Investigation of oxy-fuel combustion for methane and acid gas in a diffusion flame
2024-04-22SonglingGuoXunTaoFanZhouMengyanYuYufanWuYunfeiGaoLuDingFuchenWang
Songling Guo,Xun Tao,Fan Zhou,Mengyan Yu,Yufan Wu,Yunfei Gao,Lu Ding,Fuchen Wang
Institute of Clean Coal Technology,East China University of Science and Technology,Shanghai 200237,China
Keywords: Acid gas Methane Oxy-fuel combustion Oxidation Chemical analysis Carbon sulfides
ABSTRACT Co-combustion of methane(CH4)and acid gas(AG)is required to sustain the temperature in Claus reaction furnace.In this study,oxy-fuel combustion of methane and acid gas has been experimentally studied in a diffusion flame.Three equivalence ratios (ER=1.0,1.5,2.0) and CH4-addition ratios (CH4/AG=0.3,0.5,0.7)were examined and the flame was interpreted by analyzing the distributions of the temperature and species concentration along central axial.CH4-AG diffusion flame could be classified into three sections namely initial reaction,oxidation and complex reaction sections.Competitive oxidation of CH4 and H2S was noted in the first section wherein H2S was preferred and both were mainly proceeding decomposition and partial oxidation.SO2 was formed at oxidation section together with obvious presence of H2 and CO.However,H2 and CO were inclined to be sustained under fuel rich condition in the complex reaction section.Reducing ER and increasing CH4/AG contributed to higher temperature,H2S and CH4 oxidation and CO2 reactivity.Hence a growing trend for CH4 and AG to convert into H2,CO and SO2 could be witnessed.And this factor enhanced the generation of CS2 and COS in the flame inner core by interactions of CH4 and CO2 with sulfur species.COS was formed through the interactions of CO and CO2 with sulfur species.The CS2 production directly relied on reaction of CH4 with sulfur species.The concentration of COS was greater than CS2 since CS2 was probably inhibited due to the presence of H2.COS and CS2 could be consumed by further oxidation or other complex reactions.
1.Introduction
Hydrogen sulfide,a flammable and toxic gas,is widely spotted in crude natural gas,petroleum well and coal seams and is a common by-product during a number of industries such as coal or biomass gasification,oil refining,gas sweetening,biogas utilization,waste gas purification,waste water treatment and other desulfurization processes[1–4].Generally,acid gas(AG)mainly consists of H2S and CO2and other impurities with low concentration such as hydrocarbons,NH3and CS2and COS [3,4].Meanwhile,it is well known that venting acid gas or its combustion products (mainly SO2) directly into atmosphere without any post processing is forbidden since it can cause various environmental pollution and health hazards[5].Therefore,acid gas must be processed properly to meet the increasingly strict regulations and consideration of environment and safety.Currently,the modified Claus process is widely used for the treatment of acid gas and the recovery of economically valuable sulfur products [1,6].This process consists of three consecutive sections.In the first step,the oxidation of H2S to SO2(Eq.(1)) and the Claus reaction between H2S and SO2(Eq.(2)) to form sulfur (mainly S2) occur simultaneously at over 1273 K in the reaction furnace [7].The second part is a further Claus reaction (Eq.(3)) occurring over catalyst bed at about 573 K in the catalytic converters,rendering S8as the primary product[8,9].The final section is the tail gas treatment or incinerating to meet emission standards.The reactions of the Claus process can be described as follows:
The partial oxidation of H2S in the reaction furnace is deemed as the most critical and complex section for the Claus process[10–13].It includes the generation of up to approximately 70% sulfur and adjustment of the suitable H2S/SO2ratio for subsequent catalytic reaction [14,15].Meanwhile,the impurities in acid gas need to be destroyed to mitigate the negative impact on the catalytic section.However,this process is hard to achieve since the CH4co-combustion is required to maintain the furnace temperature especially when the H2S content is below 50% [16,17].The presence of additional CH4and pre-existing CO2can significantly complicate the reactions in the reaction furnace and further adversely affect the Claus reaction.Not only is there competitive oxidation of H2S and CH4,but unwanted COS and CS2from various side reactions and soot also proceed in the combustion progress,which can degrade catalyst performance and reduce the sulfur recovery efficiency once existing in the catalytic section due to the sulfate corrosion and coverage on the catalytic pores [18,19].
