Biotin-modified Galactosylated Chitosan-gene Carrier in Hepatoma Cells Targeting Delivery
2024-04-11CHENGMingrongZHANGFengLIQingWANGHua
CHENG Mingrong ,ZHANG Feng ,LI Qing ,WANG Hua
(1.Department of General Surgery,Nanxiang Branch of Ruijin Hospital,Shanghai 201802,China;2.Minhang District Pujin Community Health Service Center,Shanghai 201112,China;3.Hepatobiliary Surgery Center,Tongji Hospital Affiliated to Tongji University,Shanghai 200065,China)
Abstract: Our previous studies have successfully grafted biotin and galactose onto chitosan (CS)and synthesized biotin modified galactosylated chitosan (Bio-GC).The optimum N/P ratio of Bio-GC and plasmid DNA was 3:1.At this N/P ratio,the transfection efficiency in the hepatoma cells was the highest with a slow release effect.Bio-GC nanomaterials exhibit the protective effect of preventing the gene from nuclease degradation,and can target the transfection into hepatoma cells by combination with galactose and biotin receptors.The transfection rate was inhibited by the competition of galactose and biotin.Bio-GC nanomaterials were imported into cells’ cytoplasm by their receptors,followed by the imported exogenous gene transfected into the cells.Bio-GC nanomaterials can also cause inhibitory activity in the hepatoma cells in the model of orthotopic liver transplantation in mice,by carrying the gene through the blood to the hepatoma tissue.Taken together,bio-GC nanomaterials act as gene vectors with the activity of protecting the gene from DNase degradation,improving the rate of transfection in hepatoma cells,and transporting the gene into the cytoplasm in vitro and in vivo.Therefore,they are efficient hepatoma-targeting gene carriers.
Key words: gene vector;hepatocellular carcinoma;nanoparticles;sustained release;gene therapy
1 Introduction
Gene therapy consists of the key gene technology,which includes viral and non-viral vectors.Viral vector has a high transfection efficiency because it can effectively uptake the exogenous gene into the cells with the advantage of the host cell’s high infection for the virus.Although viral vectors have made considerable progress in the treatment of cancer and genetic diseases,its safety and efficiency are still not fully ascertained[1].Some inherent defects of the virus vector,such as the immunogenicity of the virus and the carcinogenic and non-specific transfection of germ cells,have not been entirely improved[2].An ideal gene carrier shall have the following characteristics[3-6]:drug delivery system is targeted,and can be directed and efficiently transferred to the interior of the cellular lesion;will transfer the target gene into the cells,and effectively release to express the related products;compatible vector for its stability and can form stable complexes with DNA,with a simple preparation method for easy commercial production and operation;the carrier material is less toxic and can be effectively degraded,and its degradation products are safe for the patients and the environment.In recent years,non-viral gene vectors in gene therapy have resolved the security issues caused by virus carriers.The nano-controlled release system has several advantages for the delivery of nucleotides,such as conservation of nucleotide and preventing its degradation,aid in the transfection of a nucleotide into the cells,and targeted nucleotide transport[7,8].It has currently become the hotspot in gene delivery research.
The developing nanotechnology is significant in addressing the issue of plasmid DNA degradation in gene delivery.Common nanomaterial chitosan (CS),a new type of non-viral vector,possesses advantages of non-toxic,no immunogenicity,and relative target.When combined with plasmid DNA,it further imports the DNA into various cells,which is evidenced in vitro and in vivo without any significant cell toxicity[9,10].Moreover,it can effectively combine with plasmid DNA to form nanoparticles and then avoid nuclease degradation to protect the plasmid DNA[11,12].Studies proved that modified chitosan not only retained the excellent chitosan biodegradability,but also has the reduced relative molecular mass,increased water solubility,and targeting property[13,14].In our previous study,we modified the molecular structure of chitosan with galactose ligand and synthesized galactosylated chitosan (GC) by the characteristics of galactose,which can specifically combine with the asialoglycoprotein receptor (ASGPR) on liver cell membranes.These synthesized particles exhibit a liver-targeting gene transfer function,thereby serving as in vitro and in vivo vectors[15].Currently,hepatoma cells are known to have a significant number of biological hormone receptors on its surface,which is 39.6 times than in the regular liver cells[16].HepG2 hepatoma cells have robust adsorption effects on biotin modified nanoparticles,and with the increasing content of biotin in those nanoparticles,the adsorption effects enhanced[17].We have utilized galactose (liver target) and biotin (cancer cells target),combined with chitosan,to synthesize biotin-modified galactosylated chitosan (Bio-GC),which consists of liver targeting and hepatoma targeting functions.The Bio-GC nanomaterials in our studies showed active hepatoma targeting function,and when acted as a 5-FU drug carrier,they can efficiently target and inhibit the growth of hepatoma cells without obvious toxic side effects[18].However,it is still not clear whether the Bio-GC vector can target genes into the target cell.In our previous gene therapy studies,pGM-CSF-GFPIRES-Rae-1-IL-21 (plasmid DNA) was spliced with granulocyte-macrophage set colony stimulating factor(GM-SCF),interleukin-21 (IL-21),retinoic acid early transcription factor 1 (Rae-1),and green fluorescent protein (GFP) through gene splicing technology,and it was found to exhibit favorable curative effect on mouse liver subcutaneous model[16].In this study,we wrapped plasmid DNA with Bio-GC nanomaterial and then investigated the effect of the nanomaterials on the protection of gene and its characteristics in vivo and in vitro.
