolium tetrachloroferrate ([OMIM][FeCl4],CAS 887305-68-2,>98%)were acquired from the Shanghai Chengjie Chemical Co.,Ltd.(Shanghai,China).Hydrochloric acid aqueous solution(CAS 7647-01-0,36%(mass))was obtained from the Beijing Chemical Works(Beijing,China).
2.2.Preparation of metal chloride-based ionic liquids
[HNMP][MClx+1] was synthesized via a two-step method according to previous literature[24,25].A certain amount of NMP was added to ethanol in a flask,which was in an ice bath to avoid intense heat release.Then an equimolar HCl aqueous solution was added dropwise to the flask.The mixture was vigorously stirred at 45°C for 24 h.Ethanol and water were removed from the mixture under the vacuum of 0.08 MPa at 85°C with a rotary evaporator.The obtained[HNMP][Cl]was washed with ethyl acetate.The ethyl acetate was removed with a rotary evaporator under the vacuum of 0.08 MPa.An equimolar mixture of [HNMP][Cl] and MClxwas stirred vigorously at 90°C for 24 h to give [HNMP][MClx+1].The synthetic process is shown in Fig.1.
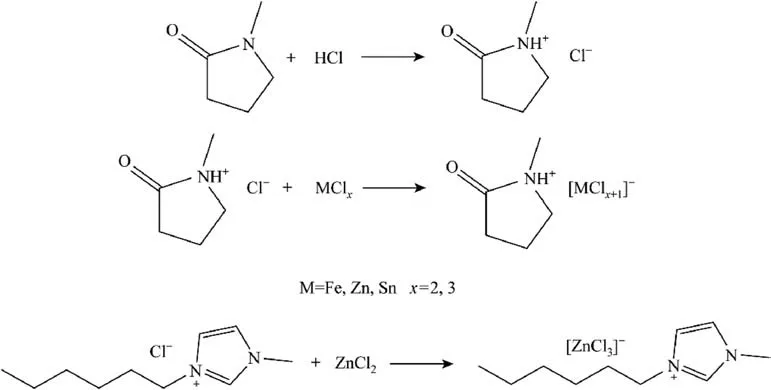
Fig.1.Synthesis processes of [HNMP][MClx+1] and [HMIM][ZnCl3].
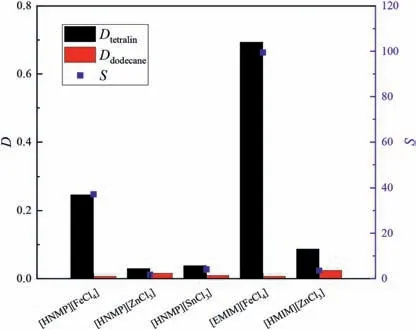
Fig.2.Effects of different extractants on separation performances in a tetralin/dodecane system(equal volumes of extractant and feed,extraction temperature 30 °C,equilibrium time 30 min,50% (mol) aromatics in initial system).
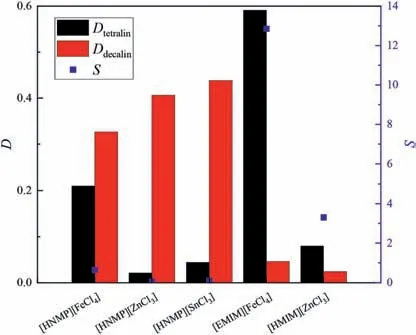
Fig.3.Effects of different extractants on separation performances in tetralin/decalin system (equal volumes of extractant and feed,extraction temperature 30 °C,equilibrium time 30 min,50% (mol) aromatics in initial system).
[HMIM][ZnCl3] was synthesized by vigorously stirring an equimolar mixture of[HMIM][Cl]and ZnCl2at 60°C for 24 h according to previous literature[26].The synthetic process is shown in Fig.1.
2.3.Liquid-liquid extraction experiments
Liquid-liquid extraction experiments were executed by adding predetermined amounts of feed and extractant to a conical flask at the targeted temperature.The extraction process was performed by vigorously shaking the mixture for 30 min to achieve phase equilibrium.The mixture was settled to ensure entire phase separation.Samples from upper and lower layers were collected for analysis.
The distribution coefficient (Di) and selectivity (S) were calculated as follows:
where xiis the mole fraction of component i,and IL and raf represent the IL and raffinate phases,respectively.
