Simultaneous removal of sulfur dioxide and nitrogen oxide from flue gas by phosphorus sludge: The performance and absorption mechanism
2024-04-22YuanyuanYinXujunWangLeiXuBinbinHeYunxiangNieYiMei
Yuanyuan Yin ,Xujun Wang ,Lei Xu ,Binbin He ,Yunxiang Nie ,* ,Yi Mei ,*
1 Faculty of Chemical Engineering,Kunming University of Science and Technology,Kunming 650500,China
2 Yunnan Provincial Key Laboratory of Energy Saving in Phosphorus Chemical Engineering and New Phosphorus Materials,Kunming 650500,China
3 The Higher Educational Key Laboratory for Phosphorus Chemical Engineering of Yunnan Province,Kunming 650500,China
Keywords: Absorption Oxidation Multiphase reaction Phosphorus sludge Yellow phosphorus Low temperature
ABSTRACT Developing low-cost and green simultaneous desulfurization and denitrification technologies is of great significance for sulfur dioxide (SO2) and nitrogen oxide (NOx) emission control at low temperatures,especially for small and medium-sized coal-fired boilers and furnaces.Herein,phosphorus sludge,an industrial waste from the production process of yellow phosphorus,has been developed to simultaneously eliminate SO2 and NOx from coal-fired flue gas.The key factors affecting the experimental results indicate that desulfurization and denitrification efficiency of over 95% can be achieved at a low temperature of 55 °C.Further,the absorption mechanism was investigated by characterizing the solid and liquid phases of the phosphorus sludge during the absorption process.The efficient removal of SO2 is attributed to the abundance of iron (Fe3+) and manganese (Mn2+) in the absorbent.SO2 can be rapidly catalyzed and converted to by them.The key to NOx removal is the oxidation of NO toward watersoluble high-valent nitrogen oxides by oxidizing reactive substances induced via yellow phosphorus,which are then absorbed by water and converted to .Meanwhile,yellow phosphorus is oxidized to phosphoric acid(H3PO4).The spent absorption slurry can be reused through wet process phosphoric acid production,as it contains sulfuric acid (H2SO4),nitric acid (HNO3),and H3PO4.Accordingly,this is a technology with broad application prospects.
1.Introduction
In recent decades,fossil energy such as coal,has injected inexhaustible impetus into China's industrial development,but sulfur dioxide(SO2)and nitrogen oxides(NOx)have inevitably discharged into the atmosphere,which poses a great threat to the ecosystem and human health [1,2].Since the implementation of ultra-low emissions of flue gas in China,SO2and NOxemissions have decreased significantly [3,4].Nevertheless,the efficient and lowcost removal of these pollutants from the flue gas of small-and medium-sized coal-fired boilers is still a difficulty.
At present,there are hundreds of control technologies for SO2and NOxremoval.For SO2control,wet flue gas desulfurization techniques still dominate the market,among which calcium-based processes are the most widely used.However,the calcium method will solve the problem of waste gypsum and acid waste water.It would not only take up a lot of ground but also pollute the environment and groundwater resources [5-7].In terms of denitrification,catalytic reduction of NOxwas the most widely used denitrification method.The selective catalytic reduction (SCR) purification method uses ammonia (NH3) as a reducing agent to reduce NOxto nitrogen (N2),which is very popular in thermal power plants[8,9].Unfortunately,this technology is difficult to adapt to the flue gas of small and medium-sized coal-fired boilers because the low-temperature SCR catalysts are expensive [10-12].Additionally,the traditional flue gas desulphurization and denitrification technology is difficult to widely introduce in medium-and small-sized enterprises due to the high investment costs and operating expenses.Based on the