2 Experimental
2.1 Raw materials
Chitosan (CS,with >85% deacetylation),1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride(EDC),N-hydroxysuccinimide (NHS),and RNase were purchased from Sigma,USA.Biotin was provided by Beijing Jiakangyuan Pharmaceutical Co.,Ltd,China.Tetramethylethylenediamine (TEMED) was obtained from Sinopharm Chemical Reagent Co.,Ltd,Shanghai,China.HCl was purchased from Shanghai Medpep.RPMI 1640 powder was purchased from Gibco,USA.Calf serum was obtained from Hangzhou Sijiqing Pharmaceutical Co.,Ltd,China.DNase I was provided by TaKaRa Biomedicals,Tokyo,Japan.GFP plasmid and plasmid DNA(pGM-CSF-GFP-IRES-Rae-1-IL-21)were previously synthesized and preserved by our laboratory[19].The galactosylated chitosan (GC) and the biotin modified galactosylated chitosan (Bio-GC)were also synthesized and stored in our laboratory.All animals were treated following the protocol approved by the Institutional Animal Care and Use Committee at the Shanghai Tianyou Hospital.
2.2 Experimental animals and cell lines
The human hepatoma cell line SMMC-7721,human colon cancer cell line SW480,human liver cell line LO2,and mouse hepatoma cell line H22 were obtained from the Cancer Institute of Fudan University.Balb/c mice,females,age 7weeks,weight 25g,were purchased from the experimental animal center of Fudan University in Shanghai.
2.3 Synthesis of Bio-GC/plasmid DNA nanoparticles
Bio-GC/plasmid DNA nanoparticles were synthesized using an ionic crosslinking technique as follows: a certain mass of Bio-GC nanomaterials was placed into a centrifuge tube at 45-50 ℃ water bath for 5-10 min.The plasmid DNA were also treated similarly and rapidly added into Bio-GC nanomaterials in accordance with the N/P (molar ratio of amino in Bio-GC and phosphate in plasmid DNA) at 0.5:1,1:1,2:1,3:1,4:1,5:1,and 6:1,respectively.The mixture was thoroughly mixed at 2000 rpm for 30 s,and incubated at room temperature for 30 min.The supernatant was collected after the suspensions of nanoparticles were centrifuged at 4 000 rpm,washed,precipitated by de-ionized water,and further centrifuged.The drugloaded nanoparticles were obtained from the freezedried residues,and the requisite concentrations of nanoparticles solutions were prepared.SMMC-7721 cells in the logarithmic growth phase were cultured at a density of 5×105cells per well in a 5% CO2humidified incubator at 37 ℃ for adherence.The cells were further treated with varying N/P ratios of the nanoparticles for 48 h.The transfection efficiency was determined by flow cytometry (FACSCalibur,BD Biosciences,San Jose,CA,USA).The particle size and zeta potential of the nanoparticles was measured by Zetasizer nano-ZS determinator (Malvern Instruments,Malvern,UK),and the morphology of nanoparticles was observed by transmission electron microscope(Philips Company,Philips,Netherlands).
2.4 Standard curve of Bio-GC/DNA nanoparticles
The plasmid DNA was solubilized with standard body fluid (SBF) at different concentrations (x) of 0.01,0.02,0.05,0.1,0.2,0.5,1,2,and 5 µmol/L,whose absorbance were respectively determined asyat 260 nm.The linear regression of the standard curve was:y=0.0361x+0 0172 (R2=0.9982).The encapsulation efficiency was determined using ultraviolet spectrophotometer (Beckman;DU series 650,INC,USA) to detect the DNA concentration in the supernatant,and the amount of free DNA was calculated according to the volume.Encapsulation efficiency (%)=(total amount of DNA added -the amount of DNA within the supernatant)/ total amount of DNA added × 100%;Drug loading (%)=the total mass of DNA within the nanoparticle/ nanoparticle mass × 100%.
