lncRNA-MSTRG.7889.1竞争性结合bta-miR-146a靶向Smad4调控牦牛颗粒细胞的凋亡
2024-03-08孟朝轶王运路姚一龙席广银牛家强索朗斯珠郭敏徐业芬
孟朝轶,王运路,姚一龙,席广银,牛家强,索朗斯珠,郭敏,徐业芬
lncRNA-MSTRG.7889.1竞争性结合bta-miR-146a靶向4调控牦牛颗粒细胞的凋亡

1西藏农牧学院动物科学学院,西藏林芝 860000,2中国农业科学院深圳农业基因组研究所,广东深圳 518000,3中国农业大学动物科学技术学院,北京 100193
【背景】卵泡颗粒细胞(Granulosa cells,GCs)凋亡是导致卵泡闭锁的重要原因,竞争性内源RNA(competing endogenous RNAs,ceRNA)机制已被证明参与调控GCs的凋亡过程。前期牦牛卵巢转录组测序发现lncRNA-MSTRG.7889.1与bta-miR-146a具有结合位点,而bta-miR-146a和4存在靶向关系。【目的通过探究影响牦牛卵泡GCs凋亡的ceRNA分子调控机制,为揭示牦牛生殖调控的奥秘奠定基础。【方法】采集和分离成年母牦牛的健康和闭锁卵泡,利用RT-qPCR法检测lncRNA-MSTRG.7889.1、bta-miR-146a和4在卵泡中的表达。制作牦牛健康和闭锁卵泡组织切片,TUNEL检测健康卵泡和闭锁卵泡中GCs的凋亡情况;荧光原位杂交分析lncRNA-MSTRG.7889.1、bta-miR-146a和4在卵泡中的定位。体外分离和培养牦牛GCs,分别转染4过表达载体和bta-miR-146a mimics,流式细胞术检测GCs细胞凋亡率,Western blot检测促凋亡蛋白CASPASE3和BAX,抑凋亡蛋白BCL-2的表达;GCs共转染4过表达载体和bta-miR-146a mimics后,探究bta-miR-146a是否靶向4影响牦牛GCs的凋亡。将lncRNA-MSTRG.7889.1过表达慢病毒载体感染GCs,流式细胞术和Western blot检测lncRNA-MSTRG.7889.1对GCs凋亡的影响;lncRNA-MSTRG.7889.1载体和bta-miR-146a mimics共转染GCs,进一步分析lncRNA-MSTRG.7889.1是否竞争性结合bta-miR-146a靶向4影响牦牛GCs的凋亡。【结果】lncRNA-MSTRG.7889.1和4 mRNA在牦牛健康卵泡中的表达极显著高于闭锁卵泡(<0.01),而bta-miR-146a的表达则相反(<0.01)。TUNEL检测结果显示,在闭锁卵泡中GCs的荧光强度极显著高于健康卵泡(<0.01),说明闭锁卵泡中GCs凋亡显著高于健康卵泡。荧光原位杂交结果显示4、bta-miR-146a和lncRNA-MSTRG.7889.1在牦牛卵泡中共表达,其在健康和闭锁卵泡中的表达与RT-qPCR结果基本一致,提示可能存在ceRNA机制参与牦牛健康卵泡发育和卵泡闭锁过程。在牦牛GCs过表达4使细胞凋亡率显著降低(<0.01),CASPASE3和BAX蛋白的表达显著降低(<0.01),BCL-2蛋白的表达显著升高(<0.01);过表达bta-miR-146a使GCs凋亡率显著升高(<0.01),CASPASE3和BAX蛋白的表达显著升高(<0.01),BCL-2蛋白的表达显著降低(<0.01);bta-miR-146a靶向抑制4的表达(<0.01);4和bta-miR-146a共转染GCs,结果显示bta-miR-146a靶向抑制4降低前者对GCs凋亡的促进作用(<0.01)。过表达lncRNA-MSTRG.7889.1使牦牛GCs凋亡率显著降低(<0.01),CASPASE3和BAX蛋白的表达显著降低(<0.01),BCL-2蛋白的表达显著升高(<0.01);lncRNA-MSTRG.7889.1显著抑制bta-miR-146a的表达(<0.01)。将lncRNA-MSTRG.7889.1和bta-miR-146a共转染GCs,结果表明lncRNA-MSTRG.7889.1靶向bta-miR-146a降低后者对GCs凋亡的促进作用(<0.01);而且RT-qPCR法和Western blot检测结果表明lncRNA-MSTRG7889.1竞争性结合bta-miR-146a促进4 mRNA和蛋白水平的表达(<0.01)。【结论】lncRNA-MSTRG.7889.1竞争性结合bta-miR-146a促进4的表达抑制牦牛GCs的凋亡。
lncRNA;bta-miR-146a;凋亡;颗粒细胞;牦牛
0 引言
