Importance of oxygen-containing functionalities and pore structures of biochar in catalyzing pyrolysis of homologous poplar
2024-04-22LiQiuChaoLiShuZhangShuangWangBinLiZhenhuaCuiYongguiTangObidTursunovXunHu
Li Qiu ,Chao Li ,Shu Zhang ,Shuang Wang ,Bin Li ,Zhenhua Cui ,Yonggui Tang ,Obid Tursunov ,Xun Hu ,*
1 School of Material Science and Engineering,University of Jinan,Jinan 250022,China
2 Joint International Research Laboratory of Biomass Energy and Materials,College of Materials Science and Engineering,Nanjing Forestry University,Nanjing 210037,China
3 School of Energy and Power Engineering,Jiangsu University,Zhenjiang 212013,China
4 Shandong BASAN Graphite New Material Plant,Zibo 255311,China
5 Department of Power Supply and Renewable Energy Sources,National Research University TIIAME,Tashkent 100000,Uzbekistan
Keywords: Poplar wood Catalytic pyrolysis Char catalyst Volatile-char interaction Bio-oil
ABSTRACT Biochar and bio-oil are produced simultaneously in one pyrolysis process,and they inevitably contact and may interact,influencing the composition of bio-oil and modifying the structure of biochar.In this sense,biochar is an inherent catalyst for pyrolysis.In this study,in order to investigate the influence of functionalities and pore structures of biochar on its capability for catalyzing the conversion of homologous volatiles in bio-oil,three char catalysts (600C,800C,and 800AC) produced via pyrolysis of poplar wood at 600 or 800 °C or activated at 800 °C,were used for catalyzing pyrolysis of homologous poplar wood at 600 °C,respectively.The results indicated that the 600C catalyst was more active than 800C and 800AC for catalyzing cracking of volatiles to form more gas(yield increase by 40.2%)and aromatization of volatiles to form more light or heavy phenolics,due to its abundant oxygen-containing functionalities acting as active sites.The developed pores of the 800AC showed no such catalytic effect but could trap some volatiles and allow their further conversion via sufficient aromatization.Nevertheless,the interaction with the volatiles consumed oxygen on 600C (decrease by 50%),enhancing the aromatic degree and increasing thermal stability.The dominance of deposition of carbonaceous material of a very aromatic nature over 800C and 800AC resulted in net weight gain and blocked micropores but formed additional macropores.The in situ diffuse reflectance infrared Fourier transform spectroscopy characterization of the catalytic pyrolysis indicated superior activity of 600C for removal of-OH,while conversion of the intermediates bearing C=O was enhanced over all the char catalysts.
1.Introduction
Biochar is homologous with bio-oil,which is produced by the same pyrolysis process[1].The interaction of the organics in bio-oil with biochar is inevitable during pyrolysis as the majority of volatiles are generated in the inner structures of a biomass particle[2,3].The travel of volatile organic components from the inner section of a particle in pyrolysis would contact biochar and might interact with biochar as biochar is not an inert material [4,5].Similar to the organics in bio-oil,biochar has its own oxygencontaining functionalities and skeleton structures,which can be regarded as a “macro” reactive molecule [6-9].The interaction between bio-oil and biochar shapes the composition of bio-oil and the structure of biochar [2,4,10,11].
There have been many studies about the catalytic effects of biochar in the pyrolysis of biomass[12-22].For instance,Ren et al.[12] investigated catalytic pyrolysis of Douglas Fir pellets over biochar catalyst and found that the biochar catalyst reduced the production of organic acids in bio-oil while enhancing the formation of hydrocarbons and phenols.The study by Yang et al.[13]showed that corn straw biochar activated with H3PO4could also promote the formation of phenolic compounds and aromatics (up to 67%) at the expense of oxygenic compounds and acetic acid in pyrolysis of Douglas Fir sawdust pellets.Bai et al.[14] performed catalytic pyrolysis of sewage sludge with self-derived char and found that the char catalyst could promote the formation of aliphatics while suppressing the generation of nitrogen-containing organics.Liu et al.[15] conducted catalytic pyrolysis of anise in a fixed-bed reactor,and the results showed that the Fe/biochar catalyst had high catalytic activity.Xu et al.[16]prepared three Ni/biochar catalysts with different K content for toluene steam reforming,and the results showed that K presence accelerated the consumption of biochar support at higher temperatures.These results demonstrated the catalytic effects of biochar on the evolution of the organics in bio-oil in pyrolysis,but further studies are required for understanding the structural evolution of biochar catalysts during catalytic pyrolysis.
In fact,except for the influence on bio-oil,the interaction between volatiles in bio-oil and biochar also impacted the structural features of biochar [10,23].In various aspects of the properties of biochar,functionality and pore structure are important features that play essential roles in the determination of the reactivity of biochar with organics in bio-oil [4].Nevertheless,in such an interactive process,the functionality and pore structure of biochar can also be shaped,which would also impact the reactivity of biochar with volatiles [24-26].This is because the oxygencontaining groups on the surface of biochar could be modified or removed,while pores of biochar could be filled [27-30].Such a process could take place in catalytic pyrolysis with biochar as a catalyst or in pyrolysis of biomass [31-33].Understanding the impacts of volatiles-char interactions on the evolution of the structure of biochar is of importance for clarification of the mechanism for the formation of biochar in pyrolysis and for further tailoring the structure of biochar.