2.4.Analytical methods
After equilibrium was reached,a sample was removed and analyzed by using a gas chromatography (GC-2014C,Shimadzu,Japan) system with a flame ionization detector and HP-5 column(30 m × 0.32 mm,film thickness: 0.25 μm),and decane as an internal standard.Ethanol was added to the samples to avert phase splitting and to maintain a homogeneous mixture.The carrier gas was nitrogen at a flow of 1 ml∙min-1,which equates to a split ratio of 40:1.The injector and detector temperatures were 280 and 270°C,respectively.The oven temperature was initially maintained at 100°C for 1.5 min and was then increased to 200°C at 8°C∙min-1;this temperature was maintained for 0.5 min.
3.Results and Discussion
3.1.Screening of separation performances of extractant
In Figs.2 and 3,the separation performances of[HNMP][FeCl4],[HNMP][ZnCl3],and [HNMP][SnCl3] show that an extractant that contains[FeCl4]gives good selectivity.However,[HNMP][FeCl4]has a low distribution coefficient.We therefore considered replacing[HNMP] with [EMIM].A comparison of the performances of[HNMP][FeCl4] and [EMIM][FeCl4] shows that [EMIM][FeCl4] gives higher selectivity and has a superior tetralin distribution coefficient.This may be because the imidazole ring structurally resembles an aromatic ring.[EMIM][FeCl4] was therefore used to investigate the effects of other factors.
3.2.Interactions of [EMIM][FeCl4] with aromatics and aliphatics
The interactions of [EMIM][FeCl4] with tetralin,dodecane,and decalin were calculated by using Gaussian 09 to intuitively reveal the separation mechanism.All density functional theory calculations were performed at the TPSSh/def2TZVPP level of theory.The optimal structures and interaction energies of [EMIM][FeCl4] with tetralin,dodecane,and decalin are shown in Fig.4 and Table 1,respectively.The data in Table 1 clearly show that the interaction energy of [EMIM][FeCl4]-tetralin is larger than those of [EMIM][FeCl4]-dodecane and [EMIM][FeCl4]-decalin.This indicates that[EMIM][FeCl4]could be used to separate tetralin from dodecane or decalin.
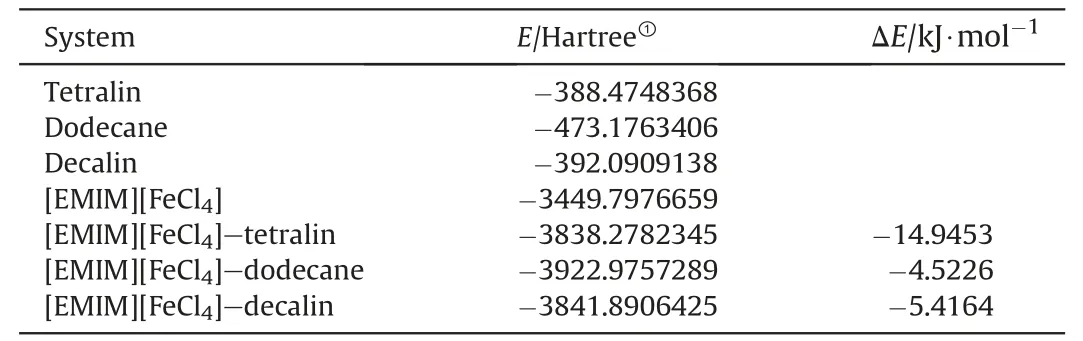
Table 1 Interaction energies between [EMIM][FeCl4] and tetralin,dodecane,and decalin
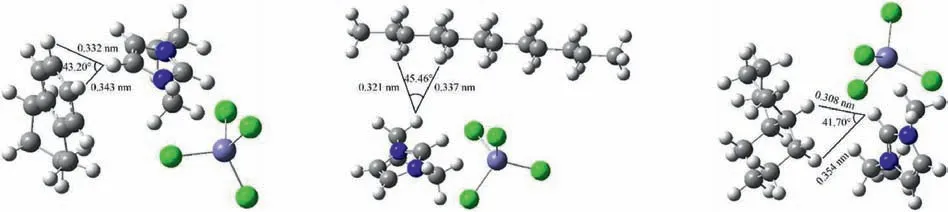
Fig.4.Optimal geometries for [EMIM][FeCl4]-tetralin (left),[EMIM][FeCl4]-dodecane (middle),and [EMIM][FeCl4]-decalin (right).