【研究意义】牦牛是在高寒地区经过自然选择的特有牛种,在青藏高原地区牦牛养殖业对牧民的生活和经济发展具有重要意义。然而,牦牛性成熟晚、发情期短、实际繁殖能力低,一般仅为两年一胎或三年两胎,高产者一生也仅能产犊4—5头[1]。卵巢中仅有少量卵泡发育成熟,大多数卵泡未能排卵而发生闭锁现象[2]。这种低繁殖力制约着种群扩繁也远远满足不了牧民的需求。因此,阐明牦牛卵泡闭锁机制对于揭示生殖调控的奥秘、提高卵巢利用率和牦牛繁殖效率,均具有重要的理论意义和实用价值。【前人研究进展】目前已经发现,在卵泡发育的不同时期细胞凋亡均有发生,它们不同程度的影响卵泡的命运(闭锁或是排卵)[3-4]。凋亡是指细胞受内在遗传机制调控而自动结束生命的过程[5-6],卵泡颗粒细胞(granulosa cells,GCs)的凋亡是导致卵泡闭锁的重要原因[7]。转录调控研究一直是生命科学领域研究的重中之重,微小RNA(microRNA, miRNA)是一类内源性的非编码小RNA分子,大小一般为18—24个核苷酸,主要是通过转录后调控的方式实现对基因表达的调控,在细胞分裂、分化、迁移以及凋亡等生理病理过程中扮演重要角色[8-9]。目前大量研究已经发现miRNA通过调节靶基因mRNA参与调控卵泡GCs的凋亡[10-11],对于影响miRNA发挥作用的因素也备受关注。长链非编码RNA(long non-coding RNA,lncRNA)具有miRNA“海绵”作用,其作为内源性竞争RNA(competing endogenous RNAs,ceRNA)降低miRNA活性而间接影响靶基因mRNA的调控网络引起了生命科学领域的广泛兴趣,更是成为非编码RNA转录调控的焦点[12-15]。目前,在牦牛的肌肉[16]、乳腺[17]和卵巢[18]等组织已发现多种lncRNAs,然而它们在竞争性内源性RNA的共表达网络还有待全面分析。在哺乳动物的繁殖方面,通过RNA测序发现牛的卵母细胞中大量候选lncRNAs参与阶段特异性卵母细胞的成熟过程[19];在绵羊的卵泡期或黄体期的卵巢组织发现多个差异表达的lncRNAs,预测其与绵羊繁殖力高低之间具有关联性[20];在猪的早期闭锁卵泡中发现77个lncRNA上调,其中lncRNA-NORHA可能参与卵泡闭锁过程[21]。但是,对牦牛卵泡GCs凋亡及卵泡闭锁的ceRNA调控机制尚不清楚。【本研究切入点】本课题组前期根据牦牛健康和闭锁卵泡的转录组测序发现,lncRNA-MSTRG.7889.1(简写为lnc-MSTRG.7889)和bta-miR-146a存在紧密结合位点[22],而在牦牛上已有研究证明bta-miR-146a和4存在靶向关系[23],在此基础上提出以下科学假设:4、bta-miR-146a和lnc-MSTRG.7889形成ceRNA作用机制,可以通过调控牦牛卵泡GCs的凋亡影响卵泡闭锁过程。【拟解决的关键问题】阐明lnc-MSTRG.7889竞争性结合bta-miR-146a靶向4调控牦牛卵泡GCs凋亡过程以影响卵泡闭锁的ceRNA机制。
1 材料与方法
1.1 材料与仪器
Trizol Reagent™试剂(15596018CN,Invitrogen); FastKing cDNA第一链合成试剂盒(KR116-01,天根);miRNA合成第一链cDNA(加尾法)试剂盒(MR201-01,诺唯赞);SYBR Premix Ex Taq试剂;Lipofectamine 2000 Reagent(11668-019,Thermo Fisher);Ⅰ和Ⅰ限制性内切酶(Thermo Fisher);无内毒素质粒提取试剂盒(D6915,Omega);连接酶(EL0011,Thermo Fisher);LV-EF1a-EGFP-2A载体(武汉枢密科技),PCR产物回收试剂盒(D6492,Omega);DMEM培养液(#D0819,SIGMA),OPTI-MEM培养液(50985091,Thermo Fisher)PBS 缓冲液(#C14190500BT,Gibco)、PS(#15140-122,Gibco)、L-谷氨酰胺(#A2916801,Gibco)、胎牛血清(#16000085,Gibco)、胰蛋白酶(#25200-056,Gibco),Annexin V-FITC/PI试剂盒(#E606336)、TUNEL 细胞凋亡检测试剂盒(#E607172,上海生工),BCA蛋白定量试剂盒(Sigma-Aldrich);CASPASE-3抗体(#14220,CST公司)、BAX抗体(#14796,CST公司)、BCL-2抗体(#3498,CST)、LC3B抗体(#3868,CST)、BECLIN-1抗体(#3495,CST)、ATG-5抗体(#12994,CST),β-actin抗体(#CW0096,北京康为世纪公司),RIPA裂解液、BSA、ECL显影液、NC膜、多聚甲醛等均购自美国SIGMA公司。
1.2 试验方法
本试验于 2020年9月至2022年12月在西藏农牧学院兽医生理与药理实验室、西藏农牧学院高原动物疫病检测中心细胞实验室和中国农业大学动物遗传育种与繁殖重点实验室完成。
