Insight into the experiment and extraction mechanism for separating carbazole from anthracene oil with quaternary ammonium-based deep eutectic solvents
2024-04-22XudongZhangYanhuaLiuJunShenYugaoWangGangLiuYanxiaNiuQingtaoSheng
Xudong Zhang,Yanhua Liu,Jun Shen*,Yugao Wang,Gang Liu,Yanxia Niu,Qingtao Sheng
College of Chemical Engineering and Technology,Taiyuan University of Technology,Taiyuan 030024,China
Keywords: Carbazole Model anthracene oil Deep eutectic solvents COSMO-RS Extraction mechanism
ABSTRACT Carbazole is an irreplaceable basic organic chemical raw material and intermediate in industry.The separation of carbazole from anthracene oil by environmental benign solvents is important but still a challenge in chemical engineering.Deep eutectic solvents (DESs) as a sustainable green separation solvent have been proposed for the separation of carbazole from model anthracene oil.In this research,three quaternary ammonium-based DESs were prepared using ethylene glycol (EG) as hydrogen bond donor and tetrabutylammonium chloride (TBAC),tetrabutylammonium bromide or choline chloride as hydrogen bond acceptors.To explore their extraction performance of carbazole,the conductor-like screening model for real solvents (COSMO-RS) model was used to predict the activity coefficient at infinite dilution (γ∞) of carbazole in DESs,and the result indicated TBAC:EG (1:2) had the stronger extraction ability for carbazole due to the higher capacity at infinite dilution (C∞) value.Then,the separation performance of these three DESs was evaluated by experiments,and the experimental results were in good agreement with the COSMO-RS prediction results.The TBAC:EG (1:2) was determined as the most promising solvent.Additionally,the extraction conditions of TBAC:EG (1:2) were optimized,and the extraction efficiency,distribution coefficient and selectivity of carbazole could reach up to 85.74%,30.18 and 66.10%,respectively.Moreover,the TBAC:EG(1:2)could be recycled by using environmentally friendly water as antisolvent.In addition,the separation performance of TBAC:EG (1:2) was also evaluated by real crude anthracene,the carbazole was obtained with purity and yield of 85.32%,60.27%,respectively.Lastly,the extraction mechanism was elucidated by σ-profiles and interaction energy analysis.Theoretical calculation results showed that the main driving force for the extraction process was the hydrogen bonding((N–H...Cl)and van der Waals interactions(C–H...O and C–H...π),which corresponding to the blue and green isosurfaces in IGMH analysis.This work presented a novel method for separating carbazole from crude anthracene oil,and will provide an important reference for the separation of other high value-added products from coal tar.
1.Introduction
Carbazole is an indispensable organic chemical raw material and intermediate,mainly derived from crude anthracene oil [1].Due to its special rigid molecular structure,carbazole exhibits numerous unique properties,making it highly valuable and extensively utilized in various industries,including dyes[2],pharmaceuticals [3],synthetic resins [4],pesticides [5],and photoelectric materials [6].With the further expansion of its application,the demand for carbazole in the market has increased rapidly.Therefore,developing carbazole production technology with a low-cost and environmentally friendly has become a hot topic for many researchers.
Although carbazole can be chemically synthesized [7],more than 90% of carbazole is still obtained by separation and purification of crude anthracene oil due to its lower cost.The structure and many physicochemical properties of components in anthracene oil are very similar,which poses a major challenge to the separation of carbazole.The common methods for carbazole separation include sulfuric acid and potassium fusion methods,distillation,emulsion liquid membrane [8],zone melting [9],supercritical fluid extraction [10],and solvent crystallization[11,12].Generally,sulfuric acid and potassium fusion methods have been abandoned due to the use of strong acids and alkalis,which result in the generation of significant amounts of industrial wastewater.Additionally,separating carbazole by distillation is time and energy-consuming due to the close boiling points of components in crude anthracene oil.The new supercritical fluid extraction technique requires rigorous conditions and specialized equipment.The zone melting technology demands strict temperature control to prevent crystal agglomeration.The emulsion liquid membrane method is limited by the poor stability of the membrane system.These methods mentioned above are immature and still in the laboratory research stage.In chemical industry,solvent crystallization is the most frequently used method for separating carbazole.However,the organic solvents employed in this process exhibit poor performance for isolating the target component,leading to significant problems in the current process.These problems include the need for multiple washing and crystallization,low product yield,high solvent usage,and environmental pollution,etc.Therefore,in order to address the mentioned challenges,it is urgent to develop an environmentally friendly and highly efficient solvent to replace the traditional solvents to achieve the green production of carbazole from crude anthracene oil.