In this study,the influence of volatiles on structures of biochar of the same origin was investigated by passing the volatiles from pyrolysis of poplar wood through the biochar of the same biomass feedstock prepared via pyrolysis at 600°C and 800°C,as well as the biochar activated at 800°C with K2C2O4.The biochar obtained at 600 or 800°C has a varied abundance of oxygen-containing functionalities,while the activated biochar has more developed porous structures [34-36].Thus,the purpose of this study was to investigate the potential effects of the volatiles of the same origin on the transformation of the oxygen-containing functionalities and the pore structures of the biochars.Hence,the change of the nature of the biochar during pyrolysis was paid particular attention.The results showed that oxygen-containing functionalities and pore structures of the char exhibited varied activity for catalyzing the evolution of the volatiles.The volatile-char interaction during the catalytic pyrolysis also impacted the reconstruction of elemental composition,distribution of functionalities,thermal stability,crystallinity,pore structures,and morphologies of the varied char catalysts in different ways.
2.Materials and Methods
2.1.Materials
Finger-sized branches of a poplar tree (P.alba) in autumn(October) were obtained from the park of the University of Jinan(China).The bark of the branches was peeled off with a knife,leaving white wood tissue that was further ground to a particle size of 0.05-0.25 mm.The crushed particles were washed with deionized water,were oven-dried at 105°C for 24 h,and were then ready for use in pyrolysis at 600 or 800°C,with a heating rate of 5°C∙min-1and a holding time of 30 min to obtain the 600C or 800C,in which the number refers to the temperature for pyrolysis while the “C” refers to char.In addition,the wood sample was also mixed with an aqueous solution of K2C2O4(:mwood=2:1) for impregnation.The impregnated sample was dried at 105°C and then activated through heating from room temperature to 800°C at 5°C∙min-1and held for another 30 min in a N2flow (60 ml∙min-1) [37,38].The activated char was then washed with 2 mol∙L-1HCl to obtain the activated carbon [38],named 800AC.Before the catalytic experiments,the feedstocks and catalysts were dried in the oven at 105°C to constant mass.
2.2.Experimental setup
The catalytic pyrolysis experiments were conducted in a fixedbed reactor with an inner diameter of 20 mm and an outer diameter of 25 mm.Before the start of the experiment,wood (in total 5 g,ensure uniform heating during the reaction) was wrapped in an aluminum foil in a small cylinder shape (about 5 mm in diameter and 10 mm in height),placed in a glass vial,and connected to one end of a three-way connecter with a hose,as depicted in Fig.S1 (in Supplementary Material).Non-in situ catalytic pyrolysis was used in this experiment.The quartz tube was filled with char catalyst (0.5 g,evenly spread over the entire section of the quartz tube) and mounted on a vertical tube furnace.Before the experiment,the reactor was purged with nitrogen atmosphere for 20 min,and the flow rate of nitrogen during the experiment was 60 ml∙min-1.The tube furnace was equipped with a silicon carbon rod for heating,and the temperature control system of the equipment adopted a proportion integration differentiation regulator.The heating program was set to ramp up to 600°C (heating rate: 5°C∙min-1) and was held at 600°C for 30 min.For the catalytic experiments,when the temperature rose to 600°C,the small cylinders in the glass vials were fed one after another into the quartz tube for fast pyrolysis to generate the volatiles passing through the biochar bed.The blank experiment without a biochar catalyst was also conducted to serve as a base for comparison.In the meantime,four gas-liquid separators prepared to collect the bio-oil produced by pyrolysis were quickly connected in tandem with the first three being empty and the fourth one containing a mixture of methanol and chloroform for further condensing the volatiles.A gas bag (volume: 15 L) was connected to the last gas-liquid separator to collect the non-condensable gas from the pyrolysis.After the experiment,the biochar catalyst and the bio-oil were collected.
The yields of biochar,bio-oil,and gas were calculated according to Eqs.(1)-(3),respectively.
m1is the total mass of biochar in the aluminum foil cylinder after the reaction;m is the mass of feedstock before pyrolysis.
m2was the mass of bio-oil produced from pyrolysis;m is the mass of feedstock before pyrolysis.
