Mussel-inspired Methacrylic Gelatin-dopamine/Ag Nanoparticles/Graphene Oxide Hydrogels with Improved Adhesive and Antibacterial Properties for Applications as Wound Dressings
2024-04-11SUZhengnanHUYanruMENGLihuiOUYANGZhiyuanLIWenchaoZHUFangXIEBinWUQingzhi
SU Zhengnan,HU Yanru,MENG Lihui,OUYANG Zhiyuan,LI Wenchao,ZHU Fang,XIE Bin,WU Qingzhi
(State Key Laboratory of Advanced Technology for Materials Synthesis and Processing,Biomedical Material and Engineering Center of Hubei Province,Wuhan University of Technology,Wuhan 430070,China)
Abstract: A novel strategy was developed to prepare the methacrylic gelatin-dopamine (GelMA-DA)/Ag nanoparticles (NPs)/graphene oxide (GO) composite hydrogels with good biocompatibility,mechanical properties,and antibacterial activity.Mussel-inspired DA was utilized to modify the GelMA molecules,which imparts good adhesive performance to the hydrogels.GO,interfacial enhancer,not only improves mechanical properties of the hydrogels,but also provides anchor sites for loading Ag NPs through numerous oxygencontaining functional groups on the surface.The experimental results show that the GelMA/Ag NPs/GO hydrogels have good biocompatibility,and exhibit a swelling rate of 202±16%,the lap shear strength of 147±17 kPa,and compressive modulus of 136± 53 kPa,in the case of the Ag NPs/GO content of 2 mg/mL.Antibacterial activity of the hydrogels against both gram-negative and gram-positive bacteria is dependent on the Ag NPs/GO content derived from the release of Ag+.Furthermore,the GelMA/Ag NPs/GO hydrogels possess good adhesive ability,which is resistant to highly twisted state when stuck on the surface of pigskin.These results demonstrate promising potential of the GelMA-DA/Ag NPs/GO hydrogels as wound dressings for biomedical applications in clinical and emergent treatment.
Key words: GelMA;dopamine,graphene oxide;adhesion;antibacterial ability
1 Introduction
The skin as the barrier plays crucial roles to isolate the internal and external environments and protect the human body against infection by foreign pathogens[1-3].Wound dressings are one class of most extensive medical consumables for various skin injuries in routine and emergent treatment,which provide defense barriers against bacterial infection and accelerate the reconstruction and healing of injured regions[4,5].Therefore,it is crucial to impart multi-functions to wound dressings,including good biocompatibility and self-adhesive ability,high mechanical strength and antibacterial activity[6,7].
Biopolymers-based hydrogels have been demonstrated with good biocompatibility and bioactivity,which are considered as ideal materials in biomedical fields,such as drug delivery carriers,tissue engineering scaffolds,and artificial organs,etc[8,9].Among various biopolymers,gelatin attracts considerable attention due to its abundant source and low cost,good biocompatibility and bioactivity,and low immunogenicity[10,11].In order to adjust the degradation rate and improve the mechanical property of gelatin hydrogels,one of the major strategies is to modify gelatin molecules with methacrylic anhydride(MA),which impart the photo-linking ability to methacrylic gelatin (GelMA) hydrogels.Both the degradation rate and mechanical strength of GelMA hydrogels can be tuned through adjusting the grafting ratio of MA.GelMA-based hydrogels have been widely used as the matrix materials for preparation of various wound dressings[12,13].Several strategies have been developed through further modifying bioactive molecules or mixing with other functional components to endow bioactivity and antibacterial activity to the hydrogels in order to accelerate wound repair and reduce inflammation and scarring[14-17].So far,it is still a challenge to improve both the self-adhesive ability and antibacterial activity of the GelMA hydrogels for applications as wound dressings[18,19].