1.2.1 牦牛卵巢的采集和处理 在西藏林芝临河牲畜屠宰场,选择体重约为300 kg、4—6岁的健康雌性牦牛,屠宰后立即采集卵巢,置于37 ℃ PBS的保温杯内2 h带回实验室。在带有温台的体式显微镜下,使用无菌的眼科剪镊剥离卵泡,依据参考文献[24]的具体标准区分健康和闭锁卵泡,用于后续试验。
1.2.2 RT-qPCR 利用Trizol ReagentTM试剂法提取卵泡总RNA,使用超微分光光度计测定浓度和纯度;按照FastKing cDNA第一链合成试剂盒和miRNA合成第一链cDNA(加尾法)试剂盒说明书进行反转录,将产物-80℃保存备用。使用SYBR Premix Ex Taq试剂分别检测牦牛健康和闭锁卵泡中lnc-MSTRG.7889.1、bta-miR-146a表达和4 mRNA表达水平,引物序列见表1。每组cDNA样品重复3次,通过熔解曲线分析并确定RT-qPCR产物的特异性。lnc-MSTRG.7889.1和4以-为内参、bta-miR-146a以U6为内参,2-△△Ct法计算相对表达量。
1.2.3 TUNEL法 牦牛卵巢健康和闭锁卵泡制作石蜡固定切片,65℃烘箱中烘片50 min,石蜡切片分别在梯度乙醇中脱蜡。组织切片使用0.01 mol·L-1枸橼酸钠缓冲液中加热进行抗原修复。使用PBS洗涤切片,3%BSA覆盖切片,室温封闭30 min。在切片上滴加稀释的一抗,4℃孵育过夜。玻片经PBS脱色后,滴加相应的二抗,避光孵育50 min。洗涤后的玻片滴加DAPI,孵育10 min;再使用抗淬灭剂封片。在荧光倒置显微镜下观察,所有GCs细胞核均呈蓝色荧光,凋亡细胞核呈绿色荧光,两者重叠后凋亡细胞核呈蓝绿色荧光,使用Image Pro Plus软件对凋亡细胞的荧光强度进行统计分析。
1.2.4 荧光原位杂交 将采集的牦牛健康和闭锁卵泡组织分别制作石蜡切片。65℃烤片,蛋白酶K消化10 min,切片脱蜡后的依次在100%乙醇、85%乙醇、75%乙醇中复水,再放入DEPC水中3 min。切片在50 ℃的30%酸性亚硫酸钠中处理20 min,漂洗后蛋白酶K孵育15 min,再于0.1 mol·L-1的HCl中浸泡5 min。将切片脱水后,浸入丙酮溶液中2 min,自然干燥。由吉玛基因生物公司合成荧光原位杂交探针(序列见表1),探针在75 ℃下温育5 min,立即置于冰上变性5—10 min。将探针混合液滴于标本上,覆盖玻片后封片,置于潮湿暗盒中37 ℃杂交过夜。切片洗涤后自然干燥,DAPI复染。在荧光显微镜下进行分析,使用Image Pro Plus图像分析系统采集同一视野下的图片,统计分析不同探针标记基因的荧光强度。
1.2.5 lnc-MSTRG.7889慢病毒载体和4过表达载体的构建 将lncRNA-MSTRG.7889.1目的片段连接至LV-EF1a-EGFP-2A载体中,重组质粒与慢病毒包装系统(pVSVG:pMDL:pRev)感染293T细胞,48—72 h后提取细胞上清液,经纯化浓缩即为lncRNA慢病毒载体。根据牦牛4(GeneBank ID:102265995)扩增目的片段,将产物与载体pEX-3酶切验证,回收产物经T4-DNA连接酶连接过夜,产物转化DH5α感受态细胞,经抗性筛选的单克隆菌液PCR和酶切验证后,送生物公司测序。
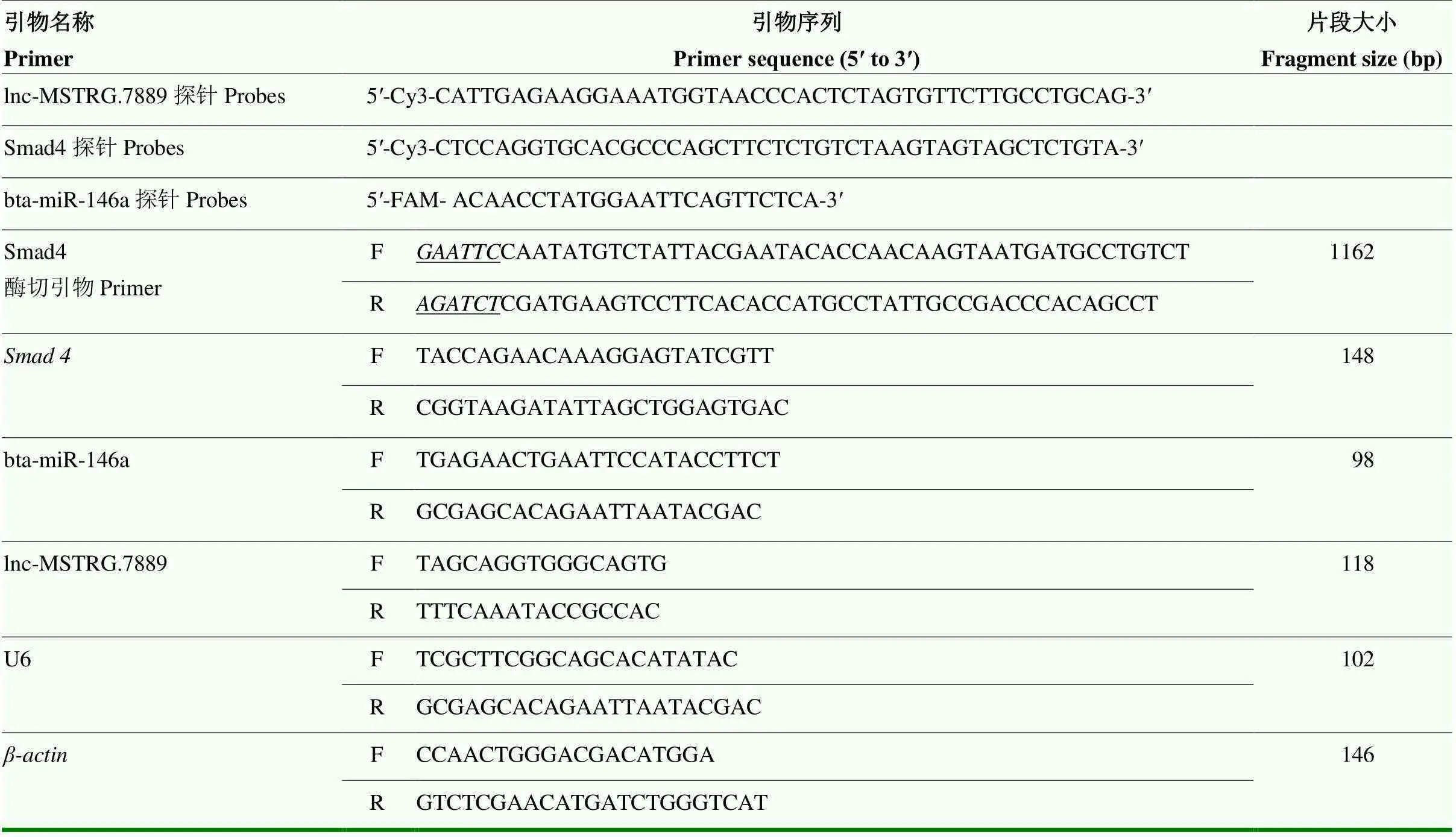
表1 引物和探针序列表
1.2.6 牦牛卵泡GCs的分离培养和转染 牦牛GCs的分离培养参考王玉恒等[25]的方法进行。按照2.6×106个将GCs接种于60 mm培养皿中,在37 ℃、5%CO2、饱和湿度的培养箱中培养,在细胞贴壁后密度达到60%后,按照Lipofectamine 2000转染试剂说明书进行转染,转染6 h后更换为新鲜细胞培养液,继续培养48 h时收集细胞用于流式细胞术和Western blot检测。
1.2.7 流式细胞术检测牦牛卵泡GCs的凋亡率 分离培养的牦牛GCs调整细胞密度为5×105个/mL,1 000 r/min离心5 min弃上清。按照Annexin V-FITC/PI凋亡检测试剂盒处理细胞,1 h内使用流式细胞仪检测细胞凋亡率。
1.2.8 Western blot 收集牦牛卵泡GCs,加入1× SDS-Loading Buffer,100 ℃金属浴10 min,瞬离取上清,使用BCA蛋白定量试剂盒检测蛋白浓度。经SDS-PAGE电泳分离、转膜和封闭。分别加入特异性抗体孵育过夜,再加入二抗孵育1 h。将ECL试剂滴于膜上避光反应,全自动化学发光成像系统曝光显影。
1.2.9 统计分析 采用SPSS软件进行数据分析,采用单因素方差分析和Tukey检验分析多个独立样本之间的差异。数据结果采用GraphPad prism 5.0软件绘图。<0.05有统计学意义。
2 结果
2.1 lnc-MSTRG.7889、bta-miR-146a和Smad4在牦牛卵巢健康和闭锁卵泡中的共表达