2.3.Characterization methods
Gas products were analyzed by gas chromatography(GC9790II)with high-purity argon as a carrier gas.The gas samples were injected by using a microinjector (size: 10 μl),and the spectra obtained were analyzed to calculate the percentage of H2,CO,CO2,CH4,and C2H4.The instrument was calibrated with standard gas before use.The composition of bio-oil was determined by using an ultraviolet fluorescence spectrophotometer (RF-6000,Shimadzu)and Gas chromatography-mass spectrometry (GC-MS) (QP2020,Shimadzu).Before the characterization with ultraviolet fluorescence,the bio-oil was diluted to 0.04% (mass) with methanol as a solvent.Two-dimensional spectrum (wavelength range:250-500 nm)and three-dimensional spectrum(wavelength range:X: 250 -500 nm,Y: 200 -400 nm) of bio-oil samples were obtained to analyze the abundance of aromatic rings,and bio-oil was diluted to the same concentration with a mixed solution of methanol and chloroform of the volumetric ratio of 1:4 and was packed into a 1.5-ml sample bottle for GC-MS characterization.During the test,a 0.5-μl sample was injected into the capillary column(30 m in length and 0.25 mm in inner diameter),in which volatile compounds could be separated.The column was kept at 50°C for 3 min,then heated from 50 to 250°C at 20°C∙min-1and held at 250°C for 10 min.The organics were identified by a standard library(NIST14 and NIST14s) and normalized by dividing the peak area of an internal standard (methyl tetradecanoate).
The elemental composition,crystal structure,thermal stability,surface functional groups,and hydrophilicity/hydrophobicity of fresh and spent catalysts were characterized using an elemental analyzer (EuroEA3000-Single),an X-ray diffraction spectrometer(Rigaku Ultima IV,Japan),a thermogravimetric analyzer (HCT-1,Hengjiu,China),a Fourier infrared spectrometer (FT-IR),and a contact angle-measuring instrument (JC2000D1,China),respectively.In situ diffuse reflectance infrared Fourier transform spectroscopy(DRIFTS,Nicolet iS50,USA)was used to heat the feedstock and catalyst mixture (5:1) to analyze the evolution pattern of functional groups during the heating from room temperature to 700°C at 10°C∙min-1.The specific surface area and morphology of the char catalysts were determined by the pore analyzer (Kubo X100,China) and scanning electron microscopy (SEM,Phenom,ProX),respectively.
3.Results and Discussion
3.1.Overall yields
The yields of the main products from the pyrolysis of wood over the char catalysts at 600°C are shown in Table 1.Compared with the blank control sample,the yield of bio-oil with the presence of char catalyst decreased significantly by 24.1% over the 600C catalyst.This was accompanied by an increase in the production of gases,as evidenced by the yield increased by 40.2%(Entry 2,Table 1).The 600C catalyst was obtained at lower temperatures,and it is more oxygen rich than the 800C catalyst,which will be characterized later.The abundance of the oxygenrich functionalities on the surface of the 600C catalyst interacted with the volatiles by promoting their cracking to form more gases and more light components in bio-oil,as also indicated by the lower content of tar in the bio-oil from catalytic pyrolysis.The distribution of gases further confirmed the promotional effects of the 600C catalyst on the dehydrogenation reactions to form more H2and the cracking reactions to form more CH4,CO,and CO2(Table S1).
The cracking of the oxygen-rich functionalities on the surface of the 600C catalyst should generate more reactive oxygen-containing or other aliphatic radicals,which might interact with organics in bio-oil,facilitating their conversion via degradation reactions [39,40].The 800C catalyst showed similar catalytic effects to that of the 600C catalyst,but the activity was lower.In comparison,the 800AC with the more developed pore structures did not effectively promote the cracking of volatiles as that over the 600C catalyst.Functionalities rather than pore structures played a more pronounced impact on enhancing the catalytic activity for cracking reactions.In addition,the mass change in the 600C catalyst was insignificant,suggesting the existence of probably a balance of deposition of carbonaceous material and their removal via gasification.The mass increase of the 800C and 800AC catalysts was probably due to the formation of carbonaceous deposits of a very aromatic nature,creating difficulty for their further gasification.
3.2.Characterization of bio-oil
The abundance of detectable organics in the bio-oil is presented in Fig.1(a).The catalytic pyrolysis decreased the production of the anhydrate sugars and their derivatives (alcohols,ketones,aldehydes,and even carboxylic acids),especially over the 600C catalyst.This was in line with the most significant decrease in the yield of bio-oil and the superior activity for cracking over this catalyst.Nevertheless,the char catalyst generally promoted the formation of phenolics such as phenol and p-cresol via promoting aromatization of the sugar derivatives,especially over the char catalyst produced at 800°C[41].Table S2 shows the detailed list of the organics from the pyrolysis.The abundance of the aldehydes such as glycolaldehyde dimer and 5-hydroxymethylfurfural as well as the ketones such as 2-hydroxy-3-methyl-cyclohexanone and 2-cyclopenten-1-one decreased significantly after the catalytic pyrolysis.They probably polymerized on the surface of the biochar catalysts.The char catalysts were reactive,and even some of the acetic acid was converted.The char catalysts also showed complex catalytic effects on the transformation of the mono-phenolics,but their production was generally enhanced.
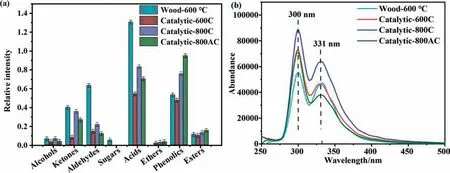
Fig.1.Analysis of the bio-oil produced from the pyrolysis of poplar wood and catalytic pyrolysis of wood with three different char catalysts at 600 °C:(a)the relative abundance of various light organics in the bio-oil to the internal standard of methyl tetradecanoate;(b) two-dimensional ultraviolet-fluorescence spectra of the bio-oil.