Recently,ionic liquids (ILs) have gained attention as promising alternatives to conventional organic solvents due to their exceptional properties,such as low saturated vapor pressure,high thermal stability and high solubility,which make them suitable for various separation processes [13–21].For carbazole,they also exhibited good separation performance.For instance,[PMPIP][Ac]showed high selectivity in separating anthracene and carbazole[22].[PM2IM][TFAc] was synthesized to separate carbazole from crude anthracene,and the carbazole with purity of 81.60% was obtained [23].Nevertheless,although ILs are effective extractants for carbazole separation from mixture,their large-scale applications are hindered due to the high cost,complicated synthesis process,poor biocompatibility,and corrosivity [24].
Deep eutectic solvent(DES)is a novel green solvent introduced by Abbottet al.[25],and has received significant attention in recent years [26–28].DESs are mixture formed by combining hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD)and they have a much lower melting point than that of the individual constituents[29].Furthermore,the physicochemical properties of DESs can be tailored by changing the nature of components and their molar ratio.DESs can be considered as potential alternatives to ILs,because DESs retain most of the advantages of ILs while overcoming many of their drawbacks.DESs are nontoxic,better biocompatible and biodegradable,less expensive and easier to prepare,which enable them more attractive for various applications in the fields of electrochemistry [30],catalysis [31],synthesis [32],material preparation [33],and separation processes [34–39].
Reports on carbazole-related DESs were mainly concentrated in the field of denitrification from liquid fuel.However,the research on the separation of carbazole from crude anthracene oil by DESs was scarce,and in the only report by our group,the carbazole was separated from model anthracene oil byin situformation of DES with tetraethylammonium chloride(TEAC)[40].Nevertheless,this approach was constrained by the stringent requirements for hydrogen bond acceptors,which required that the organic salt should form DES with the target solute and not dissolved with the raffinate[41].Another method was that the DESs were directly used as extractants to separate target component from oil mixture.This approach was performed at room temperature through liquid–liquid contact,which facilitated a faster attainment of extraction equilibrium.Quaternary ammonium-based DESs have demonstrated excellent separation performance in many different systems,including azeotropes separation [42,43],recovery of high value-added products [44–47],desulfurization and denitrification from fuel oil [48,49].
Therefore,in this work,the performance of separating carbazole from crude anthracene oil based on three quaternary ammoniumbased DESs was investigated.The effects of HBA structures on extraction performance was predicted by the conductor-like screening model for real solvent (COSMO-RS) and then validated by experiments.Moreover,the extraction conditions were also optimized.In addition,the extraction mechanisms were explained through σ-profile,interaction analysis,and FT-IR validation.Lastly,the reusability of DES was also explored.
2.Experimental
2.1.Chemicals and materials
Tetrabutylammonium chloride (TBAC,≥99.5%) was supplied from Macklin Chemical (China).Quinoline (99.7%) was purchased from Boxun Industrial (China).Tetrabutylammonium bromide(TBAB,≥99.0%),choline chloride (ChCl,≥99.0%),carbazole(≥97.0%),anthracene (≥99.0%),fluorene (97.0%) were supplied by Aladdin Chemical(China).Ethylene glycol (EG,99.7%) and toluene(99.7%) were supplied by Damao reagent (China).Crude anthracene was obtained from steelworks,Wuhan,China.The chemical reagents were employed directly without further purification.
2.2.Preparation of DESs and model anthracene oil
In this work,three quaternary ammonium-based DESs,namely TBAC:EG (1:2),TBAB:EG (1:2),and ChCl:EG (1:2),were prepared according to the method mentioned in our previous work [35].Briefly,HBA and HBD were mixed at a mole ratio of 1:2,and then vigorously stirred in a round bottom flask at 90 °C for 1 h until homogeneous liquid was formed.The as-prepared DESs were dried in a vacuum oven at 70 °C for 24 h to remove extra water.The water mass contents of three DESs after drying were measured by Karl Fischer coulometric titration,and they were all less than 0.5%.
Since the complex composition of real anthracene oil,it is difficult to directly explore DESs ability to extract carbazole from actual anthracene oil.According to Ref.[50],carbazole,anthracene,fluorene and quinoline were chosen as representatives of low acidity,aromaticity,neutrality and alkalinity to prepare model anthracene oil.Toluene was selected as the solvent.The specific preparation process can be referred to our previous work as well[40].
2.3.Extraction and reextraction process
Extraction.A certain amount of DESs and model anthracene oil were added to a graduated glass vial.The mixture was put into a water bath that was preset at the expected temperature and agitated with magnetic stirrer at 1000 r∙min-1for a certain time.Then,let it stand for a while,two phases could be clearly observed in the vial and carbazole was enriched in the DES phase (lower layer).The volume of the two phases was carefully measured and then taking a small amount of sample out from the two phases to analyze their composition.