2.5 Release of Bio-GC/DNA nanoparticles in vitro
Bio-GC/DNA nanoparticles (20 mg) were resuspended in 1 mL PBS (pH 7.4) and agitated on 37℃ water-bath thermostats oscillator.The 10 μL supernatant was collected after 1h centrifugation and again resuspended.The concentration of plasmid DNA in the centrifugal supernatant released from Bio-GC/DNA nanoparticles at different time points was estimated at 260 nm by UV spectrophotometry,and the in vitro characteristics were also observed.
2.6 Protective effect of Bio-GC nanomaterial on plasmid DNA
To determine the protective effects of Bio-GC nanomaterial on plasmid DNA,the Bio-GC/plasmid DNA nanoparticles were digested with DNase I,followed by the nanoparticles synthesis,and the protective effects observed by gel electrophoresis.Bio-GC,plasmid DNA,and PBS were used for control.Plasmid DNA,DNase I and plasma was mixed for digestion for 1h.Bio-GC/plasmid DNA nanoparticles were then added into DNase I and incubated at 37 ℃for 1,2,and 8 h,respectively.Concurrently,the Bio-CS nanoparticles were taken together with plasma for digestion for 8 h.The effects were detected by 1% agarose gel electrophoresis,observed by UV transmission instrument,and the gel image captured.Plasmid DNA,Bio-GC/plasmid DNA nanoparticles,and Bio-GC nanoparticles were stored for 60 d,before gel electrophoresis.
2.7 Receptor inhibition test
SMMC-7721 cells were incubated in 96-well plates for 24 h,and further treated with different concentrations of biotin and (or) galactose (0,0.2,0.4,0.6,0.8,1.0 mmol/L) for 4 h.After the supernatant of each well was removed and further washed twice with cold PBS,100 μL Bio-GC/plasmid DNA nanoparticles(including 4 μg plasmid DNA) were added to each well and incubated for 4 h.The supernatants were then removed,and the cells were washed again;the transfection efficiency was estimated by flow cytometry.
2.8 Cell transfection experiment
The SMMC-7721,SW480,and LO2cells at logarithmic growth phase were digested,centrifuged,counted,and cultured into 24-well plates at a density of 5×105cells per well,in a 5 % CO2humidified incubator at 37 ℃ for adherence.The cells were further treated with plasmid DNA,CS/plasmid DNA,GC/plasmid DNA,and Bio-GC/plasmid DNA nanoparticles (4 μg plasmid DNA in each well) for 48 h.Fluorescence microscopy was used to observe the transfection of the cells and flow cytometry to detect the transfection rate.Simultaneously,SMMC-7721 cells were placed in each well and treated with different nanoparticles(CS/plasmid DNA,GC/plasmid DNA,and Bio-GC/plasmid DNA) for final mass concentration of 0,4,8,12,16,20,24,28,and 32 mg/L respectively for 48 h,and the transfection efficiency was determined by flow cytometry.
2.9 Laser confocal detection
SMMC-7721 cells were incubated with FITC labeled Bio-GC/plasmid DNA nanoparticles for 1,2,and 4 h,respectively,and fixed with 4%paraformaldehyde at RT for 20 min.The nuclei were stained with fluorescent dye Hoechst33258 and washed three times with cold PBS.The glass slides were covered with coverslips and mounted with glycerol buffer solution.The fluorescence images were captured by confocal laser microscope;wherein the fluorescently labeled nanoparticles excitation wavelength was 488 nm and the fluorescence dye Hoechst33258 wavelength was 405 nm.The images were overlay processed by the NIS Elements imaging software.
2.10 Establishment of the animal model
Liver subcutaneous model mice successfully established with H22 cells were sacrificed to harvest the tumor tissues.The fresh exuberantly growing tumor tissues were selected for a tumor cell suspension at the density of 6×107/mL.After intraperitoneal anesthesia with 20% urethane and median incision,50 µL tumor cell suspension was injected into the left lobe of the liver capsule with a 1 mL syringe.Consequent to no exudation after 2 min,the abdomen was closed layer by layer.