Mussel-inspired catechol modifications recently attract increasing interests due to the exceptional adhesive ability on the surface of almost all material through non-covalent and covalent interactions[20-22].The functional groups in the molecular structure,including benzene rings,phenolic hydroxyl groups,and quinone double bonds,are primarily contributed to the exceptional adhesive ability of dopamine (DA)[23-25].For example,a hydrogel system consisting of DAmodification hyaluronic acid and reduced graphene oxide (GO) was reported to greatly reduce blood loss after injury due to the strong tissue adhesion derived from catechol[26].On the other hand,GO nanosheets exhibit excellent mechanical properties,large specific surface area,good biocompatibility and bioactivity.There are numerous oxygen-containing functional groups on the surface of GO nanosheets,which provide anchor sites for grafting the functional molecules or loading inorganic nanoparticles (NPs)[27-29].Therefore,GO nanosheets are extensively utilized as interfacial enhancers for improving the performances and functionalization of the hydrogels[30,31].
In this work,a novel strategy was developed to prepare the DA-modified GelMA (GelMA-DA)/Ag NPs/GO composite hydrogels for biomedical applications as wound dressings.Gelatin was modified with MA and DA,respectively,in order to impart photo-linking and self-adhesive ability to the hydrogels.GO nanosheets were introduced as interfacial enhancers to improve the mechanical properties of the hydrogels and load Ag NPs without agglomerations.The release of Ag+from Ag NPs has been well demonstrated with excellent antibacterial activity[32,33].The structure and morphology of Ag NPs/GO nanocomposites were characterized by XRD,SEM,and TEM.The swelling ratio,mechanical and adhesive proformances,biocompatibility,and antibacterial activity of the GelMA-DA/Ag NPs/GO hydrogels were investigated in detail.
2 Experimental
2.1 Materials
Gelatin from porcine skin,Methacrylic anhydride(MA),and 2-hydroxy-1-[4-(2-hydroxyethoxy) phenyl]-2-methyl-1-propan (I2959) were purchased from Sigma(St.Louis,MO,USA).Dopamine hydrochloride (DA)and 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) were bought from Aladdin Co.,Ltd (Shanghai,China).N-Hydroxy succinimide (NHS)was bought from Innochem Co.,Ltd (Shanghai,China).The graphite powder was purchased from Sinopharm Chemical Reagent Co.,Ltd (Shanghai,China).All the reagents and solutions were reagent grade.
2.2 Preparation of GelMA-DA
Briefly,10 g of gelatin was completely dissolved in 100 mL of phosphate buffered brine (PBS) under magnetic stirring at 60 ℃.Then,8 mL of MA was dropped into the gelatin solution and stirred at 50 ℃for 2 h under dark conditions.The mixed solution after reaction was diluted three times with PBS.Next,the mixture was dialyzed at 50 ℃ against de-ionized water using 8-14 kDa cut-off dialysis bags to remove excess MA and impurities.Finally,GelMA sponge was obtained after lyophilization.
GelMA-DA was prepared according to the Ref.[34].Briefly,6.25% (w/v) GelMA was completely dissolved in 16 mL mixed solution consisting of PBS and dimethylformamide (DMF) at a volume ratio of 1:1 under magnetic stirring at 60 ℃.1.5 g of EDC and 0.9 g of NHS were dissolved in 4 mL of DMF and added into the mixture at 60 ℃ and stirred for 30 min.Then,DA at the ratio of 1 g DA/1 g GelMA was dissolved in PBS and added into the activated mixture.Subsequently,the reacted mixture was reacted for 10 h and dialyzed at room temperature against de-ionized water (pH 5.0) using 8-14 kDa cut-off dialysis bags.GelMA-DA sponge was obtained after lyophilization.
2.3 Preparation of Ag NPs/GO nanocomposite
GO was prepared using pure graphite powder according to the modified Hummer’s method[35].Ag NPs/GO nanocomposite was prepared as follows[36].100 mg of AgNO3and 100 mg of the GO powder were added in 100 mL of de-ionized water,respectively,and sonicated for 30 min.Then,the solutions were mixed at 60 ℃ for 1 h.Subsequently,100 mg of ascorbic acid was quickly added to the mixture solution,and vigorously stirred for 1 h under dark condition[37].AgNPs/GO nanocomposites were washed using deionized water three times through centrifugation.Ag NPs/GO composites were obtained after lyophilization.