3.3.Energy decomposition analysis
Energy decomposition analysis was performed with the PSI4 program to determine the type of interaction that played the major role in [EMIM][FeCl4]-tetralin.The interaction was decomposed into dispersion,electrostatics,induction,and exchange by using the symmetry-adapted perturbation theory in the PSI4 program [27].The symmetry-adapted perturbation theory input files for PSI4 were produced by using the Multiwfn program[28].Negative and positive values mean attraction and repulsion,respectively.Fig.5 shows that electrostatics and dispersion play dominating roles,and induction cannot be neglected.

Fig.5.Energy decomposition analysis of [EMIM][FeCl4]-tetralin.
3.4.Interaction analysis of [EMIM][FeCl4]-tetralin/decalin/dodecane
IGM analysis [29,30] was used to investigate the types and strengths of the interactions between[EMIM][FeCl4]and tetralin.A repulsive interaction corresponding to sign(λ2)ρ>0 was observed,which is the same as that for exchange in the scatter diagram for the energy decomposition analysis.Simultaneously,an attractive interaction was detected at sign(λ2)ρ<0,which is consistent with dispersion,electrostatics,and induction in the energy decomposition analysis.The color-mapped isosurface of [EMIM][FeCl4]-tetralin is shown in Fig.6.The referenced color-mapped isosurface can be used to visually estimate the type of interaction in systems(Fig.6).The color indicates that VDW interactions play a major role in [EMIM][FeCl4]-tetralin.Moreover,the types of interaction among [EMIM][FeCl4]-decalin and [EMIM][FeCl4]-dodecane molecular clusters were mainly VDW interactions.

Fig.6.Scatter diagrams of [EMIM][FeCl4]-tetralin (a),gradient isosurface of [EMIM][FeCl4]-tetralin (b),[EMIM][FeCl4]-decalin (c),and [EMIM][FeCl4]-dodecane (d).

Fig.7.Effects of alkyl chain length of extractant on separation performances in tetralin/dodecane system (equal volumes of extractant and feed,extraction temperature 30 °C,equilibrium time 30 min,50% (mol) aromatics in initial system).

Fig.8.Effects of alkyl chain length of extractant on separation performances in tetralin/decalin system (equal volumes of extractant and feed,extraction temperature 30 °C,equilibrium time 30 min,50% (mol) aromatics in initial system).
3.5.Effects of alkyl chain length of extractant
The effects of the alkyl chain length of the extractant on the separation performances in tetralin/dodecane and tetralin/decalin systems were investigated.Figs.7 and 8 show that with increasing alkyl chain length of the extractant,the distribution coefficients of tetralin,dodecane,and decalin all increased,and the selectivities in the tetralin/dodecane and tetralin/decalin systems both decreased.The interactions of the alkanes with the alkyl group of the cation cause the result possibly.Alternatively,it could be because electron donation from the alkyl group to the cation of the extractant increases with increasing alkyl chain length,which results in a decrease in the positive character of the cation.The interaction between the cation and the aromatic hydrocarbon is therefore weakened,which leads to decreased selectivity[31].[EMIM][FeCl4]was therefore used to investigate the effects of other factors.
3.6.Effects of extraction temperature
Fig.9 shows that for the tetralin/dodecane system,the tetralin distribution coefficient and selectivity first increased and then decreased with increasing temperature.Fig.10 shows that for the tetralin/decalin system,as the temperature increased,the distribution coefficient of tetralin was almost unchanged,and the selectivity first decreased and then stabilized.This is possibly because ILs and aromatic compounds can organize sandwich structures,as previously reported[32],in which,the cations of the IL interact with aromatics in a commutative structure via π-π interactions,and the IL anions are placed around this structure.The intense molecular movement caused by increasing the temperature breaks the ordered sandwich structure within the IL,and this restrains the interactions between the IL and aromatics.However,thermal expansion enables organization of more aromatics between the ions [33].The results shown in Figs.9 and 10 can be explained by these effects.Considering the energy consumption,30°C was selected as the extraction temperature.