Reextraction.As mentioned above,carbazole was enriched in the DES phase.Although the DES exhibited outstanding extraction performance for carbazole,small amount of neutral oil was coextracted into the DES phase.Therefore,in order to obtain highpurity carbazole product,the neutral oil entrained in the DES extraction phase must be removed.4 ml CS2was added into a vial with DES extraction phase,after stirred at room temperature for a certain time,then settled for 3 h to ensure complete phase separation where the upper layer was DES extraction phase removed neutral oil,while the lower layer was CS2phase.The upper layer was taken out and put in another vial and then added a certain amount of deionized water,the white mixture was formed after ultrasonic shaking.Finally,filter cake (carbazole product) and filtrate(the aqueous solution containing DES)were acquired through filtering operation at room temperature.The whole separation and recovery process of carbazole was exhibited in Fig.1,DESs and all other involved solvents could be recycled,which would significantly reduce the operating cost and made the method more scalable and usable.

Fig.1.Flow diagram of separation and recovery process of carbazole.
2.4.Analytical and calculation methods
The concentrations of four components in raffinate phase,extraction phase and recovered carbazole product were determined by gas chromatograph (GC,Aligent 7820A,USA) equipped with a capillary column (HP-5),flame ionization detector (FID),and automatic sampler.The specific detection conditions were as follows: the temperature of injector and FID were both 300 °C,the column temperature was started at 100 °C and held for 1 min,then increased to 200 °C at a rate of 10 °C∙min-1and kept 2 min.The standard curves of the four components were the same as those in our previous work [40],as shown in Table 1.

Table 1 The standard curves of four components in model anthracene oil
The carbazole,DESs,DESs+carbazole and recycled DESs were analyzed with the Fourier transform infrared spectrometer (FTIR,Shimadzu IRAFFINITY-1,Japan).KBr tableting method was adopted.The instrument resolution and scanning times were 4.0 cm-1and 36 times/s,respectively.OMNIC software was adopted to amend the FT-IR spectrum.
To evaluate the separation performance of DESs,five parameters,including extraction efficiency(EE,%),distribution coefficient(DI),selectivity (SE,%),purity (%),and yield (%) were adopted and their calculation formulas were the same as reported in our previous work [40] and shown as follows:
In Eq.(1),C0andCUrepresented the concentration of carbazole in the initial model anthracene oil and in the upper layer after extraction,respectively.V0andVUrepresented the volume of the initial model anthracene oil and the upper layer,respectively.In Eq.(2),CLrepresented the concentration of carbazole in the lower layer after extraction,andVLrepresented the volume of lower layer.In Eq.(3),Cirepresented the equilibrium concentration of each component in the lower layer.In Eq.(4),C1represented the concentration of carbazole in carbazole product,andCjrepresented the concentration of the four components in the carbazole product,Vlrepresented the volume of CH2Cl2used to dissolve carbazole product.Other components were computed in the same way as carbazole.
2.5.Theoretical calculation details
2.5.1.COSMO-RS calculation
The conductor-like screening model for real solvents (COSMORS) developed by Klamt [51] is a predictive model that combines quantum chemical calculations and statistical thermodynamic approaches.At the present time,the COSMO-RS model has been widely used for the prediction of thermodynamic properties of fluid mixtures without requiring experimental data [52].In this work,the σ-profiles,the activity coefficient at infinite dilution(γ∞),the free volume(Vf),fractional free volume(FFV)and interaction energy between solutes and DESs were predicted using COSMO-RS model.According to the COSMO-RS method,the total interaction energy can be divided into three main contributions,namely: misfit interaction (EMisfit),hydrogen bonding interaction(EHB),and van der Waals interaction (EvdW).The mathematical expressions ofEMisfit,EHB,EvdWand γ∞can be referred to Klamts′works [53].The values ofC∞were used to estimate the capacity of solutes in the DESs at infinite dilution and the equation was shown below:
where γ∞is activity coefficient at infinite dilution of solute in DES at 298.15 K and the subscript solute includes carbazole,quinoline,anthracene and fluorene.
The FFV is defined as the ratio of empty space to occupied space and its formula is as follows [54]:
whereVmoleculerepresents the volume occupied by a molecule calculated by its density and molecular weight,VCOSMOandVfare the volume enclosed by the charge screening surface and the free volume of DESs,respectively.The FFV is defined as the ratio of empty space to occupied space,which reflects the compactness of DESs.TheVmoleculeandVCOSMOwere calculated by the COSMO-RS model.