above,the simultaneous desulphurization and denitrification technology has received a lot of attention in recent years,owing to its characteristics of high efficiency and low cost.Until now,more than 70 kinds of simultaneous desulphurization and denitrification technologies have been studied,such as the ozone (O3) oxidization method [13,14],the hydrogen peroxide(H2O2)oxidization method[15,16],the chlorine dioxide/sodium chlorite (ClO2/NaClO2) oxidization method [17,18],the potassium permanganate (KMnO4) oxidization method [19],the plasma method [20,21],etc.Due to factors such as high operating costs and by-product treatment,only the ozone oxidation method is widely used.However,the cost of an ozone generator and the secondary pollution of ozone have also attracted widespread attention.Considering this,Okubo et al.[22-24]developed a plasma-chemical hybrid process that can significantly reduce the cost and avoid ozone emissions.Recently,resource recycling has become a trend.Industrial raw materials or waste materials are used as desulfurization and denitrification absorbents,including pyrolusite slurry[25],copper slag[26],phosphate rock slurry[27],water-quenched manganese slag slurry [28],red mud [29],and steel slag slurry [30].Sun et al.[25] employed pyrolusite slurry to remove SO2and NOxfrom flue gas.The SO2and NOxwere converted into sulfuric acid (H2SO4) and nitric acid (HNO3),respectively,to decompose pyrolusite,and the deactivated pyrolusite slurry was further processed and utilized.Nie et al.[27,31] have built a desulfurization unit with phosphate rock slurry as an absorbent in the phosphorus chemical enterprise,realizing the recycling of SO2and phosphorus rock resources.In recognition of this,we developed phosphorus sludge,a kind of dangerous solid waste formed in the process of yellow phosphorus(P4)production,as an absorbent to remove SO2and NOxfrom the tail gas of coalfired boilers.Phosphorus sludge is composed of P4,dust,and water.Among them,the mass content of P4is as high as 5%-30%.The P4reacts with dioxygen (O2) to form trioxygen (O3),which can oxidize NO toward high-valence nitrogen oxides.These highvalence nitrogen oxides and SO2are dissolved in water to form nitric acid and sulfuric acid.Meanwhile,P4is oxidized to phosphoric acid (H3PO4).The deactivated slurry can be used for decomposing phosphate rock,realizing the purpose of recycling phosphorus,sulfur,and nitrogen resources.
In this work,phosphorus sludge is employed as an absorbent to remove SO2and NOxin a bubbling reactor.The influencing factors of SO2and NOxremoval efficiency were investigated by controlling the operating conditions.Subsequently,the absorption mechanism was discussed based on the analysis of absorbent composition during the reaction process.
2.Experimental
2.1.Materials
The phosphorus sludge used in the experiments is obtained from Yunnan Phosphate Technology Co.,Ltd.,China,and the P4mass content in phosphorus sludge was determined to be 7.95%.The higher the P4concentration,the better the denitrification effect,which was proved in our previous experimental research[31].Therefore,in order to explore the influence of process conditions on the desulfurization and denitrification effects,we diluted 5 g of phosphorus sludge in 250 ml of deionized water as an absorbent.The phosphate rock (particle size<38 μm) was provided by Sinochem Yunlong Co.,Ltd.of Yunnan,China.It is mainly composed of 9.2% (mass) magnesium carbonate calcium (CaMg(CO3)2),79.37%(mass) fluorapatite (Ca5(PO4)3F),and 4.74% (mass) silica [32].The N2(≥99.99%(vol)),SO2/N2(3%(vol)SO2,97%(vol)N2),O2(≥99.50%(vol)),and NO/N2(0.3% (vol) NO,99.7% (vol) N2) were purchased from Kunming Guangruida Industrial Trade Co.,Ltd.,China.Anhydrous calcium chloride(AR)was purchased from Tianjin Fengchuan Chemical Reagent Technology Co.,Ltd.,China.
