Preparation of Flower-like Copper Foam Supported Co3O4 Electrocatalyst and Its Hydrogen Evolution Performance
2024-04-11LIZhaoCHENGJulongWANGYananWUKunyaoCAOJing
LI Zhao,CHENG Julong,WANG Yanan,WU Kunyao,CAO Jing
(School of Materials Engineering,Xi’an Aeronautical Institute,Xian 710077,China)
Abstract: Flower-like copper foam Co3O4 catalysts (Co3O4/CF) were prepared by hydrothermal method.The crystalline structure and microscopic morphology of the prepared samples were characterized by using X-ray diffractometer (XRD) and scanning electron microscope (SEM),and the electrochemical properties were investigated by an electrochemical workstation.The experimental results show that the Co3O4 catalysts are successfully prepared on the foamed copper support by hydrothermal method,and the material's morphology is mainly flower cluster.When the current density is 10 mA·cm-2,the overpotential value of the Co3O4/CF catalyst is 141 mV,lower than that of blank support.The electrochemical impedance (EIS) spectrum shows that the Rct value of the Co3O4/CF catalyst decreases,and the Coulomb curves of double-layer show that the electrochemically active area of the Co3O4/CF catalyst efficiently increases compared with that of the blank support.Therefore,the as-obtained Co3O4/CF catalyst exhibits a good hydrogen evolution rate,showing great applicability potential in the catalytic electrolysis of water for hydrogen production.
Key words: Co3O4/CF;composite structure;electrocatalysis;hydrogen evolution reaction
1 Introduction
Hydrogen energy is promising to substitute to fossil fuels in the future,because it is clean,abundant,renewable and eco-friendly[1-4].Hydrogen production by water electrolysis is one of the effective processes for producing hydrogen.However,the hydrogen produced by this approach only accounts for 3% of the total hydrogen consumption.In the reaction process,the overpotential caused by the activation energy barrier of the anode and the cathode must be overcome.This could lead to the need to consume a higher overpotential in the electrolysis to promote the reaction of electrolyzed water.Electrocatalysts can reduce this process’s activation energy,lowering the necessary overpotential to produce hydrogen.Currently,precious metals such as Pt and Pd are mainly used as catalysts in commercial water electrolysis,and the overpotential at a current density of 500 mA·cm-2was 300 mV.However,noble metal-based catalysts have a high cost,low energy reserve,and usually poor stability,limiting their applicability[5-7].Therefore,developing electrocatalysts with low overpotential,high activity,and stability to improve the conversion efficiency of electrolyzed water systems is the key to hydrogen energy production[8-11].
In this paper,by constructing a composite structure with a flower cluster shape to increase the electrocatalytic active area and expose more active sites,a simple and efficient improvement scheme was designed based on the criteria of abundant reserves,low cost,and environmental friendliness.The Co3O4/CF catalyst was characterized by XRD,SEM,and other methods to study its phase structure and morphology.Moreover,its electrocatalytic performance was tested and studied by electronic universal testing machine.
2 Experimental
2.1 Preparation of supported Co3O4 catalyst
Co3O4catalysts were prepared by in situ growth on copper foam support by hydrothermal method.First,0.0815 g of cobalt nitrate (Co (NO3)2) and 0.1682 g of urea (CO(NH2)2) were measured and dissolved in 15 mL of distilled water.A clear solution was obtained by stirring for 20 min at room temperature.Then the obtained solution was transferred into a 25 mL autoclave,and the copper foam carrier sheet was also introduced into the autoclave obliquely.After hydrothermal reaction at 90 ℃ for 6 h,rinsed with deionized water 2-3 times,and dried at 60 ℃ for 12 h,the Co3O4/CF catalyst was obtained.
2.2 Characterization of Co3O4/CF catalysts
The crystal structure of the Co3O4/CF catalyst was determined by a Rigaku Ultima IV X-ray diffractometer.The morphology and energy spectrum was measured by a Hitachi SU5000 SEM.The electrochemical performance of the catalyst was tested by the Shanghai Chenhua CHI760E electrochemical workstation.The three-electrode system device was used,and the electrolyte solution was 1 mol·L-1KOH.The as-prepared catalyst was used directly as the working electrode.The calomel electrode (SCE) was the reference electrode,and the platinum plates placed in parallel were the auxiliary electrodes.
3 Results and discussion
3.1 Phase analysis
The phase structure characterization of the Co3O4/CF catalyst is presented in Fig.1.It can be seen that the diffraction peaks of the Co3O4/CF catalyst at 19.0°,31.1°,36.8°,44.8°,59.3°,and 65.2°,2θcould be assigned to the (111),(220),(311),(400),(511),and(440) crystal planes,consistent with the standard PDF card(43-1003).This could indicate that the material obtained during the hydrothermal method loaded onto the copper foam was a spinel-type Co3O4phase.