To understand the effects of CH4and CO2on partial oxidation of H2S in the reaction furnace,researchers have been conducting intensively studies in recent decades [20–24].Liet al.[18] has studied the oxidation process of acid gas in a non-premixed flame.As the reactivity in fuel-rich condition was mainly expressed as CO2+H ⇌CO+OH,especially at high temperature,CO2could reduce the H/OH ratio by converting atomic H into OH radical.Meanwhile enhancing oxygen concentration (OC) would raise the flame temperature,accelerate the oxidation of H2S and promote the generation of CO at near-burner region.Selimet al.[25]has investigated the effect of CO2on H2S combustion in a diffusion flame and found that the addition of CO2resulted a drop in flame temperature but contributed to raise downstream temperature.They also summarized the impact of CO2for flame temperature was attributed to its oxidizing medium role in the reaction pool.This was consistent with the results that studied by Ibrahim and Raj [26].Moreover,Leeet al.[27] concluded the primary chemical effects of CO2on flame were its collisional decomposition and pyrolysis,and highlighted the significance of CO2in preventing heat dissipation.In general,the CO2reactivity cannot be ignored during AG combustion.Moreover,compared with CO2,the impact of CH4on H2S oxidation could be more profound and complicated.Based on the study of H2S flame structure by Bernez-Cambotet al.[28],Liet al.[15] investigated the oxidation of H2S and CH4mixture at 1:1 in different ERs (1.0,1.4,2.0) and divided the diffusion flame into three zones: the initial oxidation,the intense burning and the afterburning zones.In the initial oxidation zone,H2S was dominant in the competitive oxidation with CH4injecting at the base of the flame,and it was inferred that H2S was mainly partially oxidized in this process.The mixing and combustion were contained in the second zone wherein major reaction heat was released and CO2,SO2and H2O were substantially formed.The third zone incorporated complex reactions and the Claus reaction (Eq.(2)).Meanwhile Karanet al.[29] discussed CH4was only partially consumed in the flame zone and its main conversion process occurred in the anaerobic zone at the post-flame.Colom-Díazet al.[30] further highlighted the competitive advantage of H2S over CH4to be more significant at high pressures.Similar to CH4,Ibrahimet al.[31–33] has investigated the effect of high hydrocarbon substances represented by BTEX(benzene,toluene,ethylbenzene and xylene) on H2S combustion under Claus condition and found that BTEX significantly promoted the formation of H2.However,H2hindered the oxidation of H2S and the production of SO2,which in turn affected the sulfur yield.The production of COS and CS2was also improved since the favorable production of CO and could react rapidly with S2or SO2to produce COS.It was consistent with the conclusion of Palmaet al.[34].Selimet al.[35] conducted an experimental study of the oxidation of H2S in a CH4-air flame and found trace amounts of high hydrocarbons were generated under Claus condition (ER=3) wherein SO2performed as a coupling catalyst,enhancing the combination of CH3and forming hydrocarbons.This group also considered the formation of CS2from CH4and sulfur-containing substances is more favorable in anoxic environment.
Much of the previous interest of CH4/CO2chemistry has evidently stated the relationship between CH4/CO2and COS/CS2.Chinet al.[36]studied the oxidation of H2S-CH4gas mixture in a tubular reactor at 1273–1473 K.They found the presence of CH4led to faster consumption of H2S and production of CS2.Abiánet al.[19]conducted research about the conversion of COS and CS2and emphasized the role of CS radical in the production CS2,primarilyviareacting with sulfur species such as S,S2or SH.The large amount of CH decomposed by hydrocarbon substances plays a significant role in promoting the formation of CS2viaCH+2H2-S ⇌CS2+2.5H2.Regarding the COS formation,Clarket al.[37]emphasized that CO,despite the presence of CO2,was the direct source to generate COS chiefly through CO+0.5S2⇌COS and CO+H2S ⇌COS+H2in the Claus furnace.This viewpoint was in line with the homogeneous gas phase research in H2S-CO2and H2-S-CO systems from Karanet al.[38].Besides,Liet al.[20,21] discussed the pathway of CH3→CH2S →HCS →CS →CS2in the experimental and mechanistic study of the H2S-CH4system,whereas in the H2S-CO2system the conversion of CS2from COS was emphasized.Meanwhile,the presence of reducing and oxidizing substances is an obvious factor in the tight conversion between COS and CS2[39,40].It can be assumed that the generation of COS and CS2in the H2S-CO2-CH4system could be more complex than that of simply H2S-CO2or H2S-CH4.However,little attention has been paid to this integrated system,which more closely resembles the real environment of the reaction furnace.Moreover,the effect of CH4on the oxidation of acid gas (H2S and CO2) under oxy-fuel combustion was less investigated and the generation of COS and CS2in H2S-rich diffusion flame was still not understood since the previous literatures mostly investigated the H2S reaction through detailed mechanisms or adding rare amount of H2S to other combustibles [13,19,24,29,37].To fill this gap,we aim to study the body knowledge involving CH4-AG rich diffusion flame and oxyfuel combustion.
The experiment was conducted using a coaxial burner in a vertical reactor wherein three equivalence ratios (ER=1.0,1.5,2.0)and CH4addition ratios (CH4/AG=0.3,0.5,0.7) were examined to explore the oxy-fuel combustion behavior of CH4and AG.The reasons for choosing pure oxygen as oxidant also include its ability to make the combustion easier to observe and to overcome the concentration errors caused by interference from N2in the air or oxygen-enriched air.The temperature and concentration distribution of species(H2S,CH4,CO,CO2,SO2,H2,COS,CS2)in central axis were measured and a detailed description regarding the conversion and formation of species was presented under varies feeding modes.