The phenolics were further characterized by ultraviolet fluorescence analysis(Fig.1(b)and Fig.2).The two-dimensional spectra of Fig.1(b) showed that the fluorescence of the bio-oil produced over the 600C catalyst was higher than that of the blank experiment at a wavelength above 350 nm.The three-dimensionalspectra of Fig.2 showed that region V was bigger in the bio-oil produced over the 600C catalyst.This suggested that the 600C catalyst promoted the formation of the phenolics with fused ring structures.The integration of the small fragments from the deposition of volatiles aided aromatization to phenolics.The fluorescence of the bio-oil from the 800C catalyst was the highest,suggesting the superior catalytic activity for promoting aromatization.The 800C catalyst was carbon rich,which might serve as a reservoir of hydrocarbon radicals.This promoted aromatization reactions.Differing from that over the 800C catalyst,the 800AC catalyst suppressed the formation of heavy phenolics.The 800AC catalyst had developed pore structures.After entering into the pores,the reaction intermediates might polymerize and undergo aromatization,forming heavier organics that could not diffuse away from the pores.The more significant increase in mass of the 800AC catalyst was the evidence of this.

Fig.2.Three-dimensional ultraviolet-fluorescence spectra of the bio-oil produced from the pyrolysis of poplar wood and catalytic pyrolysis of wood with three different char catalysts at 600 °C:(a)pyrolysis of the poplar wood,no catalyst;(b)catalyzed by the char-600 °C;(c)catalyzed by the char-800 °C;(d)catalyzed by the activated carbon produced from wood activation with K2C2O4 at 800 °C.The samples were diluted to 0.04% (mass) in methanol before the analysis.
3.3.Characterization of biochar
3.3.1.Elemental analysis
The results of elemental analysis of the char catalysts before and after the catalytic pyrolysis are shown inTable 2.For the used catalyst,the C content increased while the O content decreased correspondingly(i.e.,increased by 5.5%for carbon content while decreasing by 50%for oxygen content for the 600C catalyst).This indicated that the interaction between the volatile fraction and the char catalyst promoted the deoxygenation reaction and also formed the coke deposit with a highly aromatic nature (e.g.,polycyclic aromatic hydrocarbons),resulting in the higher C/O ratio and heating value of biochar.The mass gain of the used 800C and 800AC catalysts was remarkable,which was due to the formation of the external source of carbon.In converse,the mass of the used 600C was similar to that of the fresh catalyst,while the elemental contents changed remarkably.This suggested that the gasification of some volatile fractions such as aliphatic components and the formation of the aromatic-rich carbon deposit took place simultaneously during the catalytic pyrolysis.The low volatile fraction over the 800C and 800AC catalysts resulted in the net mass gain in the catalytic pyrolysis.
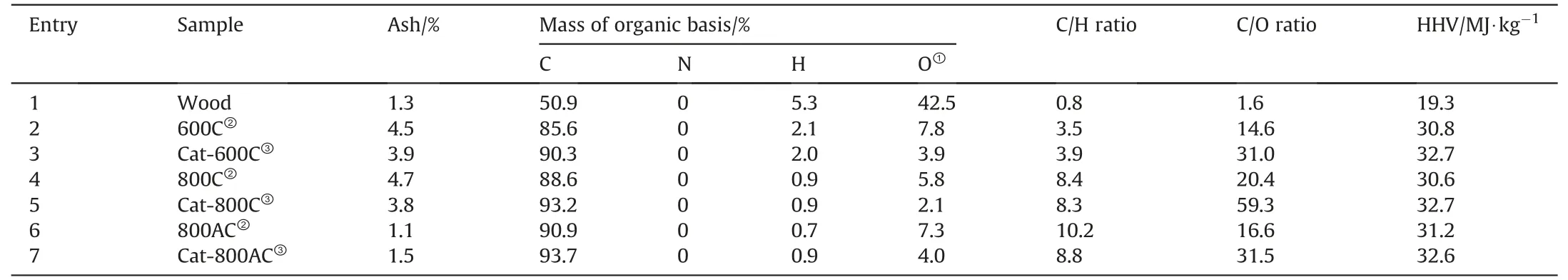
Table 2 Elemental analysis of the feedstock,fresh,and spent char catalysts
3.3.2.Thermogravimetric analysis
The thermal stability of the three char catalysts before and after the catalytic pyrolysis was characterized by thermogravimetry in a nitrogen flow(Fig.3).The overall mass loss of the used char catalyst was significantly lower than that of the fresh catalyst(i.e.,21.2% for fresh 600C,whereas 6.6% for the used catalyst).Similar results were observed for the 800C and 800AC catalysts.The catalytic pyrolysis formed the very aromatic carbonaceous deposit,which together with the deoxygenation of the char catalyst led to a remarkable increase of the thermal stability.This was consistent with the elemental analysis results in Table 2.Additionally,the used 800AC catalyst showed the highest thermal stability with only a mass loss of 0.53%.The volatiles entering into the pores of the char could not diffuse out.The prolonged reaction time led to sufficient deoxygenation,producing the char of high carbon content and thermal stability.