DESs were represented in the COSMO-RS calculation using the electroneutral approach,which was the same as that of ILs [55].All COSMO-RS calculations were performed using the COSMOthermX software package (version 21.0) with BP_TZVP_21 parametrization and the interaction energy between DESs and solutes were obtained through the “mixture” properties calculation.All COSMO files,including carbazole,quinoline,anthracene,fluorene,toluene,HBA,and HBD,were directly obtained from the standard database.
2.5.2.Quantum chemistry calculation
In this work,the extraction mechanism was further explored at the molecular level.A series of substances,including TBAC,TBAB,ChCl,EG,toluene,fluorene,anthracene,carbazole,and fluorene,were optimized using TmoleX2021 at the B3LYP-D3(BJ)/def-SVP theoretical level to obtain the geometric configuration with the lowest energy[56].The vibration frequencies of all molecules were calculated at the above theoretical level to ensure that there was no imaginary frequency,and then Gussian 09 software [57] was performed to calculate the single-point energy at the M06-2X/def2-TZVP level.Then,independent gradient model based on Hirshfeld partition of molecular density (IGMH) [58] was carried out for complex through the Multiwfn 3.8 program [59] and the sign(λ2)ρ colored δginterisosurfaces were visualized by VMD [60],which could visualize the intermolecular interaction regions intuitively.
3.Results and Discussion
3.1.Exploration of extraction performance of different DESs
For the separation process of carbazole,the selection of DESs extractant is extremely important.In this work,three quaternary ammonium based-DESs,including TBAC:EG (1:2),TBAB:EG (1:2),and ChCl:EG (1:2),were discussed and evaluated for their ability to extract carbazole.
For the structure of studied DESs,fractional free volume(FFV)is an important parameter,which reflects the compactness of the structure.The DES with larger FFV is favorable for extracting target solute[61].The free volume(Vf)and FFV of three DESs were calculated using COSMO-RS model,as shown in Table 2.As seen,the FFV of three DESs greatly decreased with the order of TBAC:EG (1:2)(17.85%) >TBAB:EG (1:2) (17.57%) >ChCl:EG (1:2) (10.51%).Therefore,TBAC:EG (1:2) had the highest potential to extract carbazole due to its larger FFV.

Table 2 The fractional free volume of three DESs calculated by COSMO-RS at 25 °C
The activity coefficients at infinite dilution(γ∞)of solute in the studied DESs were also predicted by COSMO-RS.The value of γ∞described the interaction between the solute and DESs when the concentration of the solute approaches zero and it could be further used for calculating the capacity (C∞) of DESs at infinite dilution.C∞defined the ability of DESs to extract particular component from a mixture.LargerC∞value indicated that DES had a stronger affinity for solute and made it easier to extract the solute from model anthracene oil to the DES phase.As exhibited in Fig.2,for three DESs,theC∞values of carbazole were much higher than that of quinoline,anthracene and fluorene,which indicating that the extraction performance of three DESs for carbazole were all higher than other components.Moreover,TBAC:EG(1:2)had the stronger extraction ability of carbazole ascribed to its higherC∞value.Based on the above theoretical analysis,among the three DESs,the TBAC:EG (1:2) was determined as the most potential solvent for extracting carbazole.In order to verify experimentally,the extraction of carbazole from model anthracene oil using the three DESs were conducted.The experimental extraction performances of DESs for carbazole were evaluated by extraction efficiency(EE),distribution coefficient (DI),selectivity (SE).As shown in Fig.3,all the values of EE,SE and DI of carbazole followed the order of TBAC:EG(1:2)>TBAB:EG(1:2)>ChCl:EG(1:2),which indicating that the TBAC:EG (1:2) showed the highest extraction ability for carbazole among the three DESs and its EE,SE and DI of carbazole could reach up to 85.74%,66.10% and 30.18,respectively,which was consistent with the theoretical analysis.Therefore,the TBAC:EG (1:2) was finally selected as a promising extractant for subsequent experiments.

Fig.2.The infinite dilution capacity (C∞) of different solutes in three DESs predicted by COSMO-RS.