2.11 Curative effect of Bio-GC/plasmid DNA nanoparticles on mice orthotopic liver transplantation model
5 d after the orthotopic liver transplantation model in mice were established,the tumor tissues reached about 4-6 mm in diameter.On the sixth day,the experimental models were randomly divided into 6 groups: control,Bio-GC,plasmid DNA,CS/plasmid DNA,GC/plasmid DNA,and Bio-GC/plasmid DNA nanoparticles group.The control group received an intravenous injection of normal saline (200 µL).The Bio-GC group received an empty nano tail vein injection of 200 µL.The plasmid DNA group,CS/plasmid DNA group,GC/plasmid DNA group,and the Bio-GC/plasmid DNA groups were respectively administered with 200 µL of the corresponding drug(including 100 µg plasmid DNA).The mice received continuous tail vein injection for 5 d,following which 9 mice from each group were observed for tumor weight and pathological alterations.
2.12 Statistical analysis
All data were expressed as mean±standard deviation.One-way analysis of variance (ANOVA)was used to analyze between the groups data.Least significance difference (LSD) was used for comparisons of the inter-group data.Kaplan-Meier survival plots were used for survival data.P<0.05 was as statistically significant.
3 Results
3.1 Synthesis of Bio-GC/plasmid DNA nanoparticles and its release in vitro
As shown in Fig.1(a),when the ratio of N/P changed from 0.5:1 to 3:1,the transfection rate of Bio-GC/plasmid DNA nanoparticles on SMMC-7721 cells increased significantly,whereas when from 3:1 to 6:1,the transfected rate decreased significantly.These results indicated that Bio-GC/plasmid DNA nanoparticles transfected most efficiently at the N/P ratio of 3:1.Transmission scanning electron microscopy showed that the Bio-GC/plasmid DNA nanoparticles have regular spherical shapes,a smooth surface,absence of agglomeration,superior dispersion,and average particle diameter 87.1 nm,for which the encapsulation efficiency was 86.53%,the drug loading was 32.7%,and the Zeta potential was 14.9 mV (Fig.1(b)).As shown in Fig.1(c),the release curve of Bio-GC/plasmid DNA nanoparticles exhibited the release in 2 stages.The first stage was a rapid release stage observed from 0 to 6 h,in which the plasmid DNA release rate was 14.6% attributed to the release on nano or shallow surface.The second stage was a steadily release period occurring from 6 to 168 h,which resulted in a cumulative release percentage of 60.72% because of the degradation of Bio-GC nanomaterials and the release of plasmid DNA.
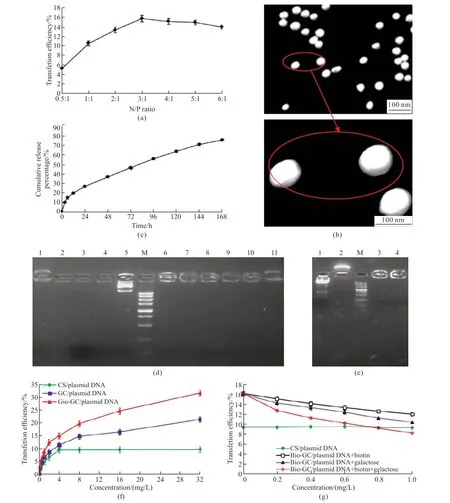
Fig.1 Synthesis of Bio-GC/plasmid DNA nanoparticles and its properties in vitro: (a) Transfection efficiencies for Bio-GC/plasmid DNA nanoparticles with different N/P ratios of SMMC-7721 cells;(b) TEM images of Bio-GC/plasmid DNA nanoparticles;(c) Release curves of Bio-GC/plasmid DNA nanoparticles in vitro;(d) Protection of Bio-GC/plasmid DNA nanoparticles on gene (1: Bio-GC,2: PBS,3: plasmid DNA+DNase I,4: plasmid DNA+plasma,5: plasmid DNA,M: Maker from top to bottom was 5 000,3 000,1 500,1 000,750,500,250,100,and 50 bp,6: Bio-GC/plasmid DNA,7: Bio-GC/plasmid DNA+DNase I digested at 37 ℃ for 1 h,8: Bio-GC/plasmid DNA+DNase I digested at 37 ℃ for 2 h;9: Bio-GC/plasmid DNA+DNase I digested at 37 ℃ for 8 h;9: Bio-GC/plasmid DNA+plasma digested at 37 ℃ for 8 h;10: Bio-GC+DNase I digested for 8 h);(e) Release of Bio-GC/plasmid DNA nanoparticles.The plasmid DNA,Bio-GC,and Bio-GC/plasmid DNA nanoparticles were synthesized and stored at 4 ℃ for 60 d,and then tested by gel electrophoresis (1: plasmid DNA,2.Bio-GC,M: Maker from top to bottom was 5 000,3 000,1 500,1 000,750,500,250,100,and 50 bp,3: Bio-GC/plasmid DNA,4: Bio-GC/plasmid DNA+DNase I digested at 37 ℃ for 8 h);(f) Cellular uptake of the different plasmid DNA nanoparticles;(g) Inhibition of transfection of Bio-GC/plasmid DNA nanoparticles in SMMC-7721 cells by biotin and (or) galactose
3.2 Protective effect of Bio-GC/plasmid DNA nanoparticles on gene