2.2.Experimental apparatus
The schematic of the experimental setup is exhibited in Fig.1.The simulated flue gas used in the experiment consists of four gases,including NO,SO2,O2,and N2.Each gas was metered by the flowmeter and then conveyed to the buffer bottle for mixing in order to obtain simulated flue gas with a certain SO2and NOxconcentration and flow.Subsequently,the simulated flue gas enters the bubbling reactor.The SO2and NOxfrom the flue gas are absorbed by the phosphorus sludge absorbent.The temperature and mixing strength of the absorbent are controlled by a magnetic stirring reactor.The flue gas discharged from the outlet of the bubbling reactor is dried by anhydrous calcium chloride and then enters the multifunctional flue gas analyzer.Herein,the SO2,NOx,and O2concentrations in the simulated flue gas are controlled at 2500 mg∙m-3,780 mg∙m-3,and 10% (vol),respectively.The NOxconsist of 97%(mass)NO and 3%(mass)NO2,to simulate coalfired boiler flue gas.The SO2and NOxremoval efficiency was investigated under the following experimental conditions: phosphorus sludge of 5 g(excluding water);deionized water of 250 ml;reaction temperature of 65°C;flue gas flow of 500 ml∙min-1;stirring speed of 1000 r∙min-1.It should be pointed out that when the influence of a single factor on the desulfurization and denitrification efficiency is examined,other experimental conditions except for variable factors are consistent with the experimental values specified above.
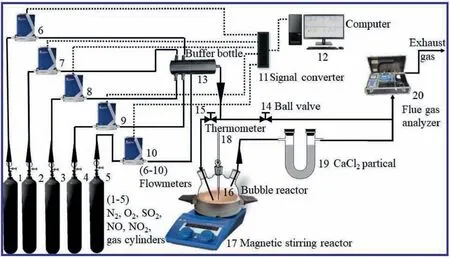
Fig.1.Framework of the experimental apparatus.
2.3.Analysis methods
During this experiment,the pH value of the phosphorus sludge absorbent was monitored by a pH meter (PHS-3C,Shanghai Leici Sensor Technology Co.,Ltd.,China).The liquid phase compositions of the absorbent were monitored by ion chromatography(IC,Model 883,Swiss Aptar,Switzerland) and inductively coupled plasma spectrometry (ICP,Avio 200,PE,America),and the solid phase material compositions were characterized by X-ray diffraction(XRD,Empyrean,PANalytical,Netherlands),Fourier-transform infrared spectroscopy(FTIR,Tensor Type 27,Bruker,Germany),and scanning electron microscopy (SEM,S-4800,Hitachi,Japan).The Rietveld method [33] was used to analyze the content of solidphase material compositions.The O3generated in the reactor is monitored by the ozone detector (964,BMT,Germany).The flow rate and concentration of SO2,NOx,and O2from the simulated flue gas at the inlet and outlet of the bubbling reactor were measured by the mass flow meter (CS200,Beijing Seven Star Huachuang Meter,China) and multifunctional flue gas analyzer (Ecom-J2KN,RBR,Germany),respectively,and the removal efficiency of SO2and NOxis calculated by the following equation:
3.Results and Discussion
3.1.Phosphorus sludge characterization
By means of XRD,ICP,and IC,the specific composition of the phosphorus sludge was analyzed in detail,and the experimental results are displayed in Fig.2.It can be seen from Fig.2(a) and (b)that the solid phase of phosphorus sludge is mainly composed of silica(SiO2,39.53%(mass)),potassium aluminum silicate(KAlSiO3,15.03% (mass)),calcium silicate (CaSiO3,8.28% (mass)) and hydroxyapatite (Ca5(PO4)3(OH),7.13% (mass)).In addition,it also contains gypsum (CaSO4∙2H2O),zinc sulfide (ZnS),plumbojarosite(PbFe6(SO4)4(OH)12),lead sulfide (PbS),phosphorus pentoxide(P2O5),iron oxide (Fe2O3),etc.Through the analysis of the liquid phase of phosphorus sludge in Fig.2(c) and (d),it was found that the phosphorus sludge liquid phase contains Fe3+,Mn2+,magnesium ion(Mg2+),aluminum cation(Al3+),calcium ion(Ca2+),,and.Among them,the contents of Fe3+and Mn2+are as high as 696.4 and 30.6 mg∙L-1,respectively,which are of positive significance for efficient SO2removal [34-36].