2.Materials and Methods
2.1.Experimental setup
Fig.1 shows the schematic diagram of the experimental setup.The system consists of gas cylinders,mass flow controllers,quartz tube reactor,motor programming controller,temperature monitor,gas component analysis and tail gas treatment.The reactor has an inner diameter of 90 mm,a length of 300 mm and a thickness of 5 mm,with a 15 mm thick layer of fine quartz cotton wrapped around the outside for insulation.A double concentric tubular burner,with an annular obtuse body at the upper end of the central passage,is installed in the center of the bottom of the reactor to ensure a stable flame.H2S (99.9%,purity),CO2(99.9%) and CH4(99.5%) are regulatedviamass flow controllers,mixed thoroughly in a longer pipe and fed into the central passage of the burner.The oxidant of O2(99.9%)is fed into the annular gap passage of the burner.N2(99.9%) is the safe gas after the experiment procedure.Noted that the cabinet for gas cylinders of H2S,CH4and CO2are placed at opposite corner of the laboratory to gas cylinders of O2and N2.

Fig.1.Scheme diagram of experimental setup.
Partial combustion of H2S/CO2/CH4produces sulfur/soot that could condense and adhere to the quartz wall,thus it is extremely difficult to illustrate the shape of the flame using a high-speed camera.Being a toxic gas,it is dangerous to combust directly without the reactor to take clear pictures of the flame.Therefore,thermocouple and quartz tube were used to obtain the central axial temperature and concentration distribution of the gas components in the reactor [18,31].A motor programming controller was used to control the up and down movement of the module on the rail.By fixing a thermocouple/quartz tube on the module,temperature measurement or sampling was achieved.Noted that the temperature measurement and gas sampling were not carried out simultaneously,but twice for the same experimental condition.The thermocouple was a K-type thermocouple with a 2.3 mm diameter bead.The detected points for gas samples were chosen to be 10,25,40,55,70,85,100,115 and 130 mm from the burner exit and for temperature measurement were considered more in the flame zone to outline a clear distribution.The combustion was considered to be stable when the temperature fluctuated by less than 3 K within 30 s and the temperature measurement data was recorded by a temperature monitor.Gas samples were taken at a rate of 2 ml∙s-1from each detected point using a thin quartz tube(2.2 mm inner diameter,4 mm outer diameter),and accessed to air bags after three consecutive gas treatments in gas washing bottles,i.e.quenching through water,sulfur separationviafine quartz wool and drying in silica gel bottle.Fine syringes were used to pump gas from air bags into GC (gas chromatograph) analysis for analyzing component concentrations.The tail gas was treated with sulfur trap and NaOH solution,then discharged into a negative pressure ventilation system.
In GC analysis,a thermal conductivity detector (TCD) in series with a flame photometric detector (FPD,column type GDX-303)was used to determine the concentration of sulfur-containing substances,where the column was maintained at 339 K.The concentration of non-sulfur components was determined in tandem with a chromatographic column.Between TCD and FID was a converter equipped with an active nickel catalyst,which contributed to accurately measure the concentration of CO and CO2at a column temperature of 333 K and a catalytic oven temperature of 593 K.
The experiment was conducted in a fume hood after checking the fixed alarms and pipeline gas tightness.Experimenters must wear gas masks and body alarms,and the laboratory need to be kept ventilated.Considering the toxicity of H2S,acid gas cannot be directly ignited at the beginning of the experiment.To deal with this difficulty,CH4was firstly injected and flamed.After the flame was stabilized,H2S and CO2were slowly injected and the flame color gradually changed from yellow to blue.This phenomenon showed the H2S started to flame.Afterwards,each gas was adjusted to the specified flow rate according to the experimental conditions.A pump with a flow meter was used to extract the gas to maintain stable pressure in the reactor,typically at a value of 0.6–0.7 times the inlet gas flow based on experimental experience.The control module was operated to adjust the K-type thermocouple (or sampling tube) to the specified position and the temperature (or gas sampling) was measured.The experiment was stopped by closing the H2S cylinder valve.The residual H2S in the pipeline has been burned out before closing the CH4cylinder valve.Then,CH4was eventually consumed and the flame was extinguished.Finally,N2was filled with the pipeline and the fume hood was ventilated for 30 min.
2.2.Parameter definition
To have good universality,the dimensionless axial distance is defined by the following equation:
whereD0is the inner diameter of the burner (D0=3 mm),andXis the axial distance from the surface of the burner to the outlet(X=0 is the surface of the burner).And equivalent ratio (ER) is given as follows:
The theoretical oxygen feed is the amount of O2that can exactly oxidize H2S to SO2and CH4to CO2which is calculated as follows:
2.3.Experimental conditions
Experimental conditions are presented in Table 1.Two variables of equivalence ratio(1.0,1.5 and 2.0)and CH4/AG(0.3,0.5 and 0.7)were considered to study their impacts on flame temperature and the conversion and formation of main species under oxy-fuel condition.