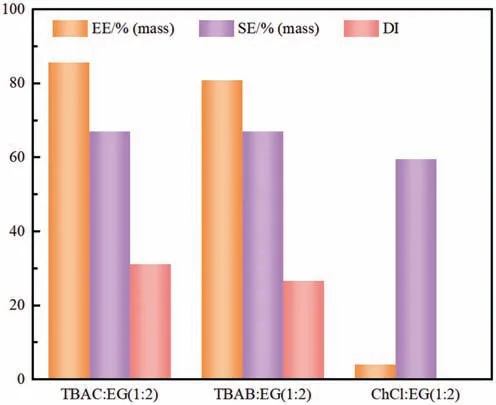
Fig.3.Effects of different DESs on the extraction of carbazole from model anthracene oil (mass ratio of DESs to model oil=1: 10;temperature,25 °C;extraction time,30 min;initial concentration of carbazole,5000 mg∙L–1).
3.2.Experimental optimization of extraction conditions
3.2.1.Mass ratio of DES to model anthracene oil
The usage of extractant is one of the important parameters in the industrial separation process.In general,it is expected that the relatively high separation performance can be obtained by using small amount of extractant.Under 298.15 K,the extraction time was 30 min,the effects of the mass ratio of TBAC:EG (1:2)to model anthracene oil (MO) on extraction performance wasinvestigated and the result was shown in Fig.4(a).As seen,when the mass ratio of TBAC:EG(1:2)to model anthracene oil increased from 1: 4 to 1:10,the extraction efficiency of carbazole decreased from 95.01% to 85.74%,while the selectivity dramatically increased from 47.87% to 66.01%,and the distribution coefficient did not change significantly.Although the extraction efficiency decreased by 9.27%,the selectivity increased significantly by 18.14%.With the mass ratio continuously increasing to 1:14,the extraction efficiency of carbazole reduced by 8.33%,but the selectivity of carbazole increased only 4.67%.Therefore,a mass ratio of 1:10 was selected and applied in the following experiments,at which the extraction efficiency,distribution coefficient and selectivity of carbazole all exhibited feasible values.

Fig.4.Effects of experimental parameters on the extraction process of carbazole:(a)mass ratio of TBAC:EG(1:2)to MO;(b)extraction temperature;(c)extraction time;(d)initial concentration of carbazole.
3.2.2.Extraction temperature
Temperature is an important factor that affects the thermodynamics of the extraction process.In our work,the effect of temperature on the extraction performance of carbazole was explored in the range of 5 to 75 °C with the mass ratio of TBAC:EG (1:2) to MO at 1:10 for 30 min as displayed in Fig.4(b).As observed,with increasing temperature,the extraction efficiency and distribution coefficient of carbazole gradually decreased,while the selectivity remained almost unchanged.It suggested that the increasing temperature was unfavorable for the extraction process of carbazole,which could be attributed to the mutual solubility of carbazole in toluene increased.A similar phenomenon was also found in the previous studies[62].Therefore,in order to reduce the energy consumption,the subsequent extraction process was carried out at room temperature.
3.2.3.Extraction time
Extraction time was investigated in the range of 1 to 60 min(1,10,20,30,40 and 60 min).The extraction experiment was conducted using the model anthracene oil at 298.15 K with TBAC:EG(1:2)-to-MO mass ratio of 1:10 and the result was shown in Fig.4(c).As seen,the extraction process was completed immediately within 1 min,which the extraction efficiency,distribution coefficient and selectivity of carbazole were around 85%,30 and 66%,respectively,and then kept almost constant as the extraction time continually increased.It indicated that carbazole was transferred rapidly from the oil phase to the DES phase.Therefore,in the following experiments,the extraction time was controlled to 20 min to ensure that the separation system could reach sufficient equilibrium.
3.2.4.Initial concentration of carbazole
Different anthracene oil may have different carbazole concentrations.Thus,different model anthracene oils with different carbazole concentrations (1,3,5,6,and 8 g∙L–1) were investigated.The extraction processes were performed at 298.15 K for 20 min,with the mass ratio of 1:10.As illustrated in Fig.4(d),when the initial concentration of carbazole increased from 1 to 5 g∙L–1,the extraction efficiency and selectivity of carbazole remained basically unchanged,with the initial concentration of carbazole continued to increase,the EE,SE,and DI of carbazole were all decreasing.This result indicated that the extraction capacity of the extractant had reached its maximum value as the initial concentration of carbazole reached 5 g∙L–1.In general,the TBAC:EG (1:2) exhibited good extraction ability for carbazole in a certain concentration range.
3.3.σ-surfaces and σ-profiles analysis
The σ-surfaces and the σ-profiles are obtained based on COSMO-RS theory and can be used to explore the possibility of forming hydrogen bonds between substances.The charge distribution of molecule can be visually observed through the color on its surface and the local polarity of molecular surface can also be determined by analyzing its σ-profiles.Generally,the σ-profile curve is divided into three regions,namely HBD region (σ <–0.82 e∙nm-2),HBA region (σ >0.82 e∙nm-2),and nonpolar region(–0.82 e∙nm-2<σ<0.82 e∙nm-2),which corresponding to the blue,red,and green of the molecular surface,respectively [63].