2.4 Preparation of GelMA-DA/Ag NPs/GO hydrogels
Ag NPs/GO aqueous solution at different concentrations (0,0.5,1,2,5,and 10 mg/mL) were prepared under ultrasonic treatment for 30 min.1 mg/mL I2959 photo-initiator and 400 mg/mL GelMA-DA were added into the solutions at 60 ℃,respectively.Subsequently,the solution was stood to remove foam to prepare hydrogel precursors with different Ag NPs/GO contents.
In order to characterize the structure and properties,GelMA-DA/Ag NPs/GO hydrogels were prepared with different geometries: disc-shaped samples with a dimension of 6 mm in diameter and 4.5 mm in height for swelling ratio,SEM,and compressive testing;disc-shaped samples with a dimension of 7 mm in diameter and 1 mm in height for antibacterial performances testing;and cuboid-shaped samples with a dimension of 10 mm in width,10 mm in length,and 1.5 mm in thickness for Ag+release testing.
2.5 Physico-chemical properties of GelMADA/Ag NPs/GO hydrogels
In order to determine the changes of functional groups after the modification,FT-IR spectra of GelMA and GelMA-DA were recorded through a spectrometer(Vertex 80V spectrometer,Bruker,Germany).GelMA and GelMA-DA were dissolved in deuterium water and1H NMR spectra were obtained by a 500-MHz Proton NMR (Bruker,Germany).SEM was used to analyze the effect of dopamine modification on the micro morphology of GelMA.
X-Ray Diffraction (XRD.Empyrean,PANalytical,Netherlands) was used to confirm the reduction and formation of GO nanosheets and Ag NPs.Scanning electron microscope (SEM,JEOL,JSM-IT200,Japan)and transmission electron microscope (TEM,JEM-1400Plus,JEOL,Japan) were used to observe the particle size and distribution of Ag NPs on the surface of GO nanosheets.The content of Ag NPs in Ag NPs/GO was determined by inductively coupled plasma optical emission spectro-scopy (ICP,Prodigy7,Leeman Labs Inc,America).
The swelling rate was measured to evaluate the water absorption capacity of the GelMA-DA/Ag NPs/GO hydrogels.In brief,the GelMA-DA/Ag NPs/GO hydrogels after lyophilization were immersed in PBS at 37 ℃ for 24 h.Then the water on the surface of the hydrogels was wiped off using filter paper.The mass of the hydrogels before (W0) and after (Wi) immersion in PBS was recorded.The swelling rate (λ(w)) of the hydrogels was calculated according to the equation (1):
2.6 Mechanical properties of GelMA-DA/Ag NPs/GO hydrogels
The shear strength of as-prepared GelMA-DA/Ag NPs/GO hydrogels was tested by ASTM F2255-05[38].The surface of the glass plate was coated with gelatin(20w/w) and dried at room temperature.Then 10 µL of the hydrogel precursor was added dropwise onto a glass slide with an area of 1 cm2.The two pieces of glass were connected by the hydrogel which was irradiated under ultraviolet light for 3 min.The maximum bonding strength of the hydrogel was measured when the two pieces of glass were separated by MTX LTX 850 universal testing machine (1 mm/min,Jinan Meites Testing Technology Co.,Ltd.).The compression modulus was tested by MTX LTX 850 universal testing machine (Jinan Meites Testing Technology Co.,Ltd.)at 25 ℃ with an experimental speed of 1.0 mm/min.Triple samples were tested (n=3).
2.7 Releasing profiles of Ag+ from GelMADA/Ag NPs/GO hydrogels
To evaluate the release of Ag+,the hydrogels were placed in 30 mL of PBS (pH 7.40) at 37 ℃.At the designated time,5 mL of the solution was aspirated to detect the concentration of Ag+through inductively coupled plasma optical emission spectro-scopy (ICP,Prodigy7,Leeman Labs Inc,America),and an equal amount of fresh buffer wad added to the system.Triple samples were used to test the release of Ag+from the hydrogels (n=3)[32].