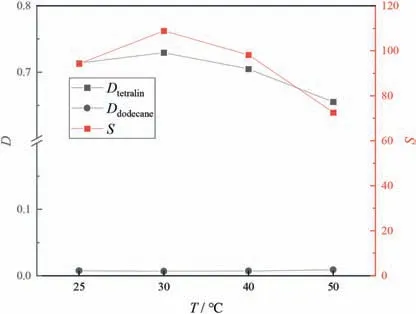
Fig.9.Effects of extraction temperature on separation performances in tetralin/dodecane system(equal volumes of extractant and feed,equilibrium time 30 min,50%(mol) aromatics in initial system).
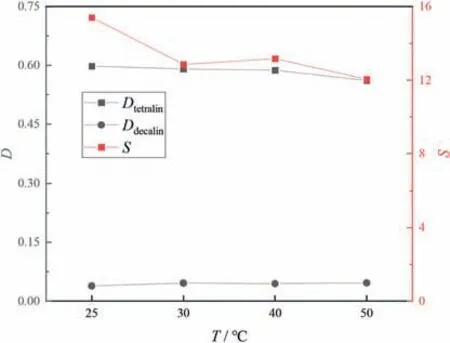
Fig.10.Effects of extraction temperature on separation performances in tetralin/decalin system (equal volumes of extractant and feed,equilibrium time 30 min,50%(mol) aromatics in initial system).

Fig.11.Effects of molar ratio of extractant to tetralin of feed on separation performances in tetralin/dodecane system (extraction temperature 30 °C,equilibrium time 30 min,50% (mol) aromatics in initial system).
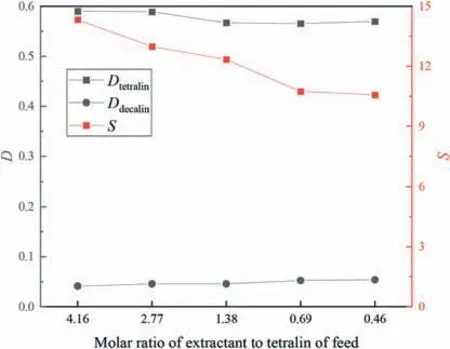
Fig.12.Effects of molar ratio of extractant to tetralin of feed on separation performances in tetralin/decalin system (extraction temperature 30 °C,equilibrium time 30 min,50% (mol) aromatics in initial system).

Fig.13.Effects of initial concentration on separation performances in tetralin/dodecane system (equal volumes of extractant and feed,extraction temperature 30 °C,equilibrium time 30 min).
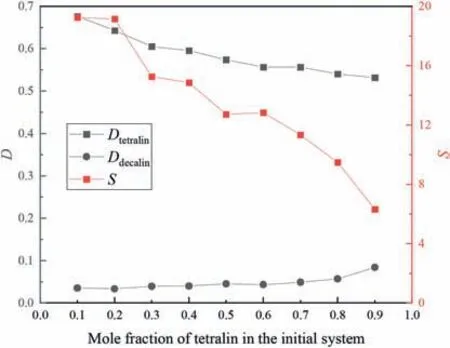
Fig.14.Effects of initial concentration on separation performances in tetralin/decalin system (equal volumes of extractant and feed,extraction temperature 30 °C,equilibrium time 30 min).
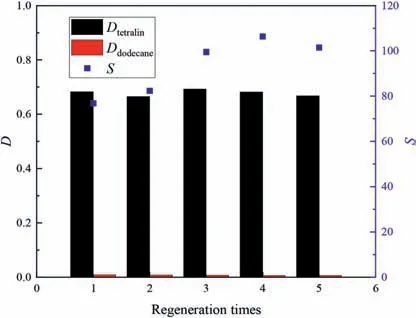
Fig.15.Effects of regeneration time on separation performances in tetralin/dodecane system (equal volumes of extractant and feed,extraction temperature 30 °C,equilibrium time 30 min,50% (mol) aromatics in initial system).
