Photocatalytic Activity Enhancement in Organic Dyes Degradation by Loading Ag Nanoparticles onto α-Fe2O3/ZnOs
2024-04-11LIBaoliuMENGXinruiYUEYifanGAOFang
LI Baoliu,MENG Xinrui,YUE Yifan,GAO Fang
(Key laboratory of Hubei Province for Coal Conversion and New Carbon Materials,Wuhan University of Science and Technology,Wuhan 430081,China)
Abstract: Fe2O3/ZnO/Ag ternary composite photocatalytic material was prepared by simple hydrothermal method,and its structure and photocatalytic properties were studied.The experimental results show that Fe2O3/ZnO/Ag exhibits better photocatalytic performance.After two hours of UV irradiation,the degradation rates of orange II and methyl orange reached 91.9% and 75.9%,respectively.The design and preparation of the photocatalyst provide a theoretical basis for the practical application of photocatalytic technology.
Key words: Fe2O3;ZnO;composites;photocatalysis
1 Introduction
All creatures on the earth are inseparable from water resources.It is one of the important conditions for maintaining life activities[1].However,the gradual maturity of economic and industrial technology has led to the increasingly prominent problem of water pollution.The water environment contains not only traditional pollutants,but also a large number of pollutants such as chemical wastes and drugs[2-4],which not only affects the water supply ecosystem,but also seriously threatens the life and health of human beings and aquatic animals and plants.As a safe and efficient environmental purification technology,semiconductor photocatalytic oxidation technology can directly use light energy to degrade organic pollutants into inorganic small molecules under normal temperature and pressure conditions,and has the advantages of low energy consumption,simple operation,mild reaction conditions and small secondary pollution[5].Its importance is self-evident when fossil energy is increasingly scarce and environmental pollution is increasingly serious.
ZnO has the advantages of high stability,strong photocatalytic ability,non-toxic and cheap[6],but ZnO has a large band width,which can not make full use of sunlight and has a low light utilization rate[7,8].In addition,the electron and hole recombination rate of ZnO is high,resulting in the low photoquantum efficiency of ZnO[9-11].α-Fe2O3has the advantages of good chemical stability and low cost,larger specific surface area,narrower band width and higher illumination efficiency[12].The heterojunction structure ofα-Fe2O3and ZnO was formed by the combination ofα-Fe2O3and ZnO,which inhibited the recombination rate of carriers and excited more photogenic radicals,thus improving the photocatalytic degradation performance[13-14].However,there are many problems such as large feed ratio,low catalytic efficiency and many influencing factors,which need to be further studied.In view of the above shortcomings,researchers have done a lot of research to enhance the photocatalytic performance of composites by increasing the specific surface area,adjusting the energy band structure,ion doping,precious metal deposition and surface photosensitization[15-17].
Silver is an important modified material for semiconductor optical catalysts,and its introduction will significantly improve the photocatalytic activity of semiconductor photocatalyst[18].Yu-Hsun[19]et alfound that deposition of noble metal Ag on TiO2surface would significantly improve the degradation efficiency of the composite material for dyes under visible light.Soner Çakar[20]et alfound that the ability of TiO2and Ag composite to degrade indigo carmine under UV light increased by about 25% compared with pure TiO2.In this study,Fe2O3/ZnO/Ag ternary complexes were constructed,and the UV photocatalytic activities of different materials catalysts were investigated with Orange II(OII) and methyl orange(MO) as the target pollutants.
2 Experimental
2.1 Materials
Sodium hydroxide (NaOH,99wt%),potassium hydroxide (KOH,99wt%),Sodium dodecylbenzene sulphonate (SDBS,98wt%),Iron chloride hexahydrate(FeCl3·6H2O,99wt%),dimethylbenzene (C8H10,98wt%),ethyl alcohol (C2H6O,99wt%),anhydrous zinc acetate (Zn(CH3COO)2,99wt%),silver nitrate(AgNO3,98wt%),methyl orange (CHN3SO3Na,98wt%),Orange II (C16H11N2NaO4S,98wt%) were purchased from Shanghai Sinopharm Chemical Reagent Co.,Ltd.and have not been further purified.