2.8 Biocompatibility of GelMA-DA/Ag NPs/GO hydrogels
To assess the cytotoxicity of GelMA-DA/Ag NPs/GO hydrogels in vitro,L929 cell suspension at a density of 2×104cells/mL per well was seeded in a 96-well plate and incubated at 37 ℃ under 5% CO2atmospheres.GelMA-DA/Ag NPs/GO hydrogels were sterilized by immersed in PBS and irradiated under UV light for 12 h.Then,the hydrogels were washed using sterilized PBS three times and immersed in DMEM to prepare leach solution.The cells were cultured in leach solution of the hydrogels for 1,3,and 5 d,respectively.The CCK-8 kits were used to measure cell viability of L929 cell.The value of optical density (OD) was measured at a wavelength of 450 nm using a microplate reader (Multiskan GO,Thermo Scientific,USA)[39].
The live/dead viability assay was performed to determine the viability and morphology when L929 cells were co-cultivated with the hydrogels at 37 ℃.After 1 and 5 d co-cultivating,the cells were stained with propidium iodide (PI) and calcein AM at 37 ℃for 30 min.The viability and morphology of cells were recorded.
2.9 Antibacterial activity of GelMA-DA/Ag NPs/GO hydrogels
Antibacterial activity of the hydrogels were evaluated using gram-negativeE.coliand grampositiveS.aureusbacteria as the models according to the agar plate diffusion method[40].0.1 mL of bacteria(1×107CFU/mL) was swabbed uniformly across a culture plate.Then,the hydrogels were placed over the culture plates.All disks containing the bacteria and the hydrogels were incubated and maintained at 37 ℃ for 24 h.After this period,the inhibition zones around hydrogel disks were analyzed to assessing the suppression on the bacterial growth.
2.10 Statistical analysis
The data were presented as mean ± standard deviation and were analyzed using SPSS 22.0 software.The statistic significant differences between different groups were analyzed using a one-way analysis of variance (ANOVA).Ap-value <0.05 was considered as significant,and ap-value <0.01 was considered as highly significant.
3 Results and discussion
3.1 Synthesis and characterizations of GelMA-DA
The synthesis process of GelMA and GelMADA is shown in Fig.1(a).MA reacted with the amino groups on gelatin to form amide bonds.Then,the amino groups on dopamine reacted with the activated carboxyl group on GelMA to form GelMA-DA.The structures of gelatin,GelMA,GelMA-DA,and DA were characterize through FT-IR spectra (Fig.1(b)).After the modification,the amide bonds (-CO-NH-)were formed with the characteristic absorption peaks at 1 640 and 1 540 cm-1.These peaks were observed in FT-IR spectra of gelatin,GelMA,and GelMA-DA owing to the extensive existance of amide bonds in the molecular structure of gelatin.It is noteworthy that the absorption peak derived from C=C and benzene ring was not observed in the FT-IR spectra of GelMA and GelMA-DA,which could be attributed to the low degree of methacrylation and DA substitution[41,42].

Fig.1 Schematic synthesis and structural characterizations of GelMA-DA: (a) Schematic diagram of GelMA-DA synthesis;(b) FT-IR spectra and (c) 1H NMR spectra of gelatin,GelMA,GelMA-DA,and DA
1H-NMR spectroscopy was utilized to further verify the successful preparation of GelMA and GelMA-DA (Fig.1(c)).The new peaks at 5.3 and 5.6 ppm in the spectrum of GelMA were assigned to the protons of H2C=C-,while the peak at 6.9 ppm in the spectrum of GelMA-DA was attributed to the protons of benzene ring.These results confirmed the sucessfully preparation of GelMA and GelMA-DA[34].
3.2 Characterizations of Ag NPs/GO nanocomposite
Fig.2(a) shows the XRD patterns of GO and Ag NPs/GO nanocomposite.A sharp diffraction peak at 8.7° was indexed to the (001) crystal plane of GO[43].Four diffraction peaks were observed at 38.2°,44.4°,64.5°,and 77.5° in the XRD pattern of Ag NPs/GO nanocomposite,corresponding to the (111),(200),(220),and (311) planes of the cubic Ag crystal(JCPDS No.04-0783),respectively.The peak at 8.7°was not observed in the XRD spectrum of Ag NPs/GO nanocomposite,which may be attributed to the reduction of graphene oxide[44].