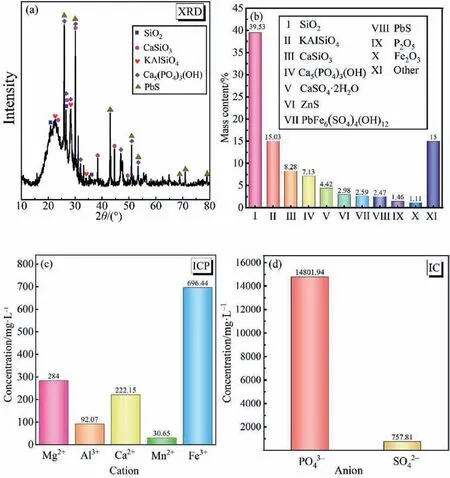
Fig.2.Analysis of the solid phase and liquid phase composition of phosphorus sludge:(a,b)XRD results of the solid phase of phosphorus sludge;(c)ICP results of the liquid phase of phosphorus sludge;(d) IC results of the liquid phase of phosphorus sludge.
3.2.Effects of process conditions on SO2 and NOx removal
3.2.1.Effects of temperature on SO2and NOxremoval
The effect of temperature on SO2and NOxefficiency was investigated with phosphorus sludge as an absorbent,and the results are shown in Fig.3.From Fig.3(a),it is seen that the desulfurization efficiency increases gradually with the ascent of the reaction temperature when the temperature is lower than 55°C.The SO2removal efficiency is maintained above 95%within 3 h at a reaction temperature of 55°C.As the temperature continues to rise,the desulfurization efficiency gradually decreases.From Fig.3(b),it is found that the denitrification efficiency is kept above 90% for 3 h,when the reaction temperature is lower than 55°C.The peak of NOxabsorption efficiency was introduced at 55°C.Obviously,the denitrification efficiency drops significantly when the temperature exceeds 65°C.When the reaction temperature drops below 55°C,the removal efficiencies of SO2and NOxshow a similar increasing trend as the temperature increases.As the reaction temperature increases from 35 to 55°C,the amount of active substance (O,O3,HO∙,etc.) generated by the reaction between P4and O2gradually increases.These active substances oxidize NO into water-soluble high-valent nitrogen oxides (NO2,NO3,etc.),which are then absorbed by phosphorus sludge and converted into HNO3[37].At the same time,a portion of SO2is oxidized to SO3by the active substance.The desulfurization efficiency is improved because SO3is more easily absorbed by water and converted into H2SO4[38].The sharp decline in NOxremoval efficiency is mainly due to the rapid reaction of P4with O2to generate P2O5at a higher temperature,which leads to a significant reduction in the duration of P4and only a small amount of active substance generated [37,39].Meanwhile,the P2O5is dissolved in water to form a large amount of,which also has a negative impact on the absorption of SO2[40].
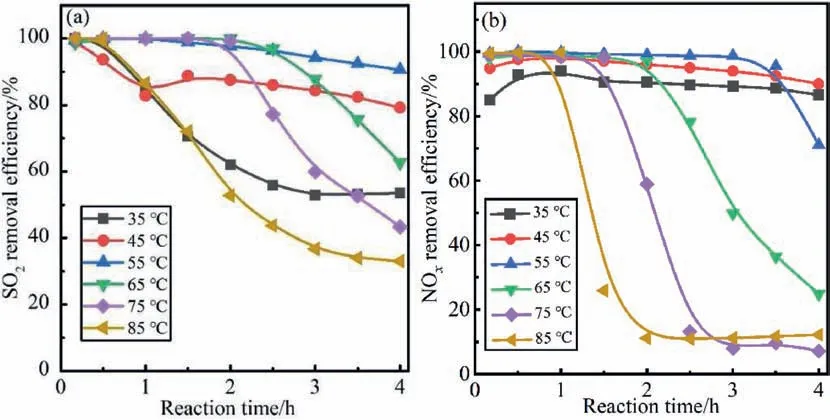
Fig.3.Effects of reaction temperature on simultaneous (a) desulfurization and (b) denitrification efficiency.