As shown in Fig.1(d),plasmid DNA group mapped on the gel electrophoresis displayed a bright band,but in the Bio-GC/plasmid DNA group,the plasmid DNA was unable to migrate from the sample loaded well,which indicated that Bio-GC and plasmid DNA formed a tight complex.After the digestion of Bio-GC/plasmid DNA with DNase I at 37 ℃ for 1-8 h,gel electrophoresis still showed a band,which continued to persist further in the digestion of plasma for 8 h.The plasmid DNA band disappeared when digested with DNase I and serum at 37 ℃ for 30 min.DNase I added to Bio-GC in the above condition displayed positively charged half-moon bands,which indicated that Bio-GC nanomaterials were positively charged and could not be digested by enzymes.Fig.1(e)showed that plasmid DNA and Bio-GC/plasmid DNA nanoparticles stored for 60 d remained intact,and the plasmid DNA in Bio-GC/plasmid DNA nanoparticles group was yet not resolved on the gel from the sample well.Furthermore,after digestion with DNase I for 8 h,the band did not disappear,which indicated that even after 60 d of storage,the plasmid DNA in the package of Bio-GC nanomaterials remained very stable.
3.3 Receptors-mediated transfection of Bio-GC/plasmid DNA nanoparticles on liver cancer cells
As shown in Fig.1(f),the transfection rate of SMMC-7721 cells significantly increased with the increasing concentrations of CS/plasmid DNA nanoparticle.At the concentration of 4 mg/L,the transfection rate reached a plateau,during which the efficiency was not continually improved.At the concentrations of 0-32 mg/L in GC/plasmid DNA and Bio-GC/plasmid DNA group,the transfection rates in SMMC-7721 cells significantly increased,respectively.The transfection rate in Bio-GC/plasmid DNA group was substantially higher than that in the GC/plasmid DNA group,which suggested that the receptor-mediated intracellular transfection was significantly superior to that of the CS/plasmid DNA,and the double receptors-mediated cell transfection was significantly greater than that of the single receptor without a transfection saturation phenomenon at these concentrations.As shown in Fig.1(g),when the concentration of galactose and biotin increased,the transfection of Bio-GC/plasmid DNA nanoparticles on SMMC-7721 cells appeared significantly decreased.At 0.6 mg/L concentrations of galactose and biotin,the transfection rate was considerably lower than that of the CS/plasmid DNA.On the other hand,when the cells were added with galactose and biotin alone,although the transfection rate of Bio-GC/plasmid DNA nanoparticles was significantly decreased,it was still significantly higher than that of the GC/plasmid DNA nanoparticles.
3.4 Targeting transfection of Bio-GC/plasmid DNA nanoparticles in hepatoma cells
As shown in Figs.2(a) and 2(b),the transfection rates of CS/plasmid DNA and plasmid DNA in SMMC-7721 and SW480 cells were similar,mainly because the CS nanomaterial was a non-targeted nanomaterial.The transfection rate of GC/plasmid DNA on SMMC-7721 cells was higher than that in SW480 cells (P<0.01),which indicated that GC nanomaterials showed obvious hepatoma targeting.The transfection rate of Bio-GC/plasmid DNA nanoparticles on SMMC-7721 cells was significantly higher than that in SW480 cells,and the latter was also significantly higher than on LO2cells (P<0.01).In addition,the transfection rate of Bio-GC/plasmid DNA nanoparticles on SW480 cells was significantly higher than that of GC/plasmid DNA nanoparticles (P<0.01),which suggested that Bio-GC nanomaterial has a dual targeting property,i e,having two ligands for hepatoma targeting and cancer targeting,and exhibiting active hepatoma targeting.Fig.2(c) demonstrats the process of gene transfected into the nucleus by the nanomaterial.The laser scanning confocal microscopy detected the green FITC labeled DNA that had already entered into the cells in 1h,since most of it was still in the cytoplasm,surrounding the nucleus,labeled by blue Hoechst 33258.After 2 h,a small amount of green fluorescence and blue nuclei appeared to overlap,and the green fluorescence increased around the nucleus.At 4h,the green fluorescence aggregation was more evident at the periphery of the nucleus,and a small amount of green fluorescence and blue nucleus overlapped less,which indicated that a large number of Bio-GC/plasmid DNA nanoparticles had entered into the cytoplasm.