The σ-profiles of carbazole,fluorene,quinoline,anthracene,and toluene were provided in Fig.5.As seen,anthracene,fluorene and toluene had large peaks distributed in the nonpolar region,which corresponding to the green of their σ-surface in Fig.6(a)–(c).This result showed that they had no possibility to form hydrogen bond with others.For quinoline,it had obvious peaks distributed in the HBA region due to its N atom on the pyridine ring,which corresponding to the red part of Fig.6(d).Among four solutes,only carbazole had a large distribution in the HBD donor area,which attributed to the H atom of the —NH group and corresponding to the blue part of Fig.6(e).The peaks located at the HBD region indicated that carbazole was strongly polar and had strong HBD ability.Therefore,in order to achieve a higher extraction performance for carbazole,it is better for DES having a stronger peak distributed in the HBA region,which will more easily interact with carbazole by forming hydrogen bond.

Fig.5.The σ-profiles of carbazole,quinoline,anthracene,fluorene and toluene.
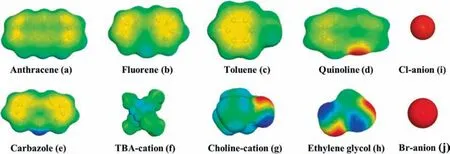
Fig.6.The σ-surfaces of solute,solvent,and components of DESs.
DES is composed of cation,anion,and HBD.The effect of cation and anion structures of HBA on the extraction performance was investigated from σ-profiles analysis,as illustrated in Fig.7.For TBA cation,σ distributed in the range of–1.3 to 0.6 e∙nm-2,which mainly exhibited the nonpolar property and weak HBD ability corresponding to the green and light blue areas in Fig.6(f).However,the σ-profiles of Ch+and EG showed extensive distribution ranging from–2.0 to 2.0 e∙nm-2,and had obvious peaks in three regions,indicating that they had both nonpolar property and strong HBD and HBA abilities corresponding to the green,blue and red areas in Fig.6(g) and (h).For two anions of Cl–and Br–,they all had one strong peak that distributed in the σ range of 1.6 to 2.0 e∙nm-2,which exhibited their strong HBA abilities corresponding to the red areas in Fig.6(i),(j),respectively.

Fig.7.The σ-profiles of cation,anion of HBA and HBD of DESs.
Through the σ-surfaces and σ-profiles analysis,quinoline,EG,Cl–and Br–had the HBA ability,while carbazole,EG,Ch+,TBA+could be considered to have HBD ability.EG had the ability of HBD,which could form DESs with TBAC,TBAB and ChCl by hydrogen bond force due to the strong HBA ability of Cl–and Br–.In addition,the HBA ability of EG was benefit for separating carbazole.Through the comparison of σ-profiles,the HBA ability of Cl–and Br–were much stronger than that of quinoline,making it much easier to form hydrogen bond with carbazole,which allowed carbazole to be successfully separated from the mixture.Compared with TBA+,the HBD ability of Ch+was much stronger,which resulted in the strong hydrogen bonding interaction inside the ChCl molecule.This would weaken the interaction between ChCl and other compound,resulting in lower extraction efficiency for carbazole than TBA+-based DESs.The HBA ability of Cl–was stronger than Br–due to the abscissa of the Cl–peak position was much larger.As a result,the DES with chloride anion had a stronger interaction with carbazole than DES with bromide anion,leading to the higher extraction efficiency of chloride anion-based DES.Based on the above analysis,The DES of TBAC:EG (1:2) had the stronger extraction ability for carbazole than the other two DESs,which was in good agreement with the experimental results.
3.4.Interaction energy analysis
The interaction energies of three DESs with carbazole were calculated by COMSOthermX to explain the extraction mechanism.In COSMO-RS theory,misfit interaction (EMisfit),hydrogen bonding interaction (EHB),and van der Waals interaction (EvdW) were frequently used to describe the interactions between different substances.Generally,the values ofEHBandEvdWwere negative,andEMisfitwas positive,indicating attraction and repulsion,respectively.The interaction energies of three DESs with carbazole were depicted in Fig.8.As seen,the van der Waals interaction energies between the three DESs and carbazole were close,while the hydrogen bonding interaction energies between TBAC:EG(1:2),ChCl:EG(1:2) and carbazole were higher than that between TBAB:EG (1:2)and carbazole.The misfit interaction energy of TBAC:EG(1:2)with carbazole was much lower than that ChCl:EG(1:2)with carbazole.Therefore,TBAC:EG (1:2) was considered as the most promising extractant due to its higher hydrogen bonding interaction and lower misfit interaction.