2.2 Synthesis of spindle α-Fe2O3 with hydrothermal method
spindleα-Fe2O3was prepared by the hydrothermal method shown as follows[21]: 2.4 g NaOH and 8.1 g of FeCl3•6H2O were dissolved in 50 mL deionized water and heated to 60 ℃.NaOH solution was added to FeCl3•6H2O solution and stirred quickly at the same time,and Fe(OH)3sol was obtained after 1 h.Further,3.2 g of SDBS was added to the gel,and the mixture was stirred for 1 h to get a yellow Fe(OH)3-DBS mixed precipitate.The precipitate was dried at 60 ℃ for 6 h,and 12.5 g of a dry matter was dissolved in 100 mL of xylene,and the magnetic force was stirred for 30 min.The resulting mixture was transferred to a high pressure reaction kettle,heated to 200 ℃ for 6 hours and then centrifuged and washed,dried for 6 h at 60 ℃to prepare α-Fe2O3.
2.3 Preparation of α-Fe2O3/ZnO composite
The 375 mL of acetate ethanol solution having a concentration of 0.01 mol/L was prepared,and 375 mL was taken in a three neck flask at 60 ℃.150 mL ethanol solution with sodium hydroxide concentration of 0.03 mol/L was added and stirred for 2 h to form ZnO nanosol.1.22 g of Fe2O3powder was dispersed in 50 mL of ethanol for ultrasonic dispersion for 30 min.Fe2O3ethanol solution was transferred to ZnO sol,stirring at 60 ℃ for 40 min to separate precipitation.The precipitation was washed with anhydrous ethanol and distilled water and dried at 60 ℃ for 6 h to prepareα-Fe2O3/ZnO composites.
2.4 Preparation of α-Fe2O3/ZnO/Ag composite
1.0 g of Fe2O3/ZnO was dispersed in 150 mL distilled water and dispersed by ultrasonic wave for 30 min.At the same time,0.016,0.049,0.083,and 0.118 g of silver nitrate were respectively weighed in a balance into a beaker and 150 mL of distilled water was added to prepare silver nitrate solutions with different contents.Then the dispersed Fe2O3/ZnO was placed under UV light for 1 h,and then silver nitrate was added to the above solution to keep the light for 2 h.After the reaction,the precipitates were separated and washed with water and ethanol,respectively,and then dried at 60 ℃ for 6 h.Fe2O3/ZnO/Ag composites with Ag content of 1%,3%,5%,and 7% were prepared.
2.5 Photocatalysis experiment
In a typical process,0.15 g freeze-dried nanoparticle sample was put into 150 mL OII or MO aqueous solution with the concentration of 10 ppm.Keep them in the dark for 30 min to get well dispersed and reach an adsorption/desorption equilibrium.Then turn on the UV lamp,take sample at regular intervals,and centrifuge to measure the absorbance of the supernatant.The OII and MO degradation (D)was calculated according to the following equation:D(%)=C/C0×100=A/A0,where,C0is the initial OII and MO concentration,Cis the instantaneous OII and MO concentration,A0shows initial absorbance,andAcorresponds to variable absorbance.
2.6 Characterization
Morphology maps were examined by field emission scanning electron microscopy (SEM,Nova 400,FEI,Netherlands,where the accelerating voltage used was 80 kV).FTIR spectra of the samples were made with a KBr pellet,scannings from 4 000 to 400 cm-1were measured on a FT-IR spectrometer(Vertex70,Bruker,Germany).XRD analysis was performed using Bruker AXS D8 Advanced instrument equipped with CuKa radiation(λ=1.5418 Å).Besides,The UV-Vis absorption spectroscopy measurements were conducted using a UV-Vis spectrophotometer(MAPADA UV-1800) at room temperature.