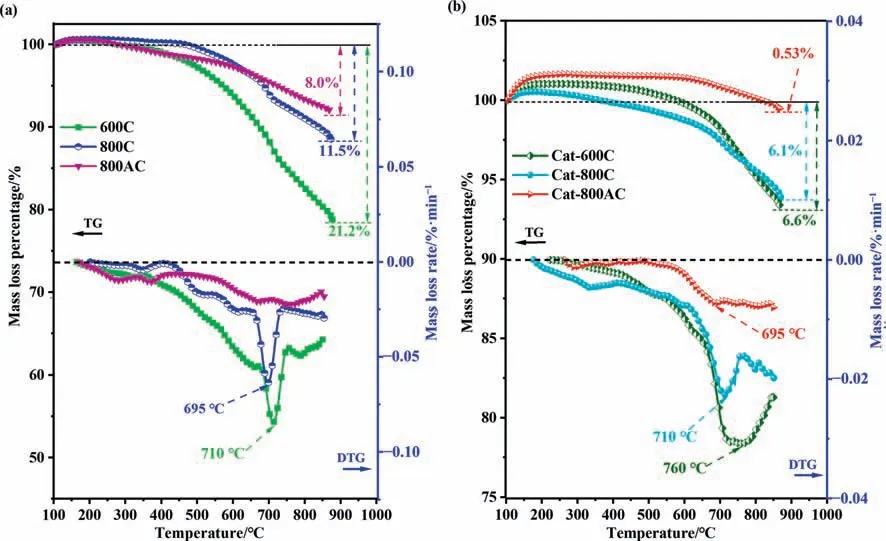
Fig.3.Thermogravimetry and derivative thermogravimetry curves of the fresh and spent char catalysts: (a) fresh char catalysts;(b) spent char catalysts.
3.3.3.Brunauer-Emmet-Teller analysis
The formation of carbon deposit on the char catalyst might affect the pore structures,which was further investigated with the N2adsorption and desorption (Table 3 and Fig.4).Thepyrolysis generated porous structures over the 600C and 800C catalysts.The further activation of the 800C catalyst produced the 800AC catalyst with more developed pore structures.However,the micropores of these chars were largely blocked by carbon deposits from the catalytic pyrolysis,even though the overall weight gain was negligible over the 600C catalyst.Fig.4 showed that the isotherm types of the catalysts before and after use belonged to the types I and III isotherms,respectively[42-44].The nitrogen adsorption of the fresh char catalysts increased rapidly in the region of low relative pressure,indicating the abundance of micropores,especially in 800AC.In addition,the adsorption curves of the used char catalysts were steeply upward at the relative pressure P/P0of 1,suggesting the formation of more macropores.The pore distribution diagram shows that the pore size was concentrated in a narrow region and was mostly microporous (Fig.4).
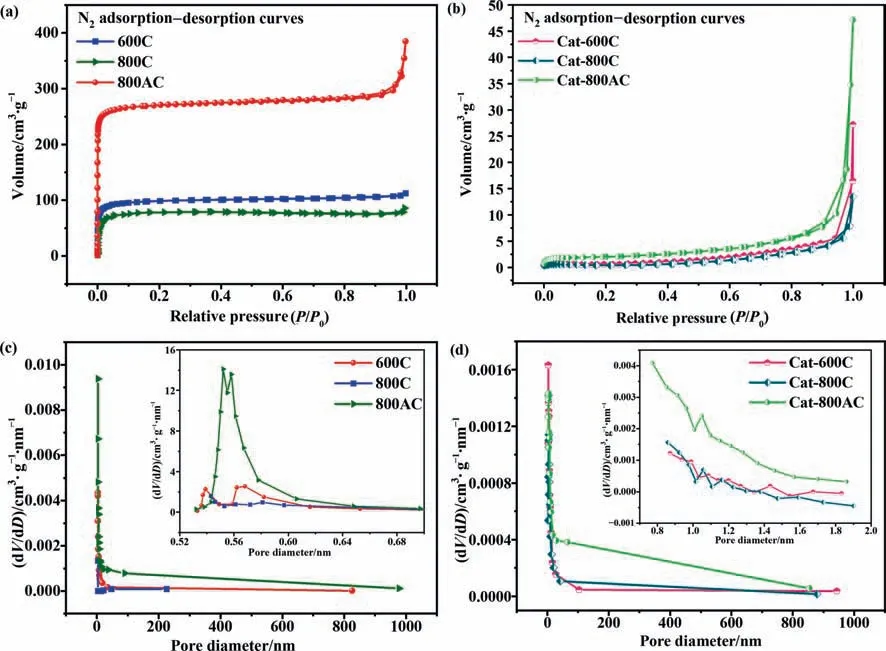
Fig.4.Specific surface area analysis of fresh and spent char catalysts: (a) and (b) N2 adsorption-desorption isotherms;(c) and (d) pore size distribution estimated from t-plot method and Horvath-Kawazoe method.