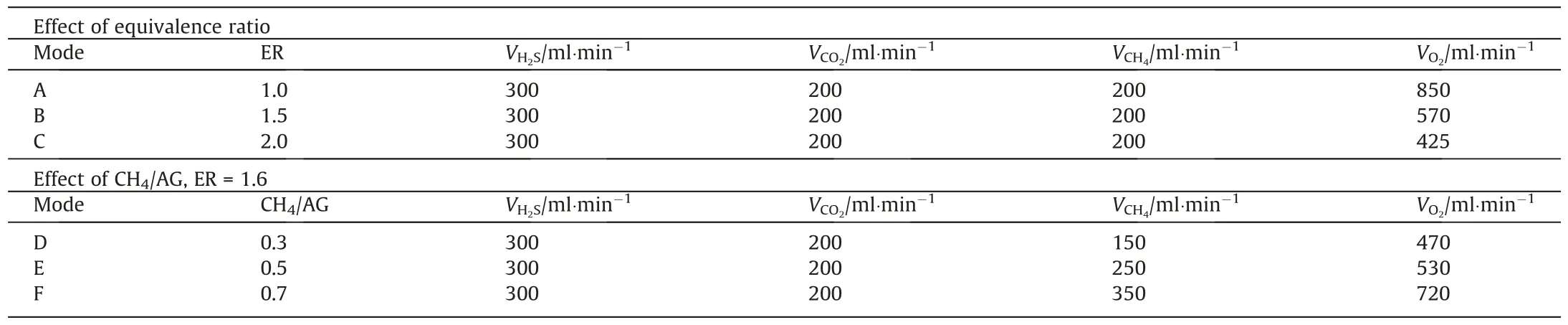
Table 1 Experimental conditions in this study
2.4.Analysis of errors on measured temperature
2.4.1.Analysis of error on measured temperature
When the thermocouple temperature is stable,the following equation of conservation of energy should be satisfied on the surface of the beads [41]:
whereTg,TbandTwdenote temperatures of the gas,the electric coupling bead and the reactor wall,respectively.The K-type thermocouple used in this experiment is a nickel–chromium alloy with a coupling bead emissivity of ε=0.31 [42].σ is the blackbody radiation constant and the value is 5.67 × 10–8W∙m-2∙K-4.The correlation equation for the fluid outer swept sphere can be determined by the following equations [41,43]:
Nu,PrandRerefer the Nussle,Prandtl and Reynolds number,respectively.μwand μbare the kinetic viscosities at wall and bead thermocouple temperatures.λ is the thermal conductivity andDbstands for the diameter of the bead thermocouple with a value of 1.5 mm.hfis the convective heat transfer coefficient between the electric coupling beads and the airflow.ρ,v andCprepresent the density,velocity and the heat capacity,respectively.
The calculation is based on the error of temperature measurement at the highest temperature.For example,the highest measured temperature that appears in mode A is 1262 K atD≈3 [18].As the gas diffusion here was not completely developed near the burner,the velocity at the measured point can be obtained as approximately 7 m∙s-1considering the gas flow,nozzle size and airflow expansion [18].The thermal properties of the gas mixture at the highest temperature are λ of 0.0833 W∙m-1∙K,ρ of 0.3416 kg∙m-3,Cpof 2453 J∙kg∙K-3and μ of 4.17×10–5Pa∙s.HenceRe,Nuandhfare 133.57,8.486 and 307.34 W∙m-2∙K-1.The gas temperature (Tg) is approximately 1388 KviaEq.(7).The relative error of mode A is 9.08% according to the following equation:
Utilizing the same method,the gas temperatures (Tg) in modes B-F are worked out to be 1174 K,995 K,1063 K,1155 K and 1198 K,respectively,and the corresponding relative errors are 6.73%,4.72%,5.93%,6.15% and 6.26%,respectively.
2.4.2.Uncertainty analysis
The uncertainty of key parameters of ER and CH4/AG ratio are calculated based on the method in Refs.[15,18].The specific calculation steps are as follows:
In the Eq.(12),mfuelandrefer the mass flow rates of fuel and O2,respectively.The subsets act and sto are the actual and stoichiometric data,respectively.represent the volumefraction of CH4and H2S,respectively.EERis the uncertainty of the key parameter ER.denote the maximum volume flow rate of O2and CH4,respectively.δVis the flow control accuracy and it is 0.001 L∙min-1for H2S and CH4and 0.01 L∙min-1for O2.Hence the uncertainty of ER can be calculated to be 1.47%.
Based on the equations above,the uncertainty for CH4/AG ratio can be worked out to be 0.71%.