本课题组前期发现lncRNA-MSTRG.7889.1和bta-miR-146a存在结合位点[22]。已有研究表明lncRNA- ENSBGRT00000000387.1与bta-miR-146a存在靶向的“海绵吸附”关系[26]。通过BLAST序列比对发现,lncRNA- MSTRG.7889.1与lncRNA-ENSBGRT00000000387.1的相似性高达99.99%,且该lncRNA与bta-miR-146a存在紧密结合位点一致,提示lncRNA-MSTRG.7889.1与lncRNA-ENSBGRT00000000387.1为同一lncRNA。已有研究发现,bta-miR-146a和4存在靶向关系[23]。本研究RT-qPCR法进一步分析lncRNA-MSTRG. 7889.1(简写为lnc-MSTRG.7889)、bta-miR-146a和4在牦牛健康和闭锁卵泡中的表达情况,结果如图1-A所示4和lnc-MSTRG.7889在健康卵泡中的表达量极显著高于闭锁卵泡(<0.01);bta-miR-146a在健康卵泡中的表达量极显著低于闭锁卵泡(<0.01)。
TUNEL检测牦牛卵巢的健康和闭锁卵泡中GCs的凋亡情况,结果显示在闭锁卵泡中GCs凋亡荧光强度极显著高于健康卵泡(<0.01,图1-B),说明闭锁卵泡中GCs凋亡显著高于健康卵泡。荧光原位杂交结果显示,在牦牛卵泡中4和bta-miR-146a共表达,而且4在健康卵泡中的表达量极显著高于闭锁卵泡,bta-miR-146a在闭锁卵泡中的表达量极显著高于4(<0.01,图1-C);在牦牛卵泡中bta-miR- 146a和lnc-MSTRG.7889共表达,且lnc-MSTRG.7889在健康卵泡中的表达量极显著高于闭锁卵泡(<0.01,图1-D),而bta-miR-146a的表达则相反(<0.01,图1-D)。上述结果表明,在牦牛卵泡中可能存在lncRNA-MSTRG.7889/miR-146a/4机制调控GCs凋亡。
2.2 bta-miR-146a靶向Smad4影响牦牛GCs的凋亡
Annexin V-FITC/PI染色分析GCs凋亡率,结果如图2-A所示GCs在转染4 过表达载体后细胞凋亡率显著降低(<0.01),而si-4使细胞凋亡率显著升高(<0.01)。Western blot检测GCs凋亡相关蛋白的表达,4过表达组的促凋亡相关蛋白CASPASE3和BAX的表达显著降低,而抑凋亡蛋白BCL-2的表达显著升高(<0.01,图2-B);si-4组促凋亡相关蛋白CASPASE3和BAX的表达显著升高,而BCL-2的表达显著降低(<0.01,图2-B)。上述结果表明,4抑制牦牛GCs的凋亡。
将合成bta-miR-146a mimics和bta-miR-146a inhibitor分别转染GCs,流式细胞术检测结果表明过表达bta-miR-146a使GCs凋亡率显著升高(<0.01,图2-C),抑制bta-miR-146a inhibitor使GCs凋亡率显著降低(<0.05,图2-C)。bta-miR-146a mimics组GCs凋亡相关蛋白CASPASE3和BAX的表达显著升高,而BCL-2的表达显著降低(<0.01,图2-D)。相反,bta-miR-146a inhibitor组凋亡相关蛋白CASPASE3和BAX的表达降低,而促进凋亡相关蛋白BCL-2的表达升高(<0.01,图2-D)。上述结果显示,bta-miR-146a促进GCs的凋亡。
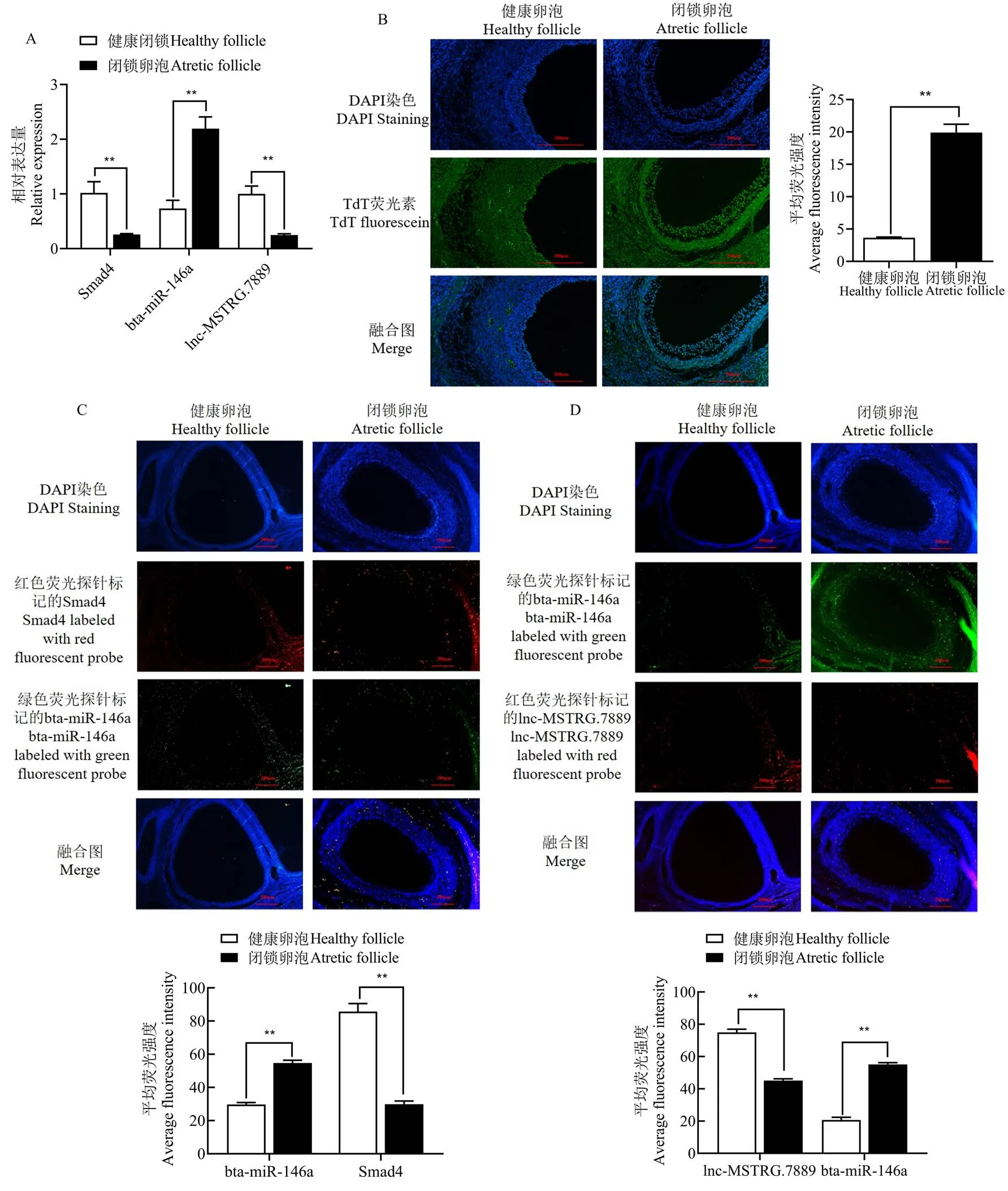
A:RT-qPCR结果;B:TUNEL法检测结果;C:4和 bta-miR-146a在卵泡中的表达;D:lnc-MSTRG.7889和 bta-miR-146a在卵泡中的表达;**表示差异极显著(<0.01)。下同
A: RT-qPCR results; B: TUNEL method detection results; C: Expression of4 and bta-miR-146a in follicles; D: Expression of lnc-MSTRG.7889 and bta-miR-146a in follicles; ** indicate significant difference (<0.01). The same as below
图14、 bta-miR-146a和lnc-MSTRG.7889在牦牛卵泡中的表达
Fig. 1 Expression of4, bta-miR-146a, and lnc-MSTRG.7889 in yak follicles