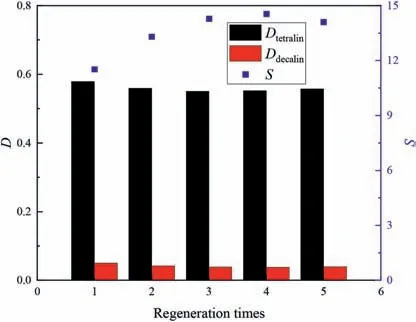
Fig.16.Effects of regeneration time on separation performances in tetralin/decalin system (equal volumes of extractant and feed,extraction temperature 30 °C,equilibrium time 30 min,50% (mol) aromatics in initial system).
3.7.Effects of extractant proportion
The desired separation performance cannot be achieved if the extractant loading is too low,and using too much extractant generates waste and increases energy consumption.The effects of the extractant proportion on the separation performance were therefore investigated;the results are shown in Figs.11 and 12.The results clearly show that the tetralin distribution coefficient and selectivity increased with increasing extractant proportion.This is probably because both dodecane and decalin lack unsaturated bonds;therefore,increasing the extractant proportion increases π-π interactions between the extractant and tetralin.
3.8.Effects of initial concentration
Figs.13 and 14 show that as the mole fraction of tetralin in the initial system increased,the tetralin distribution coefficient and selectivity generally decreased,and the distribution coefficients of dodecane and decalin increased in the tetralin/dodecane and tetralin/decalin systems.This may be because ILs and aromatic components form a sandwich structure,in which the IL cations and the aromatic components interrelate in an alternating structure via π-π interactions,with the IL anions organized around this structure.Increasing the aromatic content increases the distance between aromatic compounds and cations,which weakens the interactions and therefore the distribution coefficient.Increasing the aromatic content of the IL also enhances the aliphatic distribution coefficient by inducing the aliphatic components to array themselves between molecules [33].
3.9.Effects of regeneration time
In the regeneration process,raffinate was taken away after every extraction experiment.Then back-extraction with n-heptane was performed twice.The n-heptane was then removed by vacuum distillation at 90°C for 2 h.Figs.15 and 16 show that the distribution coefficients of tetralin in the tetralin/dodecane and tetralin/decalin systems were almost stable,those of dodecane and decalin decreased slightly,and the selectivity rose.
4.Conclusions
Metal chloride-based ILs with NMP or imidazolium as the cation were synthesized.These ILs were screened to enable the best separation performances in tetralin/dodecane and tetralin/decalin systems to be achieved.The best separation performance was achieved with [EMIM][FeCl4],which gave high selectivity,as the extractant.Calculations of the interactions between[EMIM][FeCl4]and tetralin,dodecane,and decalin showed that the interactions between [EMIM][FeCl4] and tetralin were stronger than those between[EMIM][FeCl4]and dodecane or decalin.The results suggest that [EMIM][FeCl4] could achieve separation of tetralin from dodecane and decalin.Analysis performed with the PSI4 program showed that electrostatics and dispersion played primary roles and that induction cannot be neglected in [EMIM][FeCl4]-tetralin.The IGM analysis suggested that VDW forces have a primary effect in[EMIM][FeCl4]-tetralin.The tetralin distribution coefficient and selectivity were 0.8 and 110,respectively,with 10%(mol)tetralin in the initial tetralin/dodecane system,and 0.67 and 19.5,respectively,with 10%(mol)tetralin in the initial tetralin/decalin system.Investigation of the effects of the alkyl chain length of the extractant showed that an extractant with a short alkyl chain gave high selectivity.The effects of the extraction temperature,solvent dosage,and initial concentrations of the system components were also studied.Recycling experiments showed that regenerated[EMIM][FeCl4] could be used repeatedly.The results suggest that[EMIM][FeCl4] has potential industrial applications.
Declaration of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Data will be made available on request.
Acknowledgements
This work was supported by the National Natural Science Foundation of China(22125802,22078010).The authors gratefully acknowledge these grants.We thank Helen McPherson,PhD,from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.09.004.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of PrFexCo1-xO3/Mt catalyst and study on degradation of 2-hydroxybenzoic acid wastewater by catalytic wet peroxide oxidation
- Rational surface charge engineering of haloalkane dehalogenase for boosting the enzymatic performance in organic solvent solutions
- Active MoS2-based electrode for green ammonia synthesis
- Long-term operation optimization of circulating cooling water systems under fouling conditions
- Effect of bubble morphology and behavior on power consumption in non-Newtonian fluids’ aeration process
- Preparation and properties of high-energy-density aluminum/boroncontaining gelled fuels