3.Results and Discussion
3.1.Experimental study of CH4-AG combustion: Effect of equivalence ratio
Fig.2 shows the temperature curves of modes A,B and C.Similar trends of temperature distributions for all modes could be noted as obvious typical diffusion flame characteristics.The axial temperature increased from burner exit to a peak value of 1262 K,1095 K and 948 K at ER of 1.0,1.5 and 2.0,respectively,and dropped almost linearly.This is attributed to the heat dissipation from heat convection with ambient gas and radiation with the reactor wall [18].The flame temperature increased significantly with decreasing ER,since enhanced oxygen feed favored CH4-AG combustion and released more heat.Furthermore,the peak axial temperature of mode A was reached at a relatively farther location compared with mode C.It could be interpreted as the increased oxygen flow rate raising the height of the core combustion zone.This also contributed to a shorter residence time and greater heat loss in the reactor.Consequently,the temperature gap between peak value and the reactor end was 412 K,356 K and 259 K in modes A,B and C,separately.

Fig.2.Measured temperature profiles along D.
Volume fraction distributions of H2S and SO2for modes A,B and C are presented in Fig.3.It should be noted that the initial point(D=0) is determined by species content in the premixed fuel gas and the first sampling point isD=3.3.The volume fraction of H2S in all modes decreased promptly near the burner and subsequently experienced a relatively moderate conversion downstream.For the combustion of H2S,investigators confirmed the H2S conversion was primarily classified into chemical and thermal decomposition and oxidation[28,35,44].Based on the distribution of temperature and SO2volume fraction,the chemical decomposition of H2S,regarded as the very initial step of H2S consumption,occurred at the vicinity of the burner exit from which SH and H2but not SO2was the primary product (Eqs.(23) and (24)) [45,46].However,thermal decomposition of H2S (Eq.(25)) was only likely to proceed with a relatively apparent rate in mode A since the temperature was reaching the recommend value of 1273 K [47,48].
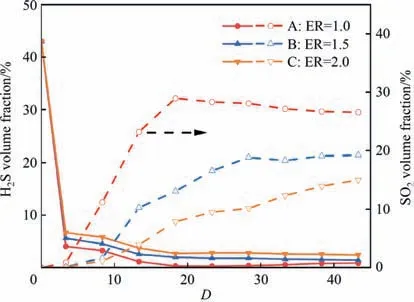
Fig.3.Measured volume concentrations of H2S and SO2 along D.

Fig.4.Measured volume concentrations of CH4 and CO along D.
As the H2S decomposition proceeded,the H2S partial oxidation took place with the aid of radicals like O and OH (Eqs.(26) and(27)) wherein the mixing of fuel and oxidant was more adequate[35].Moreover,a minor portion of H2S was also converted to COS and CS2simultaneously in the presence of CH4and CO2.The interaction with hydrocarbons is deemed as the third factor for H2S consumption [15].In this respect,Rhodeset al.[49] reported that the generation of COS and CS2could be achieved by the related reactions (Eqs.(28)–(30)) .Consequently,the H2S concentration decayed to only about 5% atD=3.3 and the temperature here indicated an intense combustion.
In the subsequent section,the remained H2S was further oxidized and stabilized at a low level.Meanwhile,SO2volume fraction monotonically increased along the central axis until reaching a steady value of 26.55%,19.26% and 15.03% in modes A,B and C,respectively.Literature reports that the generated radicals such as S,SH and HSO could be oxidized to precursor SO and finally converted to SO2depending on the abundance of the oxidants (Eqs.(31)–(33)) [15].However,SO2was almost sparse atD=8.3 with only impressive concentration in mode A and its generation rate was also dependent on ER.This revealed the addition of oxidant accelerated the rate of SO2generation and advanced its visible position.Generally speaking,the sufficient amount of oxygen at low ER can react with H2S to form SO2and H2O in the downstream and eventually affect the final conversion and production.Additionally,the Claus reaction (Eq.(2)) occurred downstream due to the reduction or levelling off in SO2concentration and the presence of condensed sulfur on the reactor wall.
To declare the relationship among H2S,CO2and CH4during combustion process,distribution of CH4,H2,CO and CO2are plotted in Figs.4 and 5.The volume fraction of CH4revealed a similar trend under modes A,B and C that finally decreased to stable values of 0.07%,1.47% and 2.57%,respectively.And the consumption of CH4was favored under oxidant sufficient condition.The literature reported that the decomposition was the initial conversion of CH4that released large amount of H and CH3radicalsviaEqs.(34) and (35) [50].It could be noted that the volume fraction of H2that increased promptly to the peak value atDof 3.3.This phenomenon verified the mentioned decomposition of H2S and CH4was the essential source of hydrogen.Afterwards the partial oxidation of CH4occurred under the role of oxidizing medium such as O and OH radicals(Eqs.(36)and(37))wherein limited oxygen spread from the nozzle bluff edge to the fuel stream.And the concentration of CH4firstly decreased by about 65% atDof 0–3.3.However,compared with H2S,CH4tended to be converted with a less impressive rate near the nozzle and experienced a monotonic dissipation till the downstream.Though the presence of CO2and H2could somewhat inhibit the conversion of CH4,researchers confirmed the primary factor was the oxidation competition between CH4and H2S [15,51].