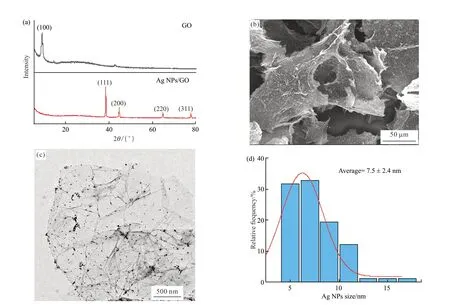
Fig.2 Characterizations of Ag NPs/GO nanocomposite: (a) XRD patterns of GO nanosheets and Ag NPs/GO nanocomposite;(b) SEM image of Ag NPs/GO nanocomposite;(c) TEM image of Ag NPs/GO nanocomposite;(d) Size distribution of Ag NPs on Ag NPs/GO nanocomposite
The morphology of Ag NPs/GO nanocomposite was observed through SEM and TEM,as shown in Figs.2(b-c),respectively.It is obvious that Ag NPs were uniformly dispersed on the surface of GO nanosheets.Numerous oxygen-containing functional groups on the GO surface provide anchor sites for the nucleation and growth of Ag NPs,and effectively decrease the agglomeration of Ag NPs.Fig.2(d) shows the average Ag NPs size of 7.5 ± 2.4 nm.The uniform dispersion of Ag NPs on the surface of GO nanosheets is benefical to improve antibacterial activity[45].
3.3 Characterizations of GelMA-DA/Ag NPs/GO hydrogels
The surface structure of GelMA-DA/Ag NPs/GO hydrogels was characterized through SEM observation,as shown in Fig.3.It is obvious that GelMA-DA/Ag NPs/GO hydrogels display three-dimensional porous structure in the lyophilization state.The pore wall of GelMA-DA/Ag NPs/GO hydrogels gradually became thinner with the increase of Ag NPs/GO content until the wall structure collapsed.It is speculate that the interaction between GO nanosheets and GelMADA molecules affects the strength of the composite hydrogels[46,47].When the content was lower than 2 mg/mL,Ag NPs/GO nanocomposites were uniformly dispersed in the hydrogels and have few effects on the crosslinking of the GelMA-DA hydrogels.The mechanical strength of the hydrogel enhanced with the increases of the Ag NPs/GO content,which is important for maintaining the stable pore wall structure[47,48].When the Ag NPs/GO content was higher than 5 mg/mL,the crosslinking degree of the GelMA-DA hydrogels decreases because the addition of Ag NPs/GO reduces the contact probability between GelMA-DA molecules[49].The agglomeration of Ag NPs/GO may also causes a decrease in the strength of the composite hydrogels[50].
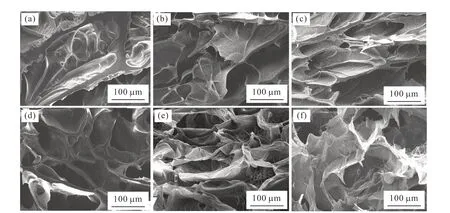
Fig.3 SEM images of GelMA-DA/Ag NPs/GO hydrogels with different contents of Ag NPs/GO: (a) 0 mg/mL;(b) 0.5 mg/mL;(c) 1 mg/mL;(d) 2 mg/mL;(e) 5 mg/mL;(f) 10 mg/mL
Fig.4 shows the digital photo and swelling ratio of GelMA-DA/Ag NPs/GO hydrogels.The hydrogels became darker when increasing the content of Ag NPs/GO nanocomposites (Fig.4(a)).The swelling rate of GelMA-DA/Ag NPs/GO hydrogels is also affected by the content of Ag NPs/GO nanocomposites (Fig.4(b)).With the increase of Ag NPs/GO content,the swelling rate of the hydrogels firstly decreased and subsequently increased.When the Ag NPs/GO content was lower than 2 mg/mL,hydrogen bonds were formed between GelMA-DA molecules and GO nanosheets,which was not conducive to the combination of the hydrogel and water,resulting in a decrease in swelling rate[51].When the Ag NPs/GO content was 2 mg/mL,the hydrogen bonds between GelMA-DA molecules and GO nanosheets may reach saturation[52],and the swelling rate of the hydrogels was minimum.Further increasing the Ag NPs/GO content higher than 5 mg/mL introduced the more hydrophilic groups derived from GO nanosheets,improving the swelling rate of the hydrogels.Moreover,the high content of Ag NPs/GO nanocomposites may reduce the crosslinking density of the hydrogels,which resulted in the larger pore structure inside the hydrogels and increased the swelling ratio of the hydrogels[53].