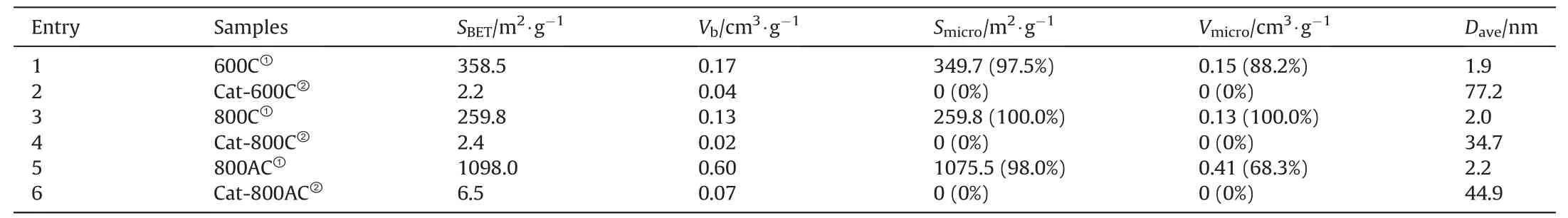
Table 3 Brunauer-Emmet-Teller analysis of the fresh or spent char catalysts
3.3.4.X-ray diffraction analysis
Fig.5(a) shows the crystal structure of the char catalyst before and after the catalytic pyrolysis.The broad peak at 22°was observed for both the fresh and used catalyst,which was related to(0 0 2) crystallographic diffraction,which corresponds to the regularity of the graphitic carbon structure of the biochar[45,46].The weak peak at 43°was characteristic diffraction of (1 0 0) facets of graphitic carbon,which indicated the size of the carbon crystals and the extent of condensation of the polyaromatic ring structures[46,47].The full width at half maxima(FWHM)of the(0 0 2)peak in the used char catalysts in Table S3 generally decreased after thecatalytic pyrolysis,suggesting the higher regularity of aromatic ring structures.The formation of carbon deposits enhanced the aromatization of the organics on the surface of the biochar catalyst.In addition,CaCO3was observed on the char surface,which decomposed to CaO over the 800C catalyst.Nevertheless,the CaO reacted with the CO2generated from the pyrolysis,forming CaCO3again in the used 800C catalyst.In the 800AC sample,the CaCO3was removed via washing with acid,and only the diffraction of carbon crystals was observed.Similarly,the catalytic pyrolysis further enhanced the aromatization and crystallinity of the carbon crystals.

Fig.5.XRD patterns and FT-IR spectra analysis of the fresh and spent char catalysts:(a)XRD patterns of the fresh and spent char catalysts;(b)FT-IR spectra of the fresh and spent char catalysts.
3.3.5.Fourier-transform infrared and hydrophilicity characterization
The functional groups of the char in Fig.5(b) showed the characteristic absorption of -OH,=C-H,-C-H,C=O,C=C,C-O-C,and Ar-H.Compared with fresh 800C,the fresh 600C showed higher absorption of the aliphatic structures-OH,-C-H,and C=O.The increase in pyrolysis temperature enhanced the removal of these functionalities through dehydrogenation,deoxygenation,and aromatization reactions [48,49].The catalytic pyrolysis did not change the distribution of the functionalities much but formed obvious adsorption peaks of CO2.The CO2from pyrolysis adsorbed on the surface of the used catalysts.The hydrophilic nature of the catalysts was further analyzed with contact angle measurement.
Fig.6 shows that the hydrophilicity of 600C and 800C catalysts increased slightly after the catalytic pyrolysis.The catalytic pyrolysis led to deoxygenation/aromatization but also formed some carbonaceous deposits that were more hydrophilic.For the 800AC catalyst,the hydrophobicity of the used 800AC increased significantly,which was possibly due to the filling of the pore structure with carbon deposit,making the surface unfavorable for absorption of water.The lipophilicity test showed that the surface of the char catalysts contained both hydrophilic and hydrophobic sites.
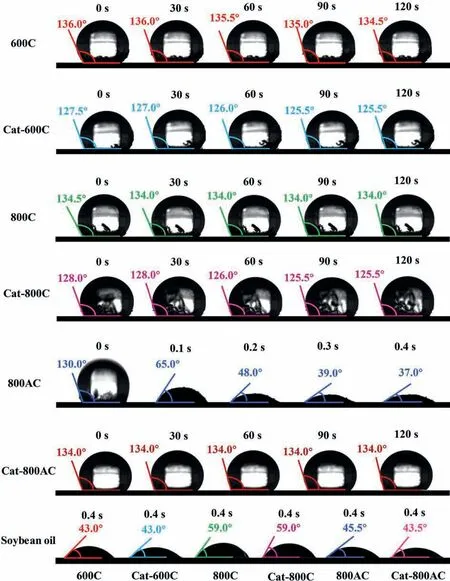
Fig.6.Contact angle of the fresh and spent char catalysts with the surface doped with (a) H2O;(b) soybean oil.