Fig.8.The interaction energies between three different DESs and carbazole calculated by COSMO-RS.
Based on the above analysis,the interaction energies between the TBAC:EG (1:2) and four solutes was further investigated.As shown in Fig.9,the interactions between TBAC:EG (1:2) and anthracene,fluorene were only van der Waals and misfit interactions,and no hydrogen bonding energies,indicating that the poor extraction ability of TBAC:EG(1:2)for them.Moreover,the hydrogen bonding interaction energy and van der Waals energy of TBAC:EG(1:2) with carbazole were much higher than those with quinoline,so TBAC:EG (1:2) had better extraction ability for carbazole than quinoline.Therefore,the TBAC:EG(1:2)would probably exhibit higher extraction efficiency and selectivity for carbazole,which was in agreement with experimental results.

Fig.9.The interaction energies between four solutes and TBAC:EG(1:2)calculated by COSMO-RS.
3.5.IGMH analysis
Through above analysis,the extraction mechanism was primarily interpreted,but the intermolecular interactions between DESs and carbazole needed to be further visualized,which could be more intuitively understood the mechanism underlying the separation process.The independent gradient model based on Hirshfeld partition(IGMH)can not only visualize the intermolecular interaction areas vividly,but also show the type and intensity of the interaction by projecting the sign(λ2)ρ function onto the isosurface through different colors.Generally,there are three main interactions between the isosurface of the molecules,including attraction,repulsion,and van der Waals interactions,which are represented by blue,red,and green,respectively,and the stronger of the intermolecular force,the deeper of the isosurface color.The intermolecular interaction of TBAC:EG (1:2) with anthracene,fluorene,quinoline,carbazole,toluene,and toluene+carbazole were visualized by IGMH,respectively,and the color-filled IGMH δginterisosurface maps were shown in Fig.10.There was only green isosurface between toluene and carbazole shown in Fig.10(a),indicating that there was a very weak vdW interaction between them.It was worth noting that a large blue isosurface was appeared between the Cl of TBAC:EG (1:2) and H atom of carbazole (shown in Fig.10(b)),indicating that a strong hydrogen bonding interaction(N—H∙∙∙Cl) was formed between them.The above results showed that the interactions of carbazole with TBAC:EG (1:2) was much stronger than that with toluene,which resulting that carbazole could be easily separated from the oil mixture.In addition,it could be seen that the δginterisosurface of TBAC:EG (1:2) with anthracene,fluorene,and toluene were similar (shown in Fig.10(c)–(e)),that was,there were only green isosurfaces,which illustrating that the existence of the weak vdW interaction between them.Furthermore,the blue isosurface of TBAC:EG(1:2)with carbazole was much deeper than that with quinoline,indicating that a stronger hydrogen bonding interaction was formed between TBAC:EG(1:2) and carbazole.Therefore,TBAC:EG(1:2)could exhibit higher extraction ability for carbazole due to its strong interaction than the others,which was consistent with the experimental results.

Fig.10.Color-filled IGMH δginter isosurface maps (isovalue=0.005) for different complexes.
3.6.FT-IR validation
From above mechanism analysis by quantum chemical calculations,the hydrogen bonding interaction between DESs and carbazole played a crucial role in the extraction process.In order to experimentally validate the existence of hydrogen bond,the FTIR spectra of carbazole,TBAC:EG (1:2) and their complex were determined at 298.15 K and presented in Fig.11.As shown in Fig.11,the spectrum of the complex had a series of characteristic peaks similar to those of carbazole or TBAC:EG (1:2),indicating that carbazole was well combined with TBAC:EG (1:2).Moreover,the N—H bond vibrational absorption peak of carbazole appeared at about 3417 cm-1,and its stretching vibration frequency of carbazole in the complex remained relatively unchanged,while the absorption intensity of the stretching vibration frequency of N—H in the complex near 3417 cm-1was much weaker than that in pure carbazole,which indicated that v-NH of carbazole in the complex underwent a change in vibrational state and further demonstrated that the hydrogen bonds was formed between TBAC:EG (1:2) and carbazole [20,50].

Fig.11.FT-IR spectra of the carbazole,TBAC:EG (1:2) and complex.