3.2.2.Effects of oxygen content on SO2and NOxremoval
The amount of O2is a crucial factor affecting the reaction between P4and O2.Since the O2content varies in the flue gas emitted by different types of coal-fired boilers and under different operating parameters,the influence of O2content on desulfurization and denitrification efficiency was investigated.From Fig.4(a),SO2removal efficiency increases with the increase in O2content.When the O2content rises from 4% to 10% at 2 h after reaction,the desulfurization efficiency improves from 64% to 100%.As the O2content continues to increase,the magnitude of the rise in desulfurization efficiency decreases.Increasing the O2content is conducive to the conversion of SO2to H2SO4,so the desulfurization effect is better under higher oxygen content conditions.It is observed from Fig.4(b)that the O2content has almost no effect on the NOxremoval within 2 h before the reaction.After that,as the reaction time prolongs,the higher the O2content,the more significant the increase in denitrification efficiency.About 20% of the denitrification efficiency is enhanced when the O2content boosts from 4% to 14% at 3 h after reaction.The reaction between P4and O2is promoted when the O2concentration increases,which leads to rapid oxidation and absorption of NOx[38].
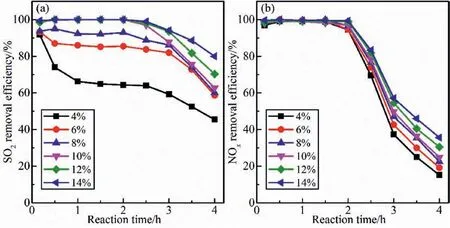
Fig.4.Effects of oxygen volume content on simultaneous (a) desulfurization and (b) denitrification efficiency.
3.2.3.Effects of stirring intensity on SO2and NOxremoval
Increasing the stirring speed can strengthen the dispersion of phosphorus sludge in the liquid phase.The P4in the colloidal center of phosphorus sludge can fully contact O2under the drive of mechanical action to generate more O,O3,and HO∙,which promotes NO oxidation.Fig.5(a) and (b) demonstrated the effect of stirring intensity on SO2and NOxremoval efficiency.The time for efficient desulfurization and denitrification efficiency shortened with the rotating speed ranging from 600 to 1400 r∙min-1.The time for desulfurization efficiency of over 90% is 3 h as long as the stirring speed is less than 800 r∙min-1.When the stirring speed reaches 1400 r∙min-1,the time is shortened to 1.5 h.For NOxremoval,the time for the removal efficiency of over 90% is shortened from 4 to 1 h when the stirring speed is improved from 200 to 1400 r∙min-1.Clearly,increasing the stirring intensity will shorten the time for efficient desulfurization and denitrification.The reason is that the high stirring intensity promotes the consumption of P4,and the P4reacts quickly with O2to generate P4O10[37,39].The P4O10dissolves in water and forms a large amount of,which inhibits the absorption of SO2[32].Hence,a stirring speed below 600 r∙min-1is preferred in order to maintain a stable and lasting desulfurization and denitrification efficiency and ease P4consumption.
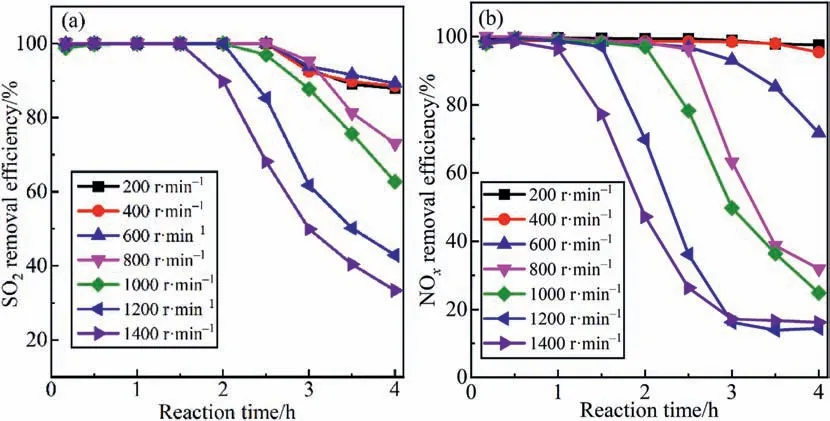
Fig.5.Effects of stirring speed on simultaneous (a) desulfurization and (b) denitrification efficiency.