Fig.2 The transfection of Bio-GC/plasmid DNA nanoparticles in hepatocellular carcinoma cells: (a) The fluorescence microscopy of Bio-GC nanomaterials for cell transfection;(b) The transfection efficiency of various cells detected by flow cytometry;(c) Laser confocal microscopy of Bio-GC/plasmid DNA nanoparticles transfected in SMMC-7721 cells
3.5 Inhibitory effect of Bio-GC/plasmid DNA nanoparticles on orthotopic liver transplantation model in mice
As shown in Fig.3(a),the tumor weights in Bio-GC/plasmid DNA,GC/plasmid DNA and CS/plasmid DNA groups were significantly lower than that of the plasmid DNA,Bio-GC,and control groups (P<0.01),in which the tumor weight in CS/plasmid DNA,GC/plasmid DNA,and Bio-GC/plasmid DNA groups,in turn,reduced (P<0.01),and in plasmid DNA,Bio-GC and control groups were no statistical significance(P>0.05).These results suggested that the active dual target Bio-GC nanomaterial as a gene vector in hepatoma therapy was significantly higher than that of single targeting GC nanomaterial and no targeting CS nanomaterial.Although a significant amount of DNase I in the blood could degrade the plasmid DNA,direct injection of plasmid DNA into the tail vein could not play a role in hepatoma inhibition.As shown in Fig.3(b),hepatoma cells in plasmid DNA,Bio-GC,and control groups exhibit vigorous growth,in which the nuclear division is more evident,and the cells arranged orderly.The hepatoma tissues in CS/plasmid DNA group demonstrated focal necrosis and cell arrangement disorder,and in the GC/plasmid DNA group,the phenomenon of bleeding and necrosis was more severe than that of the CS/plasmid DNA group.The tumor tissues in the Bio-GC/plasmid DNA group were apparently degraded,the cells were atrophic and sparse,along with fibrosis,bleeding,and infiltration of a large number of inflammatory cells.
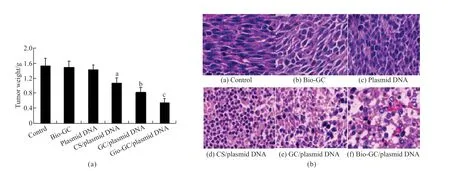
Fig.3 The inhibitory effect of Bio-GC/plasmid DNA nanoparticles on mice orthotopic liver transplantation model: (a) The effects of Bio-GC/plasmid DNA nanoparticles on the tumor weight of orthotopic liver transplantation model in mice;(b) The effects of Bio-GC/plasmid DNA nanoparticles on the pathological tissue of liver cancer (HE×400)
4 Discussion
Nanotechnology in cancer treatment has attracted much attention.the use of technology in gene transport resolved the issues of immunogenicity,safety,low transfection efficiency,and targeting property that occurred in viral vectors,thus showing a broad application prospective[20].Transfection efficiency is an important indicator for the evaluation of gene carrier,and the purpose of improving cell transfection efficiency was mainly through the modification of nanomaterials and then grafted by the ligand to form the active targeting nanomaterials[21].In our previous studies,the nanomaterial Bio-GC with the liver target and liver cancer target was synthesized,which demonstrated obvious hepatoma targeting and promoting anti-tumor effect for chemotherapeutic drugs.Our studies also showed that the N/P ratio of Bio-GC and gene is a major factor in tumor cells transfection and that the ratio directly affects the ability of nanomaterials to bind DNA,and the interactions of positively charged nanoparticles with negatively charged membrane[22].When the N/P ratio increased,the zeta potential of nanoparticles increased,which enhanced the electrostatic adsorption of the cell membrane,promoted the cellular uptake[23]and the intracellular endosomal vesicles rupture,and also precipitated the release of the nanoparticles into the cytoplasm to benefit transfection[24].The small ratio of N/P signified that the Bio-GC/plasmid DNA nanoparticles contained less Bio-GC and the zeta potential of the nanoparticles was low,which caused the instability and mutual aggregation for the nanoparticles.The large N/P ratio would lead to a high zeta potential of nanoparticles which would interfere with the release of DNA from those nanoparticles.In either case,it resulted in a decreased transfection rate[25].Therefore,choosing appropriate N/P ratio is the key to enhancing the transfection rate.