3.3.6.In situ diffuse reflectance infrared Fourier transform spectroscopy analysis of the catalytic pyrolysis
For characterization of the in situ change of functionalities of char,wood was mixed with the three char catalysts (5:1 of mass ratio),well ground,loaded in the in situ cell,and heated in a nitrogen flow.The change of the functional groups of the char with increasing heating temperature is shown in Fig.7.The distribution of the functional group of biochar varies very significantly with increasing temperature.The-OH in sugary and lignin structures was observed at 3540 cm-1[46],the abundance of which increased remarkably and then decreased with further increasing temperature,resulting from their removal via dehydration or dehydrogenation reactions.The transformation of-C-H to=C-H also started at about 400°C,which was part of the aromatization process [50].The abundance of C=O(1700 cm-1) [51,52] in ketones reached a maximum at 450°C,which then slowly decreased with increasing temperature but maintained a significant abundance.The abundance of C=O(1780 cm-1) in lactones was also obvious and thermally stable[51,53].The abundance of C=C and Ar-H increased monotonically with the increase in heating temperature,indicating the dominance of aromatization at the higher reaction temperature.
The presence of the char catalysts impacted the evolution of functional groups on the surface of the biochar.Compared with that in heating wood samples alone,the presence of the 600C catalyst could facilitate the removal of -OH through dehydration or dehydrogenation reactions,as evidenced by the maximum temperature of the abundance at 300°C.In comparison,the abundance of-OH only reached the maximum at 400°C(Fig.8).The effect of 800C and 800AC catalysts did not have a comparable catalytic effect for the conversion of-OH.Additionally,the three char catalysts catalyzed the deoxygenation of the carbonyl functionalities in esters/lactones and also in unsaturated ketones,as seen by their obvious lower absorbance.The organics bearing these functionalities should react with the biochar catalyst,while more detailed routes for their conversion cannot be extracted with the data herein.Additionally,it seemed that the presence of char catalysts did not enhance the aromatization of the biochar formed as the absorbance of C=C and=C-H was lower than that of the feedstock.

Fig.8.The absorbance of various functional groups derived from the in situ DRIFTS spectra of poplar wood pyrolysis and wood pyrolysis catalyzed by three different char catalysts:(a)poplar wood pyrolysis,no catalyst;(b)catalyst:char produced from the pyrolysis of wood at 600 °C;(c)catalyst:char produced from the pyrolysis of wood at 800 °C;(d)catalyst:activated carbon produced from wood activation with K2C2O4 at 800 °C.
3.3.7.Scanning electron microscopy analysis
Morphologies of the char catalysts before and after the catalytic pyrolysis are shown in Fig.9.The 600C prepared by pyrolysis at 600°C partially retained the original fibrous structure of wood,with an uneven surface and the presence of a tubular pore structure parallel to the fibers.The used 600C showed morphology similar to that of bitter gourd,with obvious pore structures and small particles attached to the surface.The particulate matter could be the carbon deposit formed via the volatiles-char interactions.The fresh 800C catalyst showed a rougher and more severely broken surface through the destruction of the original fibrous structure of the wood,due to its high preparation temperature.After the catalytic pyrolysis,the adhered bulky particles on the surface of the 800C became more obvious,which was attributed to the carbon deposits on the surface by the interaction of volatiles and char,clogging the pore channels.The fresh 800AC had a very rough surface with broken structures.The mesoporous and micropore structures with more regular ellipses could be observed,which was expected from the activation with K2C2O4.However,the pore channels were blocked by the presence of an adhesion substance on the surface of 800AC after the catalytic pyrolysis.The macropores could hardly be observed,and the surface became smooth.The coke deposits filled the pores from the interaction of the volatiles with the char.
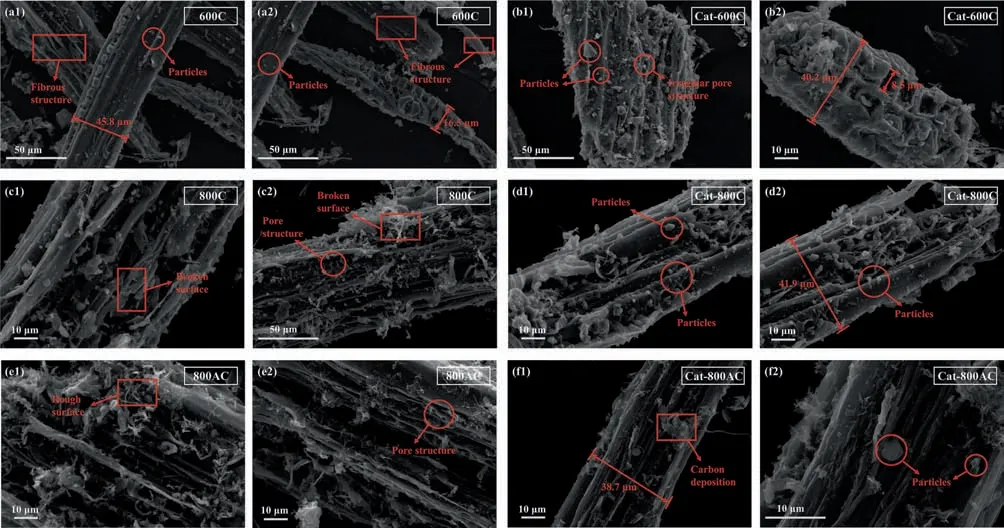
Fig.9.Surface morphology of the fresh and spent char catalysts: (a1,a2),(c1,c2),and (e1,e2): fresh char catalysts;(b1,b2),(d1,d2),and (f1,f2): spent char catalysts.