3.2.4.Effects of gas flow on SO2and NOxremoval
The effect of gas flow on the absorption reaction can be mainly attributed to the difference in residence time.The results of desulfurization and denitrification efficiency under different gas flows are displayed in Fig.6(a)and(b).In the first 2 h of continuous reaction,the gas flow has little effect on the SO2and NOxremoval efficiency due to the sufficient amount of absorbent and oxidation.After that,with the increase in gas flow rate,the removal efficiency decreased sharply.On the one hand,increasing the gas flow rate leads to an increase in the O2content per unit time,which accelerates the consumption of P4.On the other hand,the increase in gas flow will reduce the retention time of SO2and NOxin the reactor,so they will be discharged before they can be oxidized and absorbed.As a consequence,at the same time point,the total amount of unabsorbed SO2and NOxand the consumption of P4increase with gas flow,which is an important reason for the rapid decline of the removal efficiency in the late reaction period.
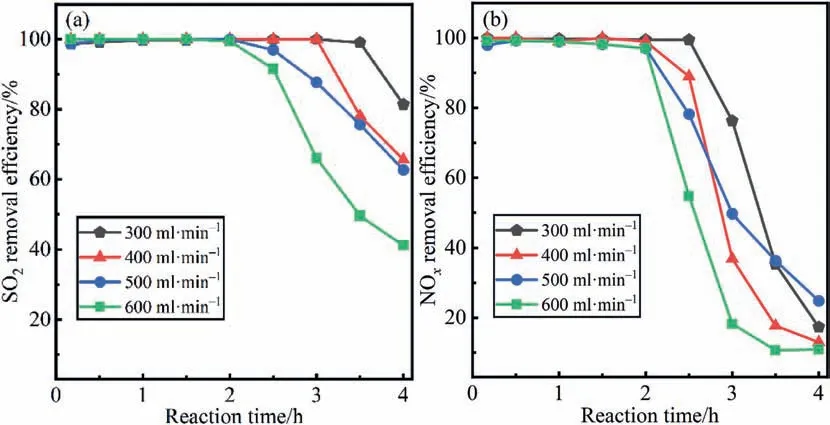
Fig.6.Effects of gas flow on simultaneous (a) desulfurization and (b) denitrification efficiency.
3.2.5.Effects of phosphate rock additive on SO2and NOxremoval
The deactivated phosphorus sludge absorbent after desulfurization and denitrification can be further used to decompose phosphate rock in the wet process phosphoric acid production section,so phosphate rock is selected as an additive[27,31].It can be seen from Fig.7(a) that the SO2absorption efficiency has considerably enhanced with the addition of phosphate rock in the phosphorus sludge absorbent.When the concentration of phosphate rock reaches 12 g∙L-1,the duration of 100% desulfurization efficiency is extended for 4 h compared with that of absorbent without phosphate rock.In our previous research[31,39],it has been established that magnesium calcium carbonate (CaMg(CO3)2),an effective component in phosphate rock,has excellent desulfurization performance.The elimination of NOxis mainly affected by the amount of P4.Therefore,as shown in Fig.7(b),the addition of phosphate rock does not greatly improve NOxremoval efficiency.Importantly,this result provides a basis for adding phosphate rock to phosphorus sludge absorbents to boost SO2absorption.

Fig.7.Effects of phosphate rock on simultaneous (a) desulfurization and (b) denitrification efficiency.