It is worthwhile to mention the study of Liet al.[15] regarding the oxidation competition of CH4and H2S.They deemed the dominant reaction paths for CH4were partial oxidation and reacting with active radicals such as S and SH through Eqs.(38) and (39).Combined with the low volume fraction of CO atD=3.3,as seen in Fig.5,this conclusion could be supported that indicated the oxidation of CH4was suppressed by H2S.Meanwhile,Clarket al.[35,51,52]considered the initial step was the H2S partial oxidation that produced H2O and S2.Subsequently CH4reacted with the formed sulfur species hence CS2and COS were producedviaEqs.(40) and (41).

Fig.5.Measured volume concentrations of CO2 along D.

Fig.6.Measured volume concentrations of CS2 along D.
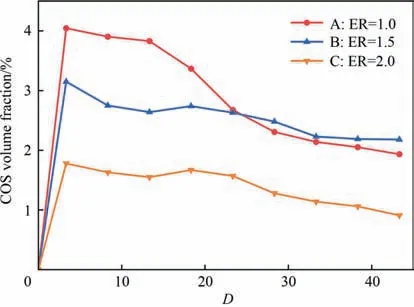
Fig.7.Measured volume concentrations of COS along D.
In relation to CO2volume fraction,it firstly decayed atDof 3.3 and gradually increased to a steady value.And the CO concentration featured the similar tendency to that of H2,but reaching the peak value at a latter detected position withD=8.3 and decreasing monotonically.The variation of CO2and CO was positively correlated with ER.It is well known that the generation of CO2was primarily from the further oxidation of CH4and CO downstream.For the CO2consumption atDof 3.3,the previous studies found that CO2proceeded the decomposition (Eq.(42)) to trigger the formation of CO at high temperature.In the present work,the high temperature in modes A and B would promote the reactivity of CO2and thus enhanced the degree of concentration attenuation.Moreover,numerous literatures indicated CO2could be mainly converted to CO with the help of H radicalviaEq.(43) and attributed it to the oxidation medium impact [18,21,25,52].This viewpoint indicated the H radical was involved in the CO2conversion and revealed CO2consumption was moderate in mode C since H2concentration was lower.Therefore,the number of reactions related to CO generation could be large,including the conversion of CO2and oxidation and steam reforming of CH4(Eqs.(44) and (45)).In addition,Chinet al.[36] considered that the presence of SO2could promote the CH3radical to COviaEq.(46).However,this viewpoint could not be verified in the current work since the SO2concentration was not sufficient atDof 0–8.3.
The distributions of CS2and COS volume fractions are exhibited in Figs.6 and 7,respectively.For all the modes studied in this section,CS2and COS were formed rapidly atD=3.3 wherein mixing of fuel and oxygen was not sufficient.Interestingly,the concentration of COS was higher than that of CS2and the rates of generation showed sensitiveness to the ER.These phenomena can be analyzed by the formation path of CS2and COS at relatively high temperature.Zhuet al.[53] considered CS2was primarily originated from the reactions of CH4and sulfur speciesviaEqs.(40) and (47).And the conversion from CO2to CS2could be complicated that the generated CO from CO2firstly converted into CS and further formed CS2[19].Besides,Liet al.[15,18] concluded the formation of COS was mainly through the reactions of CO2and radicals like SH and S in the decomposition section (Eqs.(48) and (49)),and deemed the CO as the key precursor to react with SO and SH radicals for generating COS in the oxidation stage.And the enhancement in the COS production was found in the oxy-fuel condition with rich radicals of O and OH.Based on these studies,in the present work,it could be inferred that CS2was mainly formed from CH4instead of CO2near the nozzle since the C—H single bond in CH4was generally weaker than the C—O double bond in CO2,and the formation of COS was possibly achieved by the interactions of CO and CO2with the sulfur species considering the presence of CO and consumption of CO2atDof 3.3.Meanwhile,the CS2and COS concentrations here could be explained wherein the significant presence of H2could inhibited the forward direction of these CS2-formed reactions and the COS production was enhanced in the oxy-fuel condition.
Afterwards,the volume fraction of COS decreased monotonically along the axis.It could be noted that the consumption rate of COS concentration was favored under the oxygen sufficient condition.This was attributed that the COS oxidation was the dominant channel under the role of OH,O and H radicals (reverse Eqs.(48)–(51)) [19,54].The COS hydrolysis (reverse Eqs.(29) and(30))was another approach that was usually considered in the first catalyst reactor of the Claus process[33].However,the variation of CS2concentration decreased under ER=1.0 and 1.5 and increased under ER=2.0 atD>8.3.It can be judged that CS2was gradually generated from CO and sulfur species in this section under oxygen deficient mode since CO was significantly present atDof 8.3.For the consumption of CS2in modes A and B,the oxidation might be accounted for the reduction in its concentration to form radicals such as CO,CS and SOviathe reactions of Eqs.(52) and (53).Furthermore,numerous experimental and simulation studies indicated that the tight interaction of COS and CS2could be achieved with the aid of radicals such as SH,OH and SO (Eqs.(54) and(55)) [21,35,38,40].The related reactions could proceed at an insignificant rate downstream of the reactor.