3 Results and discussion
3.1 Morphology and component
Figs.1(a)-1(f) are the SEM images of α-Fe2O3and Fe2O3/ZnO/Ag composites with different Ag contents.As can be seen from Fig.1(a),α-Fe2O3is uniformly spindle-shaped.The longitudinal dimension of a single particle is about 380 nm,and the lateral dimension is about 70 nm.In Fig(b),all spindle-shapedα-Fe2O3surfaces become rough compared with pureα-Fe2O3particles,and there are tiny particles on the surface,which can be preliminarily judged as ZnO generated during the preparation process.In Figs.1(c)-1(f),as the Ag content gradually increases,the overall regularity of the composite changes,and a certain agglomeration occurs at the same time,but the individual particles are still nano-sized.The morphology of several composites does not change much on the whole,which may be due to the less Ag content in the composites.The asprepared composite catalysts are nano-sized and thus may have strong photocatalytic activity.Fig.1(g) is the EDS diagram of the sample Fe2O3/ZnO/Ag(5%),it can be seen that it contains Fe,O,Zn,Ag and other elements,and it can be preliminarily judged thatα-Fe2O3is successfully combined with ZnO and Ag.At the same time,there are basically no peaks of impurity elements,indicating that the prepared composite material is relatively pure.And the Ag content in the composite is 5.03%,which is basically consistent with the actual Ag addition.
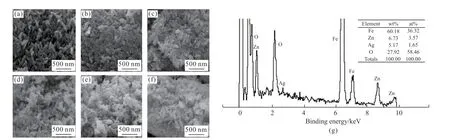
Fig.1 SEM and EDS images of Fe2O3 (a) and Fe2O3/ZnO/Ag composite materials(the content of Ag is 0(b),1%(c),3%(d),5%(e),7%(f)respectively)
3.2 IR analysis
Fig.2 shows the FTIR spectra ofα-Fe2O3,Fe2O3/ZnO,and Fe2O3/ZnO/Ag composites in the wavenumber range from 4 000 to 500 cm-1in transmission mode.The peaks located at 469 and 544 cm-1represent the Fe-O stretching vibration ofα-Fe2O3.The absorption at 667 cm-1was caused by Zn-O stretching of zinc oxide.

Fig.2 FTIR spectra of synthesized α-Fe2O3,Fe2O3/ZnO,and Fe2O3/ZnO/Ag(5%) composites
As can be seen from Fig.2,the absorption peaks of the three samples all appear near 1 050 cm-1,which correspond to the characteristic absorption peaks of sulfonic group,which is caused by the addition of sulfonic group in sodium dodecylbenzene sulfonate in the preparation ofα-Fe2O3.In addition,stretching vibration absorption peak (near 3 420 cm-1) and bending vibration absorption peak (near 1 640 cm-1)of adsorbed water can be obviously observed in the spectra of the three samples,which is due to the small particle size and large specific surface area of the composite material,which is easy to adsorb water.At the same time,due to the introduction of ZnO and Ag into the composite material,the surface hydroxyl peak of the composite material is widened,which will increase the specific surface area of the catalyst,strengthen the activity,and also contribute to improve the photocatalytic performance.
3.3 X-ray diffraction study
Fig.3 shows the XRD patterns ofα-Fe2O3,Fe2O3/ZnO,and Fe2O3/ZnO/Ag(5%).As can be seen from the figure,eight diffraction peaks with 2θangles of 24.13°,33.14°,35.61°,40.82°,49.47°,54.08°,62.44°,and 63.98° are observed in the three samples in Fig.3.They correspond to seven crystal planes of hematite phaseα-Fe2O3respectively,indicating that the three materials all containα-Fe2O3.Seven diffraction peaks with 2θangles of 31.76°,34.42°,36.25°,47.53°,56.60°,67.69°,and 69.09° also appear in Figs.3(b) and 3(c),which are consistent with the crystal plane structure of hexagonal wurtzite,indicating the presence of ZnO in these two materials.