3.3.Analysis of phosphorus sludge absorbent in the reaction process
To investigate the mechanism of desulfurization and denitrification of phosphorus sludge absorbent,an experiment lasting for 8 h was conducted at a temperature of 55°C,and the results are presented in Fig.8.As can be seen in Fig.8(a),the desulfurization and denitrification efficiency decreased from 97% and 96% to 89% and 93%,respectively,when the pH was dropped from 3.11 to 1.82.Subsequently,the absorption efficiency of SO2and NOxdescends dramatically.It can be inferred that the decrease in pH is an important reason for the attenuation in absorption efficiency[27].To further elucidate the absorption mechanism,the solid and liquid phase compositions of the phosphorus sludge absorbent during the process of desulfuriza-tion and denitrification were further analyzed by IC,XRD,FTIR,and SEM.Fig.8(b)shows the changes of anion in the liquid phase of the absorbent at different reaction times.Asthe reaction time goes on,the IC peaks ofin the liquid phase gradually strengthen,indicating that SO2and NOxare absorbed and transformed into.Besides,the increase inconcentration is mainly due to the continuous oxidation of P4.Further,from Fig.8(c),by comparing the XRD characteristic peaks of the samples,it can be found that the diffraction lines of Ca5(PO4)3(OH) weakened in the spent samples after 4 h of reaction.This result is in agreement with the IC analysis,demonstrating that a part ofin the liquid phase comes from the decomposition of Ca5(PO4)3(OH).In the reaction process,the diffraction peaks of SiO2,CaSiO3,kalsilite(KAlSiO4),and CaSO4∙2H2O have hardly changed,indicating that they do not participate in the absorption reaction.Furthermore,it can be clearly seen from Fig.8(d)that four bands at 1097,954,798,and 467 cm-1assigned to silicon species(SiO2,CaSiO3,and KAlSiO4)appear in the spectra of the fresh and spent phosphorus sludge [40].The bands observed at around 3436,1630,and 1153 cm-1are denoted as CaSO4∙2H2O.The peaks at around 1450,604,and 568 cm-1are assigned to Ca5(PO4)3(OH) [27].There is no new infrared characteristic peak found in the reacted samples,which is consistent with XRD analyses.In addition,from Fig.8(e),it is observed that the solid phase of phosphorus sludge is irregular and granular,and there is no obvious change as the desulfurization and denitrification efficiency decrease.Consequently,based on the above analysis,it can be inferred that SO2and NOxare mainly present in the liquid phase in the form ofafter being absorbed into the phosphorus sludge.