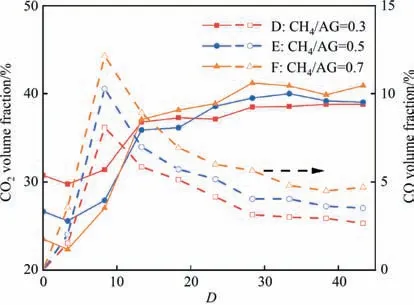
Based on the analysis of the temperature distribution and species conversion pathways under different ERs,a schematic diagram of the CH4-AG diffusion flame was portrayed as Fig.8.Depending on the intensity of oxidation,the whole reactor could be divided into two sections namely rapid and slow oxidation section.Meanwhile,it could also be interpreted to three zones according to various chemical process that flame inner core(0 Fig.8.Schematic diagram of CH4-AG diffusion flame under oxy-fuel condition. In this section,the effect of CH4on AG partial oxidation was studied under ER of 1.6.ModeD,EandFpresented here are pure oxygen with CH4/AG of 0.3,0.5 and 0.7.Similarly,variation of the temperature curves featured to rise to a peak value rapidly and decreased almost monotonically. Fig.9 reveals a positive impact of CH4on the axial temperature with peak values of 1000 K,1084 K and 1123 K for modes D,E and F,respectively.This is due to the reaction heat of CH4is greater than that of H2S at the same molar flow.Meanwhile,it could be noted that the temperature increment from mode D to E was significantly greater than that from modes E to F for equivariant CH4/AG additions.Therefore,a weaker effect of added CH4on temperature could be predicted within high CH4/AG region.Similarly,as discussed in the impact of ER,the added volume flow by higher ratio of CH4/AG showed obvious contribution on flame height and temperature gap. Fig.9.Measured temperature profiles versus CH4/AG. The distributions of H2S and SO2concentrations were summarized in Fig.10.The volume fractions of H2S were sharply decayed atDof 3.3 with increasing CH4proportion and the values were 9.53%,8.48% and 6.64%,respectively.Though the flame temperature in higher CH4/AG favored the decomposition and oxidation process of H2S,similar effect to providing sufficient O2,H2S conversion was simultaneously influenced by the subsequently enhanced competitive oxidation.As seen in SO2profiles under CH4/AG=0.7,SO2concentration finally reached up to 23.47%,which obviously was higher than that of the other two modes.The results for the SO2volume fraction distributions revealed that H2S could react preferentially with O2introduced by the raising of CH4/AG.Evidently higher complete oxidation rate of H2S could be derived under CH4/AG enriched modes. Fig.10.Measured volume concentrations of H2S and SO2 along D. Fig.11.Measured volume concentrations of CH4 and H2 along D. Fig.12.Measured volume concentrations of CO2 along D. Fig.13.Measured volume concentrations of CS2 along D. Fig.14.Measured volume concentrations of COS along D. Figs.11 and 12 depict the distributions of CH4,H2,CO2and CO volume fraction.It could be seen that increasing CH4/AG had no obvious effect on the trend in concentration or conversion rate of CH4itself.The initial conversions of CH4atDof 3.3 for modes D-F were 62%,68.57% and 66.53% and final conversions downstream were 96.15%,96.68% and 95.37%,respectively.As discussed previously,oxidation of CH4atDof 3.3 was confined due to the presence of H2S,and H2and COS were the typical products at flame inner core.However,the total reaction amount of CH4still increased with the enhancing CH4/AG.Though the initial concentration of CO2(D=0) decreased due to the reducing content in the fuel mixture,the final concentration of CO2was tightly related to CH4/AG.Meanwhile,it is noted that discernable high concentrations of H2and CO,14.89% and 12.15% are detected in CH4/AG=0.7 mode.The reactions of forming CO and H2were promoted under CH4/AG enriched condition due to richer presence of CH3,CH and H radicals and ultimately,higher temperature.Furthermore,the axial concentrations of H2and CO for all modes here were inclined to be slightly decreased and sustained downstream under anoxic atmosphere of ER=1.6.The former Section 3.1 has emphasized that oxidation is an important process for the consumption of H2and CO wherein the presence of sufficient oxygen downstream is critical.Besides to it,the behavior of diffusing outward radially in the reactor and combination of CO and sulfur species may result in the slight reduction of CO concentration as seen in Eqs.