The present study showed that when the N/P ratio increased in the range of 0.5:1-3:1,the transfection rate significantly increased,while when in the range of 3:1-6:1,the transfection rate decreased significantly,thus suggesting that the appropriate N/P ratio for Bio-GC/plasmid DNA nanoparticles was 3:1.The optimum N/P ratio for single target nanomaterial GC and gene in our previous study was 5:1[26],which suggested that the double targeting Bio-GC nanomaterials increased the receptors-mediated hepatoma cells combination and improved the transfection efficiency.In our study,when the N/P ratio was 3:1,the transmission scanning electron microscope demonstrated that the Bio-GC/plasmid DNA nanoparticles were spherical,smooth,no agglomeration,enhanced dispersion,and average particle size 87.1 nm.The definition of nanoparticles for American International Cancer Association is colloidal particles with 1-100 nm particle size,and the specific particle size is not restrictive.However,studies have shown that the particle diameter between 10-100 nm is preferable[27].The particle size greater than 100 nm or less than 10 nm is easily stranded in the liver or excreted by the kidney,and the particle size larger than 200 nm could non-specifically be removed by mononuclear and reticuloendothelial system[28,29].In the present study,the particle size is ideal at 87.1 nm.The release of Bio-GC/plasmid DNA nanoparticles in the simulated body fluid in vitro demonstrated two phases,the fast and the stable release phases.In the fast release period,the plasmid on the surface and shallow of nanoparticles were rapidly released into the simulated body fluid,within 6 h.In the stable release period,the plasmid was steadily released because of the gradual degradation of nanomaterials,over a period of 6 -168 h.These results indicated that Bio-GC/plasmid DNA nanoparticles have sustained release effect.
The Zeta of Bio-GC/plasmid DNA nanoparticles were 14.9 mV,which indicated that the nanoparticles had positive charge on its surface and was not easy to gather,which is conducive to the stability of the system.Gel electrophoresis assay showed that the Bio-GC nanoparticles migrated to the top of the halfmoon strip band,indicating that they were positively charged,and Bio-GC/plasmid DNA nanoparticles were almost completely blocked in the sample well in a tight complex.DNase I protection experiments showed that the DNA was not degraded either after interaction of Bio-GC/plasmid DNA nanoparticles and plasma for 8 h or Bio-GC/plasmid DNA nanoparticles and DNase I for 1-8 h.Since the nucleic acid enzyme concentrations in the physiological conditions were significantly lower than the DNase I concentration,these results indicated that Bio-GC/plasmid DNA nanoparticle had obvious anti-DNase I degradation ability.The DNA release experiment revealed that after 60 d storage at 4 ℃,the Bio-GC/plasmid DNA nanoparticles were still not resolved by electrophoresis,and also can resist the degradation of DNase I,which showed that the Bio-GC/plasmid DNA nanoparticles had high stability.
The cell transfection efficiency of Bio-GC nanomaterials carried with plasmid DNA is an important evaluation index.The transfection efficiency increase was not only directly affected by the N/P ratio but also depended on increasing doses of plasmid DNA.Our studies demonstrated that the transfection rate was stable at 4 mg/L gene concentration of CS/plasmid DNA nanoparticles.The transfection efficiency increased significantly when the concentrations of GC/plasmid DNA and Bio-GC/plasmid DNA nanoparticles were at 0-32 mg/L and the Bio-GC/ plasmid DNA nanoparticles had the highest transfection rate.Nontarget CS nanomaterials were imported into the cells by internalization effect.The CS then causes tight junction of the proteins,re-arrangement of filamentous actin,and increased intercellular permeability,which promotes macromolecular drugs transport between cells[30,31].When the gene concentration reached 4 mg/L,the internalization effect declined,resulting in decreased transfection rate.These results demonstrated that the increasing rate of gene transfection caused by active hepatoma-targeting nanomaterials was not only affected by the internalization effect but also depended on the cell membrane receptors to let the genes import into the cells.Hence,the nanomaterials Bio-GC with dual targeting characteristics has pronounced ability to promote gene transfer into the cells.Receptor competitive inhibition assay revealed that in the culture solution with galactose and biotin,the transfection rate of hepatoma cells declined.The transfection rate of Bio-GC/plasmid DNA nanoparticles on hepatoma cell is still higher than CS/plasmid DNA when using a single ligand (galactose or biotin) in 0-1 mg/L.Coaddition of galactose and biotin,significantly lowered the transfection rate than that of the CS/plasmid DNA at the concentration of 0.8 mg/L,which indicated that the transfection rate induced by internalization effect for Bio-GC/plasmid DNA nanoparticles was significantly lower than that of the CS/plasmid DNA when cell receptors have been fully inhibited.