在牦牛上已有研究证明bta-miR-146a和4存在靶向关系[23],因此本试验将bta-miR-146a mimics转染GCs,qPCR检测结果显示bta-miR-146a mimics组与mimics NC组相比4的表达显著降低(<0.05,图2-E(a));bta-miR-146a inhibitors组与inhibitors NC组相比4的表达显著升高(<0.01,图2-E(a));Western blot检测结果显示bta-miR-146a mimics组与mimics NC组相比SMAD4蛋白的表达显著降低(<0.01,图2-E(b));bta-miR-146a inhibitors组与inhibitors NC组相比SMAD4蛋白的表达显著升高(<0.01,图2-E(b)),上述结果说明在牦牛GCs中bta-miR-146a靶向抑制4的表达。
流式细胞仪检测结果表明bta-miR-146a mimics和4过表达载体共转染组与bta-miR-146a mimics和pEX-3载体共转染组相比GCs凋亡率显著降低(<0.01,图2-F),而且凋亡相关蛋白的CASPASE3和BAX的表达显著降低,BCL-2的表达显著升高(<0.01,图2-G)。bta-miR-146a inhibitor和si-4载体共转染组与bta-miR-146a inhibitor和si-4 NC共转染组相比GCs凋亡率显著上升(<0.01,图2-F), 而且凋亡相关蛋白的CASPASE3和BAX蛋白的表达显著升高,BCL-2蛋白的表达显著降低(<0.01,图2-G)。上述结果表明,bta-miR-146a mimics靶向4降低前者对GCs凋亡的促进作用。
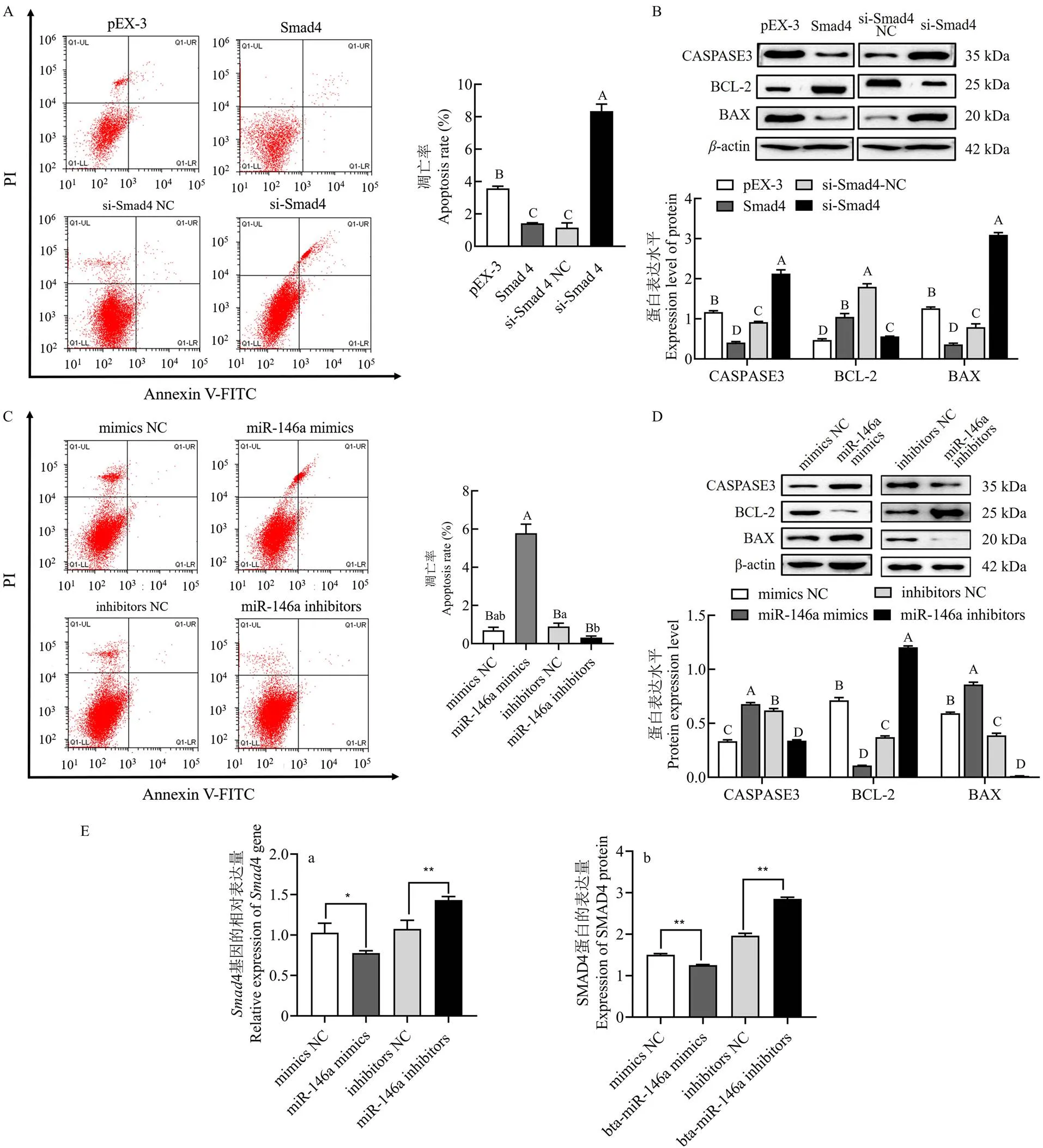

A:4影响牦牛GCs的凋亡率;B:4影响GCs凋亡相关蛋白的表达;C:bta-miR-146a影响牦牛GCs的凋亡率;D:bta-miR-146a影响GCs凋亡相关蛋白的表达;E:bta-miR-146a抑制4的表达;F:bta-miR-146a靶向4影响牦牛GCs的凋亡率;G:bta-miR-146a靶向4影响牦牛GCs的凋亡相关蛋白的表达;不同小写字母表示差异显著(<0.05),不同大写字母表示差异极显著(<0.01)。下同
A:4 affects the apoptosis rate of yak GCs; B:4 affects the expression of apoptosis related proteins in GCs; C: bta-miR-146a affects the apoptosis rate of yak GCs; D: bta-miR-146a affects the expression of apoptosis related proteins in GCs; E: bta-miR-146a inhibits the expression of Smad4; F: bta-miR-146a targeting Smad4 affects the apoptosis rate of yak GCs; G: bta-miR-146a targeting Smad4 affects the expression of apoptosis related proteins in yak GCs; Different lowercase letters represent significant differences (P<0.05), and different uppercase letters represent extremely significant differences (<0.01). The same as below
图2 bta-miR-146a靶向4影响牦牛GCs的凋亡
Fig. 2 bta-miR-146a targeting4 affects apoptosis of yak GCs
2.3 lnc-MSTRG.7889靶向bta-miR-146a影响牦牛GCs的凋亡
为了进一步探讨lnc-MSTRG.7889是否影响GCs凋亡,将lnc-MSTRG.7889慢病毒过表达载体和si-lnc-MSTRG.7889分别转染GCs,流式细胞术检测结果表明lncRNA过表达组与LV空载组相比GCs的凋亡率显著降低(<0.01,图3-A),凋亡相关基因CASPASE3和BAX蛋白的表达降低(<0.05),而BCL-2蛋白的表达升高(<0.05,图3-B)。相反,si-lncRNA组与si-lnc NC组相比GCs的凋亡率显著升高(<0.01,图3-A),而且凋亡相关基因CASPASE3和BAX蛋白的表达升高(<0.01),而BCL-2蛋白的表达降低(<0.01,图3-B)。上述结果表明,lnc-MSTRG.7889抑制GCs的凋亡。
已有研究证明lnc-MSTRG.7889和bta-miR-146a具有“海绵吸附”关系[26],本研究将lnc-MSTRG.7889过表达慢病毒载体和si-lnc-MSTRG.7889分别转染GCs,结果显示lncRNA组和LV组相比bta-miR-146a的相对表达量显著降低(<0.01,图3-C),si-lncRNA组和si-lnc NC组相比bta-miR-146a的相对表达量显著升高(<0.01,图3-C);上述结果表明lnc-MSTRG. 7889靶向抑制bta-miR-146a的表达。