(50)and(51) [18]. Figs.13 and 14 show the distributions of CS2and COS concentration under investigated modes.It exhibited CS2and COS experienced rapid generation at flame inner area and moderate consumption in the latter section of the reactor.It is interesting that CS2and COS formation were higher in mode E during the flame zone and the final concentrations downstream were dependent on the CH4/AG ratio.This showed that suitable concentration of reactants was the key to generate CS2and COS.And the high richness of carbon and sulfur species was more likely to maintain the CS2and COS concentration downstream.Meanwhile,the striking distinction was the noticeably lower formation of COS in the CH4/AG=0.3 case.This observation coincides with the remarkable lowest temperature,but also lowest concentration of CS2in mode D.As has been discussed that low temperature hinders CH4and H2S decomposition,CH and SH radicals generation and CO production,and these factors,aside from the lower reaction rate,are negative for the CS2and COS formation through mentioned reaction paths. In the current study,the combustion of methane and acid gas was conducted in a diffusion flameviaa coaxial burner.The distribution of the flame temperature and main species concentration on the central axis were analyzed under different equivalence ratios and CH4-addition ratios.The CH4-AG diffusion flame under oxy-fuel combustion was described and the conversion and generation of species were discussed. Generally,the CH4-AG diffusion flame could be divided into three sections according to the distinct chemical processes namely the initial reaction,oxidation and complex reaction sections.During the first section,the decomposition and partial oxidation of H2S and CH4occurred wherein large amount of H2were produced.Oxidation competition could also be found here that the CH4conversion was inferior to that of H2S.CH4could interact with sulfur species that contributed to formations of COS and CS2.Meanwhile CO2was initially consumed here mainlyviaCO2+H →CO+OH and subsequently generated from the complete oxidation of CH4and CO wherein mixing of the fuel and oxidant became adequate.The production of SO2was improved and advanced under sufficient oxygen condition while its obvious appearance would be postponed to the oxidation section and the final volume fraction would be promoted by reducing ER and increasing CH4/AG.The generations of H2and CO were favored by this trend.However,the formed H2and CO could be consumed mainly through oxidation reaction downstream for mode A while they were inclined to be sustained in mode B-F. COS and CS2experienced rapid generation at flame inner area.COS was mainly formed by interactions of CO and CO2with sulfur species.And production of CS2directly relied on the reaction of CH4with sulfur species.With low ER and high CH4/AG application,generation rates of COS and CS2were increased under higher temperature and enhancing carbon–sulfur-oxygen environment.The concentration of COS was higher than that of CS2since the large presence of H2prevented the main paths of CS2generation.COS and CS2could be consumed by oxidation process and the decay rates were closely related to ER. Considering the heat resistance of the materials in the practical Claus furnace,CH4co-combustion and oxy-fuel combustion are rarely applied simultaneously since high temperatures can be achieved.However,the unequal state of CH4in AG oxidation remains unchanged and the generation of COS and CS2contaminants is significant in the flame inner core.As enhanced conditions,it may provide references for the suitable applications of CH4cocombustion,oxy-fuel combustion and fitting ER in the Claus furnace,which can meet requirements including control of furnace temperature,improvement of species conversion and reduction of COS and CS2.Meanwhile,the selection of proper conditions,such as ER=1.6,CH4/AG=0.7 in this study and suitable reactor length,may achieve the production of valuable syngas (CO and H2)directly from CH4-AG combustion after exhaust gas separation. Data Availability Data will be made available on request. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgements The project was supported by the National Natural Science Foundation of China (21978092). Nomenclature Cpspecific heat capacity,J∙K-1∙mol-1 Ddimensionless axial distance EERuncertainty of the equivalence ratio,% hfconvective heat transfer coefficient,W∙m-2∙K-1 mfuelmass flow rate of H2S,kg∙min-1 NuNussle number Piproduction of speciesi,% PrPrandtl number ReReynolds number Siproduct selectivity of speciesi,% Tbelectric coupling bead temperature,K Tggas temperature,K Twreactor wall temperature,K δVflow rate controlling resolution,L∙min-1 ε emission rate μ kinetic viscosity,Pa∙s σ blackbody radiation constant,W∙m-2∙K-4 φ relative error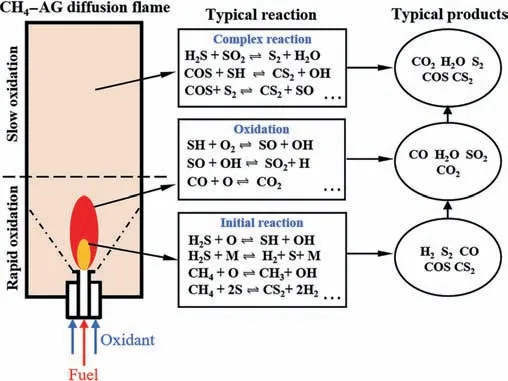
3.2.Experimental study of CH4-AG combustion:Effect of CH4/AG ratio





4.Conclusions
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