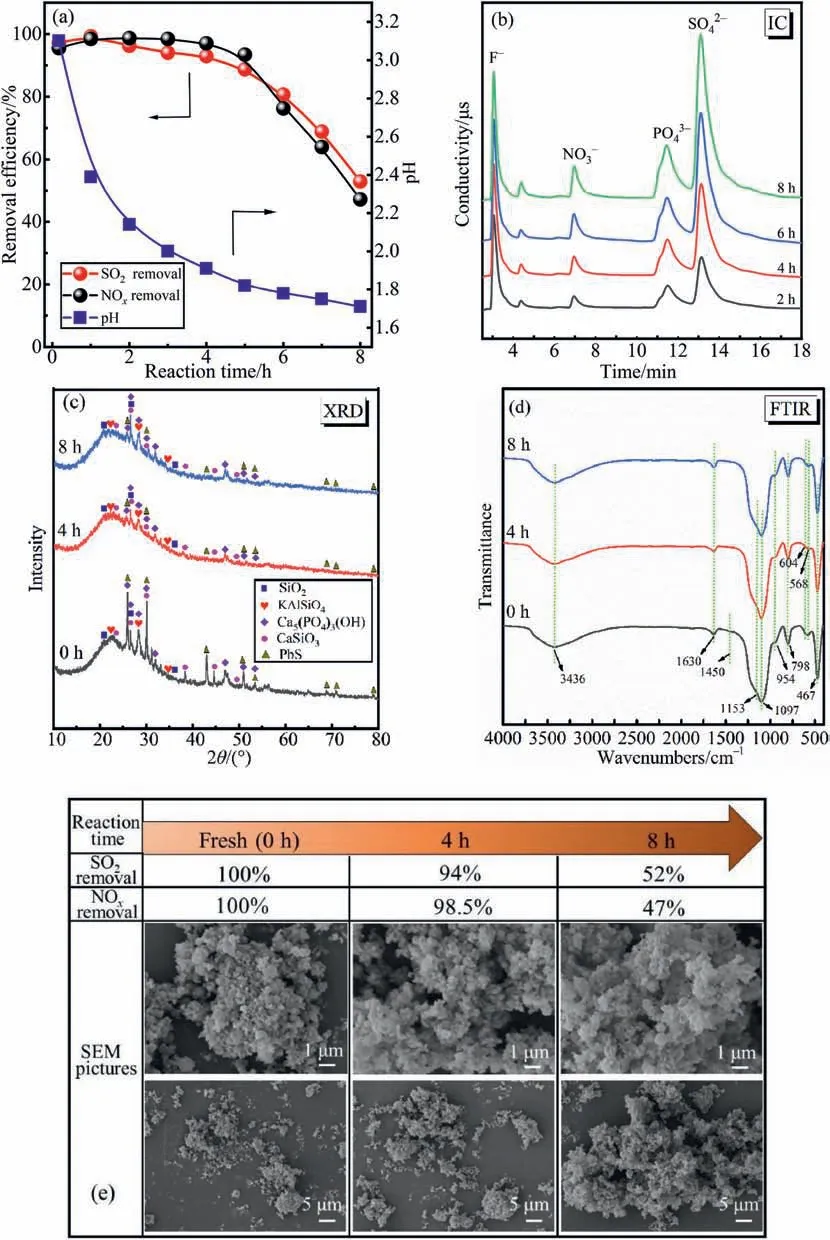
Fig.8.(a)Variations in desulfurization efficiency,denitrification efficiency,and pH value with time using phosphorus sludge as an absorbent.(b)IC results of the liquid phase of the absorbent at different reaction times.(c-e) XRD,FTIR,and SEM results of the solid phase of the absorbent at different reaction time.
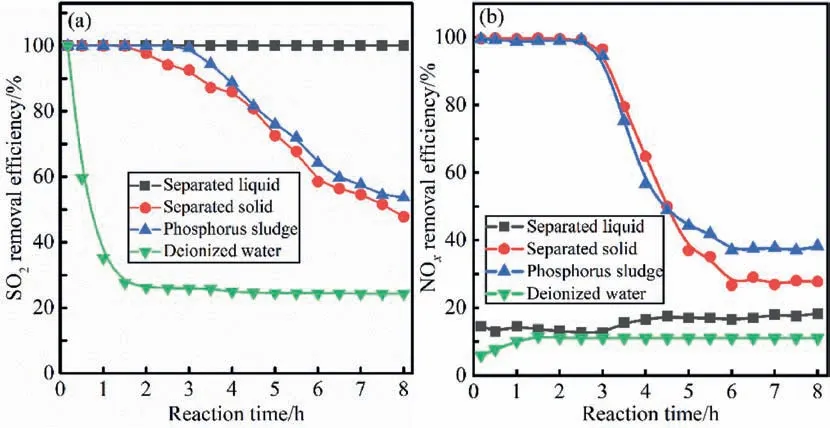
Fig.9.Effects of liquid and solid components separated phosphorus sludge on simultaneous (a) desulfurization and (b) denitrification efficiency,respectively.
3.4.Absorption mechanism for SO2 and NOx
4.Conclusions
In this study,a novel method for simultaneous removal of SO2and NOxusing phosphorus sludge as an absorbent was studied.The key factors influencing SO2and NOxabsorption are studied,including reaction temperature,stirring intensity,gas flow,and phosphate rock additive.The reaction temperature should be controlled at 55°C to achieve optimal desulfurization and denitrification effects.Because the P4from phosphorus sludge is easily oxidized to P2O5,the stirring speed and gas flow rate should be controlled within an appropriate range.Adding phosphate rock to the phosphorus sludge absorbent can prolong the time for efficient SO2removal,but it has almost no promoting effect on NOxremoval.Furthermore,the absorption mechanism of SO2and NOxhas been proposed by conducting a series of online characterizations of the absorbent during the reaction process.The reason for the efficient absorption of SO2is that the Fe3+and Mn2+ions from the phosphorus sludge can rapidly catalyze the conversion ofandtowards.The P4can induce O,O3,and HO∙radicals by reacting with O2,which oxidizes NO into high-valent nitrogen oxides that are easily soluble in water.Ultimately,it exists in the liquid phase as.This technology shows good application prospects due to its low cost and high desulfurization and denitrification performance.
Declaration of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Data will be made available on request.
Acknowledgements
The National Natural Science Foundation of China (22068019)and Yunnan Major Scientific and Technological Projects(202202AG050001) are gratefully acknowledged for financial support of this research work.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