A:lnc-MSTRG.7889影响牦牛卵泡GCs的凋亡率;B. lncRNA影响GCs凋亡相关蛋白的表达;C:GCs中lnc-MSTRG.7889影响bta-miR-146a的表达;D:lnc-MSTRG.7889和bta-miR-146a影响牦牛卵泡GCs的凋亡率;E:lnc-MSTRG.7889和bta-miR-146a影响牦牛卵泡GCs的凋亡相关蛋白的表达
A: lnc-MSTRG.7889 affects the apoptosis rate of yak follicular GCs; B. lncRNA affects the expression of apoptosis related proteins in GCs; C: lnc-MSTRG.7889 in GCs affects the expression of bta-miR-146a; D: lnc-MSTRG.7889 and bta-miR-146a affect the apoptosis rate of yak follicular GCs; E: lnc-MSTRG.7889 and bta-miR-146a affect the expression of apoptosis related proteins in yak follicular GCs
图3 lnc-MSTRG.7889抑制bta-miR-146a影响GCs的凋亡
Fig. 3 lnc-MSTRG.7889 inhibits bta-miR-146a and affects GCs apoptosis
为了进一步探讨lncR-MSTRG.7889是否通过靶向bta-bta-miR-146a影响GCs凋亡,GCs共转染lnc- MSTRG.7889过表达载体和bta-miR-146a mimics后,流式细胞术检测结果显示该组与LV和bta-miR-146a mimics共转染组相比GCs的凋亡率显著降低(<0.01,图3-D),凋亡相关蛋白表达结果表明CASPASE3和BAX的蛋白表达降低,BCL-2蛋白的表达升高(<0.01,图3-E)。上述表明, lncRNA-MSTRG.7889.1靶向bta-miR-146a降低后者对GCs凋亡的促进作用。
2.4 lnc-MSTRG.7889竞争性结合bta-miR-146a影响Smad4的表达
GCs共转染lncRNA和bta-miR-146a后,qPCR法检测4 mRNA水平的表达,结果如图4-A所示lncRNA+mimics NC组与LV+mimics NC组相比4 mRNA水平的相对表达量显著升高(<0.01),LV+bta-miR-146a mimics组与lncRNA+ bta-miR-146a mimics组相比4 mRNA水平的相对表达量降低(<0.01),而且共转染lnc-MSTRG.7889过表达载体和bta-miR-146a mimics组的GCs中4 mRNA水平的相对表达量降低(<0.01)。Western blot检测结果如图4-B所示,与LV+mimics NC组相比共转染lnc-MSTRG.7889过表达载体和mimics NC组SMAD4蛋白表达量显著升高,共转染LV载体和bta-miR-146a mimics组的GCs中SMAD4蛋白表达显著降低,而共转染lnc-MSTRG.7889过表达载体和bta-miR-146a mimics组的GCs中SMAD4蛋白表达量降低(<0.01)。上述结果说明,lnc-MSTRG.7889通过与bta-miR-146a竞争性结合调节4的表达。综上所述,牦牛GCs中lnc-MSTRG.7889.1是通过竞争性结合bta-miR-146a而促进4的表达。

A:lncRNA过表达载体和bta-miR-146a mimics共转染GCs后4的表达;B:Western blot检测lncRNA过表达载体和bta-miR-146a mimics共转染GCs后SMAD4蛋白的表达
A: The expression of4 in GCs co-transfected with lncRNA overexpression vector and bta-miR-146a mimics; B: Western blot was used to detect the expression of SMAD4 protein in GCs co-transfected with lncRNA overexpression vector and bta-miR-146a mimics
图4 lncRNA靶向bta-miR-146a影响4的表达