Fig.1 XRD patterns of Co3O4/CF catalyst
3.2 Microstructure analysis
Fig.2 shows the microstructure of the Co3O4/CF catalyst at different magnifications.It can be seen that the Co3O4catalyst supported on foam copper is in the shape of spherical flower clusters.This morphology could increase the contact area between the electrode and the electrolyte,providing more active sites for the catalyst,further improving the catalytic performance,and more conducive to the hydrogen evolution reaction.
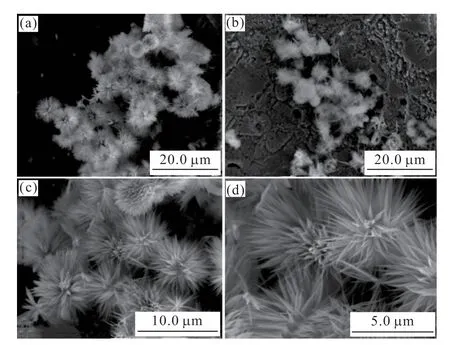
Fig.2 SEM images of Co3O4/CF catalyst
As can be seen in Fig.2(b),the elemental composition and distribution of the Co3O4/CF composite electrocatalyst were tested by EDS.The obtained EDS point scan and area scan spectra are shown in Figs.3 and 4.It can be seen from Fig.3 that the point scanning analysis of two points on the surface of the catalyst proves that the catalyst is mainly composed of two elements,consistent with the elements of the Co3O4.Fig.4 presents the element distribution diagram of the Co3O4/CF catalyst,in which the Co and O were homogeneously distributed in the composite catalyst.

Fig.3 Spot scan energy spectra of the Co3O4/CF catalyst
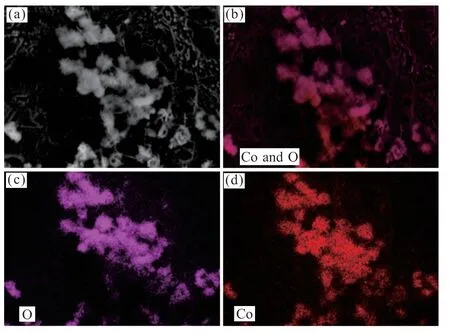
Fig.4 Mapping images of the Co3O4/CF catalyst
3.3 Electrocatalytic performance
3.3.1 Linear sweep voltammetry(LSV) curve test analysis
To investigate the catalytic performance of the Co3O4-based catalysts,the LSV polarization curves of the samples were measured.Since the precursor of Co3O4was grown on the highly conductive foamed copper substrate,the sample could be directly used as the working electrode.The LSV polarization curve of Co3O4/CF-based catalyst in hydrogen evolution reaction(HER) is shown in Fig.5(b).The overpotential value of the Co3O4-based catalyst supported on foamed copper at a current density of 10 mA·cm-2was 141 mV,and the overpotential at a current density of 100 mA·cm-2was 239 mV.The LSV polarization curve of blank copper foam in HER is also shown in Fig.5(a).It can be observed that the overpotential value of blank copper foam at a current density of 10 mA·cm-2was 200 mV,which is higher than the overpotential of the composite electrode.The overpotential is an important indicator to measure the catalytic performance of the catalyst for oxygen evolution.The smaller the overpotential value,the higher the electrocatalytic activity.Thus,the catalytic activity of the Co3O4/CF composite electrode was higher than that of the CF electrode material[12].The LSV polarization curves of the effect of concentration of Co3O4in HER is shown in Fig.6.Different concentrations have great influence on the morphology.So it can be observed that when the concentration of Co3O4is 0.015 mol/L,which is lower than the other concentration.Compare the hydrogen evolution performance of the flower-like copper foam supported Co3O4electrocatalyst with the Co3O4-NCTs electrocatalysts[13],when the current density was 10 mA·cm-2,the overpotential value of the Co3O4/CF catalyst was 141 mV,is better than the value of Co3O4-NCTs catalyst was 358 mV.

Fig.5 LSV curves of catalyst: (a) CF;(b) Co3O4/CF

Fig.6 LSV curves of different concentrations of Co3O4
3.3.2 EIS test analysis
Electrochemical impedance spectroscopy could investigate the interface characteristics of solidphase catalyst and liquid electrolyte solution,directly reflecting the kinetic law of the surface of the solidphase catalyst.To explore the interfacial electron transport performance between the catalyst and the electrolyte and to examine the kinetic parameters related to the electrocatalytic process,the EIS of the Co3O4-based catalyst was further investigated(Fig.7(b)).