Gene therapy is the most promising method in hepatoma treatments.However,the import of genes into hepatoma cells is yet an unsolved issue.The targeted gene delivery into HCC cells has become a hot topic in the treatment of HCC,and the construction of an ideal target in hepatoma gene therapy is the key to the treatment.Our studies suggested that the gene transcription rate of dual targeting nanomaterials Bio-GC on hepatoma cells was significantly higher than that of the single target nanomaterial GC and also higher than that of Bio-GC in LO2cells.This indicated that the Bio-GC nanomaterials can transfer the gene into cells by biotin and galactose receptors,and the transfection rate was significantly higher than that of single target and non-target nanomaterials.The present study demonstrated that the Bio-GC/plasmid DNA nanoparticles labeled with FITC were imported into the cells across the membrane,and the cells showed intracellular fluorescence staining,which increased significantly with the extension of time.Green fluorescence was found mainly distributed at the periphery of the nucleus in 1h,which indicated that Bio-GC/plasmid DNA nanoparticles were primarily in the cytoplasm.The green fluorescence gathered around the nucleus was more obvious in 2 to 4 h,noting that the amount of Bio-GC/plasmid DNA nanoparticles into the cytoplasm is gradually increasing.Due to technical limitations,little is known about the transport of DNA into the nucleus.Our studies demonstrated that the green fluorescence was observed in the nucleus in 2 to 4 h,mainly concentrated at the periphery,which is consistent with the results reported in the literature that DNA enters the nucleus in the form of a poly complex[24].Therefore,increasing the dissociation of Bio-GC/plasmid DNA nanoparticles in the cells can improve the transfection efficiency mediated by Bio-GC.
We verified the ability of Bio-GC nanoparticles to transport the plasmid DNA to the liver,and then observed the targeted inhibition of orthotopic hepatoma transplantation model.Our studies demonstrated that the curative effect of dual targeting Bio-GC nanomaterial carrying the gene in murine orthotopic hepatoma transplantation model was superior to the single target and non-target nanomaterial.The nanoencapsulated gene therapy was superior to the bare gene therapy,which suggested that the nanomaterials had a protective effect on the gene,and when the gene was transported to the liver through the vein,it was not digested by the enzyme in the blood.However,the transfection efficiency of the dual targeting Bio-GC gene was significantly higher than that of the single target,so the effect of Bio-GC/plasmid DNA nanoparticles was the most significant.A curative effect for naked gene therapy in the orthotopic transplantation tumor model was not observed,which suggested that the naked gene injected into the blood has been digested by the nucleic acids enzyme,and therefore,there was no obvious therapeutic effect.Pathology also confirmed that liver cancer tissue in Bio-GC/plasmid DNA group appeared degenerated,atrophic,hemorrhaged,with fiber ring and inflammatory cell invasion,while in the control group,the nuclear division of the liver cancer tissue was apparent,the structure was orderly,and the cell growth was strong.Additionally,the liver cancer tissues of plasmid DNA group were consistent with the control group,which further confirmed that the naked gene was digested with the nucleic acid enzyme and had little effect on the liver cancer cells.
5 Conclusions
Bio-GC/plasmid DNA nanoparticles not only have a sustained release effect but also have a gene protective effect of avoiding the degradation by a nuclease.Thus,the improved gene transfection efficiency of hepatocellular carcinoma actively targets the genes transfer into the cells,in which the target role is realized mainly through the combination of nanomaterials galactose and biotin and the receptors on hepatocellular carcinoma cells.Also,Bio-GC/plasmid DNA nanoparticles also exerted an inhibition effect on orthotopic liver transplantation model in mice.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Fabrication of YAG: Ce3+ and YAG: Ce3+,Sc3+ Phosphors by Spark Plasma Sintering Technique
- Preparation of Modified UiO-66 Catalyst and Its Catalytic Performance for NH3-SCR Denitration
- Effect of Molecular Weight on Thermoelectric Performance of P3HT Analogues with 2-Propoxyethyl Side Chains
- Ultraviolet Photodetector based on Sr2Nb3O10 Perovskite Nanosheets
- Fabrication of Silane and Desulfurization Ash Composite Modified Polyurethane and Its Interfacial Binding Mechanism
- Bio-inspired Hydroxyapatite/Gelatin Transparent Nanocomposites