Fig. 4 lncRNA affects the expression of4 by regulating bta-miR-146a
3 讨论
3.1 ceRNA调控卵泡GCs凋亡
卵泡是雌性哺乳动物的卵巢的基本功能单位,GCs的增殖、凋亡和类固醇激素的合成等影响卵泡的生长和成熟。在动物机体内发生的程序性、有顺序的死亡称为细胞凋亡,能保持机体细胞总体数量的均衡、细胞增殖与凋亡之间的稳定,在动物体病理、生理等生命过程中发挥重要作用[6]。卵泡闭锁是卵巢内发生的正常生理现象,卵泡中GCs和卵母细胞发生凋亡,其中GCs在闭锁过程中起主导作用[7]。越来越多的证据表明,ceRNA在不同动物GCs的凋亡过程中发挥关键的调节功能,例如在猪的GCs发现lnc2300/miR- 365-3p/CYP11A1轴参与调控细胞凋亡和卵泡闭锁[27];lncRNAs介导的ceRNA网络在水牛卵泡GCs中具有调节作用[28];lncMALAT1通过miR-205/CREB1轴调节小鼠GCs的凋亡[29]等。本研究发现lnc-MSTRG.7889、bta-miR-146a和4在牦牛卵泡中共表达,而且三者在健康和闭锁卵泡中的表达趋势表明lnc-MSTRG. 7889、bta-miR-146a和4可能存在ceRNA分子机制调控参与卵泡的闭锁过程,且与卵泡GCs凋亡相关。
3.2 bta-miR-146a靶向Smad4影响GCs凋亡
4是细胞内TGF-β信号转导通路中重要的信号级联分子[30],作为卵巢卵泡发育的调节因子影响排卵、激素分泌和细胞凋亡等[31-33]。在不同的动物中已经发现了多种miRNAs,其通过与靶基因的mRNA结合,抑制其翻译或导致mRNA降解,从而实现转录后调控基因表达[34-36]。在miRNA和mRNA结合的机制中,在mRNA上存在miRNA应答元件(microRNA response elements,MREs)能够与miRNA进行结合[8]。已有研究表明牦牛bta-miR-146a靶向4[23],本试验利用体外分离培养牦牛GCs结合细胞转染技术研究发现,4抑制GCs凋亡,bta-miR-146a促进GCs凋亡,而且在GCs中bta-miR-146a靶向抑制4的表达降低前者对GCs凋亡的促进作用,说明牦牛bta-miR-146a靶向抑制4影响GCs凋亡。目前,大量研究已通过miRNA敲除模型和转基因过表达等方法揭示了miR-146通过调节靶基因在不同细胞凋亡中重要作用[37-39]。在卵泡方面的研究发现,miR-146a可能调控GCs的功能,尤其是通过影响GCs的凋亡调控卵泡发育和闭锁过程。He等[40]的研究表明,miRNA-146表达的上调通过抑制TLR4/NF-κB信号通路降低卵巢GCs的凋亡率。因此,在转录后水平研究bta-miR-146a的作用,进一步扩展了对于GCs功能中基因表达调控网络的理解,但其具体的分子调控机制有待深入探索。
3.3 lnc-MSTRG.7889作为ceRNA参与bta-miR-146a靶向Smad4影响GCs凋亡
越来越多证据表明,长非编码RNA(lncRNA)可以作为竞争性内源性RNA与miRNA的靶向位点结合,从而影响和调节mRNA和靶基因的表达。在lncRNA上也存在MREs,同一个miRNA也可与多个多种类型的RNA结合,因而结合相同miRNA的RNA分子之间形成竞争关系[41]。lncRNA-miRNA-mRNA的ceRNA网络在许多类型的细胞凋亡中发挥着不可或缺的作用,在牦牛上是否也存在类似的ceRNA机制调控卵泡GCs凋亡还未知。为阐明mRNA、miRNA、lncRNA相互之间形成的调控机制对牦牛卵巢GCs凋亡的影响,本研究发现lnc-MSTRG.7889抑制GCs凋亡,lnc-MSTRG.7889的过表达显著降低GCs中bta-miR-146a的表达;而且在GCs中lnc-MSTRG.7889靶向bta-miR-146a降低后者对GCs凋亡的促进作用;进一步研究发现lnc-MSTRG.7889通过竞争性结合bta-miR-146a促进4的表达;上述表明上调lnc-MSTRG.7889竞争性结合bta-miR-146a促进4表达抑制牦牛GCs的凋亡。目前,已发现lncRNAs通过充当ceRNAs“海绵吸附”miRNAs调节哺乳动物的GCs凋亡,例如lncRNA NORFA通过调节miR-126/TGF-β轴抑制GCs凋亡[42],lnc_13814通过充当apla-miR-145-4“海绵”影响DDIT3促进GCs凋亡[43]。上述多项研究提示,在卵泡GCs中存在ceRNA机制调控细胞功能。在本试验中首次证实,lnc-MSTRG.7889/bta-miR-146a/4存在ceRNA并调控牦牛GCs凋亡,对了解卵泡发育和闭锁过程中的分子调控机制相关研究具有重要意义。
4 结论