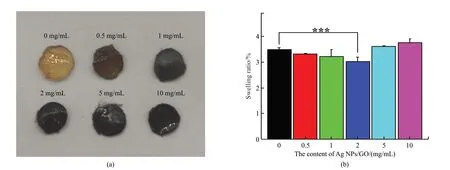
Fig.4 (a) Picture and (b) Swelling rate of GelMA-DA/Ag NPs/GO hydrogels after in PBS for 24 h.Significant effect between different groups: ***p <0.005,n=3
3.4 Mechanical properties of GelMA-DA/Ag NPs/GO hydrogels
In order to meet the needs of skin repair,hydrogels for wound repair should have compatible compressive and adhesion strength[54,55].The higher the lap shear strength of the hydrogel dressing,the stronger the connection between the hydrogel and the skin tissue,which promotes healing and limits bacterial growth[38].The lap shear strength and compression modulus of the GelMA-DA/Ag NPs/GO hydrogels were measured,as shown in Fig.5.The lap shear strength of the hydrogels was increased first and then decreased with the increased of the Ag NPs/GO content.When the Ag NPs/GO content was 1 and 2 mg/mL,the lap shear strength of the hydrogels reached appreciate 163 ± 28 and 147 ± 17 kPa,which was 1.17 and 1.06 times higher than that of the GelMADA hydrogel,respectively.The similar changes on the compressive modulus of the hydrogels were also observed.When the Ag NPs/GO content was 1 and 2 mg/mL,the compressive moduli of the hydrogels reached appreciate 162 ± 17 and 136 ± 53 kPa,which was 3.65 and 3.06 times higher than that of the GelMADA hydrogel,respectively.

Fig.5 Mechanical characterizations of GelMA-DA/Ag NPs/GO hydrogels: (a) Lap shear strength and (b) Compression modulus of GelMA-DA/Ag NPs/GO hydrogels;(c)Pulling the 10 g weight by connecting two glass plates with the hydrogel;(d) Pasting the hydrogel to the pigskin and twisting the pigskin.Significant effect between different groups: *p <0.01,***p <0.005,n=3
Fig.5(c) shows that the GelMA-DA/Ag NPs/GO hydrogels with the Ag NPs/GO content of 2 mg/mL successfully pulled up a weight of 10 g without breaking,indicating the excellent tensile and adhesion strength of the hydrogel.Fig.5(d) shows that the hydrogel was stuck to the surface of the pigskin,and the connection between the hydrogel and pigskin was remarkably firm without obvious damages under highly twisted state.Therefore,these results demonstrate that both the adhesion strength and compressive modulus of the GelMA-DA/Ag NPs/GO hydrogels with the/Ag NPs/GO content of 1 and 2 mg/mL meet the requirements of hydrogel dressings for wound repair[56-60].
3.5 Ag+ release from GelMA-DA/Ag NPs/GO hydrogels
In order to explore the release of Ag+from the hydrogels,the hydrogels with the Ag NPs/GO content of 0.5,2,and 10 mg/mL were immersed in PBS at 37℃.The suspension was collected at designed time intervals and the Ag+concentration was detected by ICP (Fig.6(a)).The results show that an initial burst release of Ag+was observed in the first 1 day,followed by a slower release.It is obvious that the Ag+release was positively proportional to the Ag NPs/GO content in the hydrogels.