4.Limitations and Future Prospects
The results showed significant changes in the properties of the spent char catalysts,such as thermal stability,hydrophilicity/hydrophobicity,aromatic properties,and morphology as well as their varied capability for catalyzing the conversion of the volatiles from the pyrolysis.Overall,the oxygen-containing functionality played more important roles than the pore structures for catalyzing the conversion of the organics in bio-oil.However,some problems remain to be further investigated.Firstly,how the oxygencontaining functionalities on 600C are involved in the conversion of the volatiles needs to be further investigated.The results herein demonstrated the high activity of the catalyst for the conversion of the sugary derivatives and also the formation of more phenolics,but a clear picture of the overall reaction network is missing.Secondly,in pyrolysis,the functionalities of biochar changed constantly versus increasing temperature,suggesting also the change of its reactivity with volatiles passing on its surface.How the dynamic change of reactivity of biochar affects the interaction with volatiles in pyrolysis deserves further attention.Thirdly,the pore structure of the char catalyst may not be active for conversion of the volatiles,but they could trap some volatiles.With prolonged reaction time,the trapped volatiles could be further carbonized themselves or with the aid of the pores.The detailed roles of the pore structures in the conversion of volatiles need to be further investigated as well.
5.Conclusions
In summary,three char catalysts termed 600C,800C,and 800AC were produced via pyrolysis of poplar wood at 600,800°C or activated at 800°C and were used to catalyze pyrolysis of the homologous biomass at 600°C,aiming to probe change of nature of the char catalysts.The results showed that the 600C catalyst with abundant oxygen-containing functionalities was more active than 800C and 800AC catalysts for catalyzing cracking of the organics in bio-oil into lighter organics or gases.This reduced tar content and also an abundance of anhydrate sugars and their derivatives (alcohols,ketones,aldehydes,and even carboxylic acids).Furthermore,the 600C and 800C catalysts enhanced the aromatization to form more phenolics of single rings or multiple rings (heavy phenolics).The developed pore structures of the 800AC did not effectively promote the cracking of the volatiles or the aromatization reactions to form the heavy organics with π-conjugated structures.Functionalities rather than pore structures played a more pronounced influence on enhancing catalytic activity for cracking reactions.Nevertheless,the organics entering the pores of the 800AC catalyst could experience sufficient carbonization,forming a very aromatic carbonaceous deposit,increasing thermal stability,and leading to weight gain.Similar mass gain after the catalytic pyrolysis was also observed over 800C,due to the formation of the carbonaceous deposits of a very aromatic nature.This was not observed over the 600C catalyst as the existence of a balance of deposition of carbonaceous material and their removal via gasification in the catalytic pyrolysis.Nonetheless,the substance exchange did result in the increase of carbon content by 5.5% while the decrease of oxygen content by 50%over the used 600C catalyst,which significantly increased the thermal stability,owing to the increased aromatic degree after the catalytic pyrolysis.The formation of the very aromatic carbonaceous deposit also blocked the micropores of these char catalysts but formed some additional macropores,which also rendered the used char catalyst with the dotted substance or adhered particulate matter on the surface.The in situ DRIFTS analysis of the catalytic pyrolysis showed that the 600C catalyst could facilitate the removal of -OH through dehydration/dehydrogenation,while the removal of the carbonyl functionalities in esters/lactones/unsaturated ketones was promoted over all the char catalysts.Given the importance of oxygencontaining functionalities in the interaction with the volatiles,the surface functional groups of a char catalyst could be further tailored to optimize the activity in catalytic pyrolysis.
CRediT Authorship Contribution Statement
Li Qiu:Conceptualization,Investigation,Data curation,Formal analysis,Validation,Visualization,Writing-Original draft.Chao Li:Investigation,Validation,Visualization,Writing-Review and Editing.Shu Zhang:Resources,Supervision,Writing-Review and Editing.Shuang Wang:Resources,Supervision,Writing-Review and Editing.Bin Li:Resources,Supervision,Writing-Reviewand Editing.Zhenhua Cui:Supervision,Writing-Review and Editing.Yonggui Tang:Supervision,Writing-Review and Editing.Obid Tursunov:Supervision,Writing-Review and Editing.Xun Hu:Resources,Methodology,Data curation,Validation,Supervision,Writing-Review and Editing,Project administration,Funding acquisition.
Declaration of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Data will be made available on request.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51876080) and the Program for Taishan Scholars of the Shandong Province Government.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.09.002.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