Fig.3 XRDpatterns of α-Fe2O3,Fe2O3/ZnO,and Fe2O3/ZnO/Ag(5%)composites
In Fig.3(c),the four diffraction peaks with 2θangles of 38.1°,44.4°,64.5°,and 77.6° correspond to the diffraction peaks of (111),(200),(220),(102),and (311) crystal planes of silver (JCPDS 04-0783)respectively,indicating that Ag has been successfully loaded in the composite material.The diffraction peaks of Fe2O3,ZnO and Ag in Fe2O3/ZnO/Ag composite do not change significantly,indicating that the material composition of the composite is relatively stable,and there is no crystal transformation during the preparation process.
3.4 UV-Vis DRS analysis
The UV-Vis diffuse reflectance characterization of the sample is shown in Fig.4(a).Fe2O3,ZnO,and Fe2O3/ZnO/Ag(5%) have absorption ability in the visible light range of 200-800 nm,and the absorption sideband of ZnO is concentrated at 380 nm,the absorption sideband of Fe2O3is concentrated at 500 nm,while the absorption sideband of Fe2O3/ZnO/Ag composite catalyst is concentrated around 580 nm.This indicates that the composite has a wider visible light absorption region than pristine ZnO and Fe2O3.In Fig.4(b),according to the Kubelka-Munk formula,the tangent to the optical system curve of photon and (αhν)2is calculated,and the forbidden band widths of ZnO,Fe2O3and Fe2O3/ZnO/Ag are calculated by the size of the cross-sectional distance to be 3.15,2.02,and 1.58 eV,respectively.The results show that the Fe2O3/ZnO/Ag heterojunction has a smaller forbidden band width,which improves the photocatalytic efficiency.

Fig.4 UV-Vis absorption spectrogram and energy gap of composites
3.5 Photo-degradation of AZO dyes
Fig.5 shows the experimental results of degradation of OII and MO by different composite materials under UV light.All the photocatalytic experiments kept the same conditions before and after,the lamp was not turned on for the first 30 min,and the material was stirred in the dark place to fully adsorb the dye in the aqueous solution.After reaching the adsorption equilibrium,the light source was turned on,and the reaction solution was fully irradiated by UV light,and the photocatalytic degradation of organic dyes was maintained for 2 h.In this experiment,we chose OII and MO,two representative azo dyes.The initial concentration of the two dyes was 5 ppm and the dosage of photocatalyst was 1 g/L.As can be seen from Fig.5,with the progress of the reaction,the concentration of dye decreases gradually.Fe2O3/ZnO/Ag composite material has the best degradation effect on OII and MO.As time goes on,the degradation rate of all photocatalysts gradually slows down.With the increase of Ag content in the composite,the photocatalytic effect first increases and then decreases.When the content of Ag increases from 1% to 5%,the photocatalytic rate increases gradually,while when the content of Ag increases to 7%,the photocatalytic effect of the composite decreases.The relationship between the photocatalytic performance of Fe2O3/ZnO/Ag composites and the content of Ag is not simple and monotonically increasing,but the content of Ag has an optimal value.After 2 h,the degradation rates of OII and MO by Fe2O3/ZnO/Ag(5%) composites are 91.9%and 75.9%,respectively.

Fig.5 Degradation of OII and MO by different composite materials
Fig.6 shows the reaction kinetics curves of different photocatalysts for catalytic degradation of OII and MO under UV light.Under UV light,the degradation of OII and MO by various catalysts conforms to firstorder reaction kinetics.The photocatalytic properties of the materials are shown as follows: Fe2O3/ZnO/Ag(5%)>Fe2O3/ZnO/Ag(7%) >Fe2O3/ZnO/Ag(3%)>Fe2O3/ZnO/Ag(1%)>Fe2O3/ZnO >Fe2O3>Blank.According to the Langmuir-Hinshelwood model,the degradation rate constants of OII and MO by Fe2O3/ZnO/Ag(5%)composites can be calculated to be 0.0197 and 0.0108 min-1,respectively.