Fig.6 Ag+ release and in vitro biocompatibility of GelMA-DA/Ag NPs/GO hydrogels with different Ag NPs/GO contents: (a) Ag+ release curves (n=3);(b) Cell viability of L929 Cells treated with the leach solution of the hydrogels at different time intervals (1,3 and 5 d)(n=5);(c) The live/dead assay of L929 cells treated with the leach solution of the hydrogels at the end of 1 and 5 day (the red arrows point to the dead cells).Significant effect between different groups: *p <0.01,***p <0.005
3.6 In vitro biocompatibility of GelMA-DA/Ag NPs/GO hydrogels
CCK-8 and live/dead assays were carried out to evaluate the biocompatibility of GelMA-DA/Ag NPs/GO hydrogels.Fig.6(b) shows that no evident cytotoxicity was observed after treating L929 cells with the leach solution of the hydrogels for 24 h.
Cell viability of the hydrogel groups was lower than that of the control group after 3 and 5 days,but still higher than 75% of that after 5 days in the case of the Ag NPs/GO content lower than 2 mg/mL,indicating that the hydrogels with the low Ag NPs/GO contents are compatible with cells as a dressing[61].It is also noticeable that the decrease of cell viability was negatively proportional to the increase of the Ag NPs/GO content in the hydrogels.Fig.6(c) shows the fluorescent images of the live/dead assay.The dead cells were pointed by red arrows in the images.The amount of the dead cells obviously increased with the enhancement of the Ag NPs/GO content in the hydrogels after treatment for 5 days.The increased cytotoxicity could be attributed to the high Ag+level released from the hydrogels with the high Ag NPs/GO content.These results indicate good biocompatibility of the GelMA-DA/Ag NPs/GO hydrogels with the Ag NPs/GO content lower than 2 mg/mL.
3.7 In vitro antibacterial activity of GelMADA/Ag NPs/GO hydrogels
Antibacterial efficacy of GelMA-DA/Ag NPs/GO hydrogels with different Ag NPs/GO contents (0,0.5,1,and 2 mg/mL) was evaluated using gram-negativeE.coliand gram-positiveS.aureusbacteria as the models.As shown in Fig.7,the inhibition zone steadily grew with the increase of the Ag NPs/GO content in the hydrogels,indicating good antibacterial activity of the hydrogels against both of bacteria.Results also show that the higher Ag NPs/GO content is beneficial for improving antibacterial activity of the hydrogels,which can be ascribed to the higher Ag+concentration releasing from the nanocomposite[62,63].

Fig.7 Inhibition zones of GelMA-DA/Ag NPs/GO hydrogels with different Ag NPs/GO contents against S.aureus and E.coli bacteria: (a)Inhibition zone experiment against S.aureus bateria;(b) Inhibition zone experiment against E.coli bateria;(c) Diameter of inhibition zone on S.aureus bateria;(d) Diameter of inhibition zone on E.coli bateria.Significant effect between different groups: *p <0.01,***p <0.005,n=3
4 Conclusions
In summary,a novel strategy is developed to prepare the GelMA-DA/Ag NPs/GO hydrogels with good mechanical properties,biocompatibility,and antibacterial activity.Mussel-inspired DA was utilized to modify GelMA and impart good adhesive performance to the hydrogels.GO nanosheets play crucial roles as interfacial enhancers not only to improve the mechanical strength of the hydrogels,but also load Ag NPs for antibacterial activity.The GelMA-DA/Ag NPs/GO hydrogels with the Ag NPs/GO content of 1 and 2 mg/mL possess high mechanical properties,including the lap shear strength,tensile and compressive strength.The loading of Ag NPs imparts good antibacterial activity against both gram-negative and gram-positive bacteria to the hydrogels through releasing Ag+.These results demonstrate promising potential of the GelMA-DA/Ag NPs/GO hydrogels as wound dressings for applications in clinical and emergent therapy.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Biotin-modified Galactosylated Chitosan-gene Carrier in Hepatoma Cells Targeting Delivery
- Effect of Polyvinyl Alcohol in Inner Aqueous Phase on Stability of Millimeter-scale Capsules
- Synthesis and Characterization of Nonionic Waterborne Polyurethane and Application to Wool Fabric Finishing
- Fluorescent Double Network Hydrogels with Ionic Responsiveness and High Mechanical Properties for Visual Detection
- Damage Mechanism of Ultra-thin Asphalt Overlay (UTAO)based on Discrete Element Method
- Preparation of Laser Cladding Coating Undercooling Cu-based Alloy and Co on Non-equilibrium Solidification Structure