3.7.Recovery process of carbazole
In order to recover carbazole from DES extraction phase,it was necessary to break the hydrogen bonds that formed between them.As was well known,water was a strong polar compound,which could break the stable hydrogen bond network structure of DES,and the HBA and HBD could be distributed nonstoichiometrically between aqueous phases [64,65],while the solute was“released”from aqueous phase.Therefore,in this work,water was employed as the antisolvent at room temperature to recover carbazole.As described in Section 2.3,the obtained carbazole product (filter cake) dried at 106 °C for 2 h and dissolved in the known volume of dichloromethane,and the concentrations of the four components in recovered carbazole product were measured by GC and the results were shown in ESI(Table S1 in Supplementary Material).Based on the process,the carbazole product with purity and yield of 96.76%,64.31%was finally obtained experimentally from model anthracene oil.
3.8.Reusability of the DES
Considering the economic and environmental aspects,it is necessary to recycle and reuse of DESs after the separation process.Some researchers had demonstrated that hydrogen bonds formed between HBD and HBA were regenerated in DESs when the water was removed [35,66].In our work,the TBAC:EG (1:2) was recovered by rotary evaporation of the filtrate (the aqueous solution containing DES) at 70 °C,and then dried under vacuum at 70 °C for 12 h.The regenerated extractant was used for five successive cycles,and the results were shown in Fig.12.It indicated that the recycled TBAC:EG (1:2) remained its high separation ability for carbazole after five regeneration cycles.Meanwhile,the FT-IR spectra of recycled TBAC:EG (1:2) had the same characteristic peaks as the fresh (shown in Fig.13),which demonstrated its excellent reusability again.

Fig.12.Effects of recycle times of TBAC:EG(1:2)on the extraction process of carbazole(a)and purity and yield of carbazole(b)(mass ratio of TBAC:EG(1:2)to model oil=1:10;temperature,25 °C;extraction time,20 min;initial concentration of carbazole,5000 mg∙L–1).

Fig.13.FT-IR spectra of recycled and fresh TBAC:EG (1:2).
3.9.Investigation separation performance of carbazole with real crude anthracene
According to the above research,TBAC:EG (1:2) was identified as the most promising DES extractant for carbazole separation from model anthracene oil.Considering the industrial application of DES,exploring extraction performance of TBAC:EG (1:2) in separating carbazole from real crude anthracene was necessary.The separation processes were similar to those conducted with the model anthracene oil (described in Section 2.3).After completing a single extraction,the carbazole product was obtained with the purity and yield of 85.32%,and 60.27%,respectively.The good extraction performance of TBAC:EG(1:2)for real crude anthracene demonstrated its suitability as an efficient extractant for carbazole separation and had the potential to achieve large-scale applications in the chemical industry.
4.Conclusions
In this work,three quaternary ammonium-based deep eutectic solvents were applied to separate carbazole from model anthracene oil and real crude anthracene.Through COSMO-RS calculation and experimental verification,TBAC:EG(1:2)was identified as the optimum DES for the separation of carbazole,and the key parameters of the extraction process were optimized.Under the optimal conditions (25 °C,20 min,mass ratio 1:10),the extraction efficiency,selectivity and distribution coefficient of carbazole were 85.74%,66.10% and 30.18,respectively.Moreover,the purity and yield of carbazole product were 96.76% and 64.31%,85.32% and 60.27%,separated from model anthracene oil and real crude anthracene,respectively.In addition,the TBAC:EG (1:2) could be regenerated by water and its extraction performance for carbazole did not change obviously after reusing for five times.Finally,the hydrogen bonding and van der Waals interactions between DESs and carbazole were interpreted by σ-surfaces and σ-profiles,and they were quantitatively calculated by using the COSMO-RS model.The hydrogen bonding interaction of TBAC:EG(1:2)with carbazole was also validated by FT-IR analysis.Comprehensively,TBAC:EG(1:2)can be served as a promising solvent for extracting carbazole from anthracene oil,and will present greater potential application in the separation of other high value-added components from coal tar.
CRediT Authorship Contribution Statement
Xudong Zhang:Formal analysis,Investigation,Writing– original draft.Yanhua Liu:Software,Investigation.Jun Shen:Conceptualization,Writing– review &editing.Yugao Wang:Methodology,Validation.Gang Liu:Resources,Validation.Yanxia Niu:Data curation.Qingtao Sheng:Writing– review &editing.
Data Availability
The data that has been used is confidential.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by Shanxi Province Natural Science Foundation of China (20210302123167);NSFC-Shanxi joint fund for coal-based low carbon (U1610223);and Shanxi-Zheda Institute of Advanced Materials and Chemical Engineering(2021SX-TD006).Besides,we greatly appreciated the research group of Prof.Baojun Wang in Taiyuan University of Technology for their valuable guidance on the quantum calculations of the manuscript.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.08.003.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