Fig.6 The reaction kinetics of degradation of OII and MO by different composite materials
The photocatalytic mechanism of Fe2O3/ZnO/Ag under UV light is explained as follows: Under UV light,the electrons in the valence band of Fe2O3and ZnO semiconductor will undergo energy level transition to the conduction band,forming free electrons,and the valence band will generate holes accordingly,thus forming electron hole pairs.Since Fe2O3and ZnO have higher Fermi energy levels than Ag,the electrons in Fe2O3and ZnO will be redistributed after they combine,and the electrons will move from Fe2O3and ZnO to the metal surface until their Fermi energy levels are stable.ZnO is a wide-band gap semiconductor and Fe2O3is a narrowband gap semiconductor.According to the energy level structure of Fe2O3and ZnO,some electrons in the conduction band of ZnO and Fe2O3will transfer to the surface of Ag.Moreover,the electrons in ZnO conduction band will also transfer to Fe2O3conduction band,which will promote the electron hole pairs generated by ZnO and Fe2O3excited by UV light,reduce the recombination rate of carriers,and improve the quantum efficiency.Photogenerated electrons on the Fe2O3conduction band and silver surface will react with oxygen in the solution to form superoxide anions.Meanwhile,holes in the Fe2O3and ZnO valence bands can oxidize water and hydroxide ions into hydroxyl radicals.Superoxide anion and hydroxyl radical have strong oxidizing ability,which can degrade organic matter in water and achieve the purpose of treating wastewater.
3.6 Photocatalysis repeated experiments
To evaluate the reusability of Fe2O3/ZnO/Ag(5%)composite by five-times recycling measurements for the degradation of OII and MO are shown in Fig.7.The composite was obtained by centrifugation and washed with anhydrous ethanol and deionized water for the next test after each cycle of photocatalytic experiment.The discoloration rate of the same Fe2O3/ZnO Ag(5%) composite was only slightly reduced in the photocatalytic reaction after five recoveries,indicating that the Fe2O3/ZnO/Ag(5%) heterostructure has a good repeatability in the photodegradation of OII and MO aqueous solution.

Fig.7 The degradation of OII and MO by Fe2O3/ZnO/Ag(5%)repeated several times under UV light
4 Conclusions
In this paper,Fe2O3/ZnO composites were synthesized,and Fe2O3/ZnO/Ag composites loaded with different contents of Ag were prepared,and characterized by SEM,XRD and FT-IR spectroscopy.By comparing the experimental results of degradation of OII and MO by different photocatalysts under UV light,the following conclusions are drawn: through the characterization of materials,a series of Fe2O3/ZnO/Ag composites have been successfully prepared in the experiment;the photocatalytic properties of a series of Fe2O3/ZnO/Ag composites were tested and analyzed,Fe2O3/ZnO/Ag(5%) has the highest photocatalytic capacity,the introduction of Ag will improve the photocatalytic efficiency of the catalyst;ZnO andα-Fe2O3are photocatalytic materials with good catalytic performance,the introduction of Ag will help to separate carriers and improve quantum efficiency.The composite of these three materials will form heterojunction,which is beneficial to the improvement of photocatalytic performance of the materials.In addition,the composite material can be excited by both UV and visible light,which has the potential to utilize sunlight.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Biotin-modified Galactosylated Chitosan-gene Carrier in Hepatoma Cells Targeting Delivery
- Mussel-inspired Methacrylic Gelatin-dopamine/Ag Nanoparticles/Graphene Oxide Hydrogels with Improved Adhesive and Antibacterial Properties for Applications as Wound Dressings
- Effect of Polyvinyl Alcohol in Inner Aqueous Phase on Stability of Millimeter-scale Capsules
- Synthesis and Characterization of Nonionic Waterborne Polyurethane and Application to Wool Fabric Finishing
- Fluorescent Double Network Hydrogels with Ionic Responsiveness and High Mechanical Properties for Visual Detection
- Damage Mechanism of Ultra-thin Asphalt Overlay (UTAO)based on Discrete Element Method