Fig.7 Nyquist curves of catalyst: (a) CF;(b) Co3O4/CF
Using the equivalent circuit diagram to fit the Nyquist curve,it was found that Co3O4/CF showed a semi-circular arc shape in the test frequency region due to the presence of water molecules at the interface between the solid-phase catalyst and the liquid electrolyte.The charge transfer resistance of the electron reduction means that the electrochemical reaction could control the kinetic processes of the electrode surface.In the equivalent circuit diagram,RSis the series resistance in the solution,andRctis the charge transfer resistance of the electrocatalytic reaction[14,15].It can be seen from Fig.7(b) that theRSof the Co3O4/CF composite electrode material was 2.7 Ω·cm-2,and theRctwas 11.5 Ω·cm-2.To study the change in the catalytic activity of the Co3O4/CF composite electrode material,the EIS spectrum of the blank copper foam also tested.Fig.7(a) is the Nyquist curve of the blank copper foam.It can be seen that theRSof CF electrode material is1.13 Ω·cm-2and theRctis 30.8 Ω·cm-2.From CF to Co3O4/CF,theRctdecreased from 30.8 Ω·cm-2to 11.5 Ω·cm-2because as Co3O4in the catalyst grows on CF,the resistance of electron transfer on the surface of the catalytic material decreases,and the charge transfer rate increased.The Co3O4/CF composite electrode material showed an enhanced charge transfer rate in the electrocatalytic process,mainly attributed to the spherical flower-like structure of the electrode material.This structure could bring more active sites,making it compatible with the electrolyte.The catalytic process has a faster kinetic rate with lower resistance to charge transport[16].
3.3.3 ECSA test analysis
To understand the electrocatalytic performance of Co3O4catalysts supported on foamed copper,investigations detailing the number of active sites are also essential.The analysis of the electrochemically active area (ECSA) could reflect the number of catalytically active sites on the catalyst’s surface.Moreover,the size of the ECSA is proportional to the electrocatalyst’s electric double-layer capacitance (Cdl).Therefore,the cyclic voltammetry (CV) of the Co3O4-based catalysts was measured (Fig.8(b)).When the potential was 0,the correspondingly charged electric double layer Coulomb curves were drawn (Fig.9(b)),and the Cdl of the Co3O4/CF composite catalyst was calculated to be 1.05 mF·cm-2,ECSA is 26.25 cm2.To analyze the change in the catalytic activity of the composite,the CV of the blank copper foam CF was also measured by the same method (Fig.8(a)),and the corresponding charged electric double layer Coulomb curve was drawn (Fig.9(b)).It can be observed that theCdlof the CF material was 0.915 mF·cm-2,and the ECSA was 22.875 cm2.Compared with pure CF,the Co3O4/CF composite catalyst has a largerCdlvalue and a larger electrocatalytic active area with more active sites,so its electrocatalytic efficiency was successfully enhanced.

Fig.8 CV curves of catalyst: (a) CF;(b) Co3O4/CF

Fig.9 Coulombic curves of catalyst: (a) CF;(b) Co3O4/CF
4 Conclusions
The Co3O4/CF composite catalyst prepared by the hydrothermal method showed a spherical flowerlike cluster structure.The composite catalyst showed good catalytic activity,and the catalyst exhibited good activity at a current density of 10 mA·cm-2.The required overpotential of the Co3O4/CF catalyst was 141 mV,significantly lower than the 200 mV of the blank copper foam.TheRctvalue of Co3O4/CF was 11.5 Ω·cm-2from the EIS spectrum,smaller than the blank copper foam.The charge transfer rate of the supported catalyst could be accelerated,and the HER will be easier to obtain.From the Coulomb curve of the charged electric double layer,it was found that theCdlvalue of the electric double layer capacitance of Co3O4/CF was 1.05 mF·cm-2,significantly higher than that of the blank support.The supported catalyst will have a larger electrochemically active area and better catalytic activity.The spherical flower-like structure of the Co3O4/CF composite catalyst ensures full contact with the electrolyte solution,increases the gas escape ability,provides a larger specific surface area,and increases the number of active sites improving the composite electrocatalytic activity of catalysts.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Biotin-modified Galactosylated Chitosan-gene Carrier in Hepatoma Cells Targeting Delivery
- Mussel-inspired Methacrylic Gelatin-dopamine/Ag Nanoparticles/Graphene Oxide Hydrogels with Improved Adhesive and Antibacterial Properties for Applications as Wound Dressings
- Effect of Polyvinyl Alcohol in Inner Aqueous Phase on Stability of Millimeter-scale Capsules
- Synthesis and Characterization of Nonionic Waterborne Polyurethane and Application to Wool Fabric Finishing
- Fluorescent Double Network Hydrogels with Ionic Responsiveness and High Mechanical Properties for Visual Detection
- Damage Mechanism of Ultra-thin Asphalt Overlay (UTAO)based on Discrete Element Method