研究发现在牦牛卵泡中lnc-MSTRG.7889、bta-miR-146a和4共表达,bta-miR-146a靶向4影响牦牛GCs的凋亡,lnc-MSTRG.7889靶向bta-miR-146a影响牦牛GCs的凋亡,lnc-MSTRG.7889调控bta-miR-146a促进4的表达,因此lncRNA-MSTRG.7889.1竞争性结合 bta-miR-146a靶向4调控牦牛GCs的凋亡。本研究为完善牦牛卵泡GCs的ceRNA调控网络提供了理论基础,为进一步解析牦牛卵泡闭锁的原因提供新的见解。
[1] MANN G E. Reproduction in the yak. British Veterinary Journal, 1993, 149(6): 513-514.
[2] EVANS A C O. Characteristics of ovarian follicle development in domestic animals. Reproduction in Domestic Animals, 2003, 38(4): 240-246.
[3] TIWARI M, PRASAD S, TRIPATHI A, PANDEY A N, ALI I, SINGH A K, SHRIVASTAV T G, CHAUBE S K. Apoptosis in mammalian oocytes: A review. Apoptosis, 2015, 20(8): 1019-1025.
[4] MENG L, JAN S Z, HAMER G, VAN PELT A M, VAN DER STELT I, KEIJER J, TEERDS K J. Preantral follicular atresia occurs mainly through autophagy, while antral follicles degenerate mostly through apoptosis. Biology of Reproduction, 2018, 99(4): 853-863.
[5] GRILO A L, MANTALARIS A. Apoptosis: a mammalian cell bioprocessing perspective. Biotechnology Advances, 2019, 37(3): 459-475.
[6] OBENG E. Apoptosis (programmed cell death) and its signals - A review. Brazilian Journal of Biology, 2021, 81(4): 1133-1143.
[7] MATSUDA F, INOUE N, MANABE N, OHKURA S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. The Journal of Reproduction and Development, 2012, 58(1): 44-50.
[8] GEBERT L F R, MACRAE I J. Regulation of microRNA function in animals. Nature Reviews Molecular Cell Biology, 2019, 20(1): 21-37.
[9] MICHLEWSKI G, CÁCERES J F. Post-transcriptional control of miRNA biogenesis. RNA, 2019, 25(1): 1-16.
[10] ZHANG J B, XU Y X, LIU H L, PAN Z X. microRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reproductive Biology and Endocrinology, 2019, 17(1): 9.
[11] 刘玉芳, 陈玉林, 周祖阳, 储明星. miR-221-3p靶向BCL2L11调控小尾寒羊卵泡颗粒细胞凋亡. 中国农业科学, 2022, 55(9): 1868-1876. doi: 10.3864/j.issn.0578-1752.2022.09.015.
LIU Y F, CHEN Y L, ZHOU Z Y, CHU M X. miR-221-3p regulates ovarian granulosa cells apoptosis by targeting BCL2L11 in small-tail Han sheep. Scientia Agricultura Sinica, 2022, 55(9): 1868-1876. doi: 10.3864/j.issn.0578-1752.2022.09.015. (in Chinese)
[12] YAMAMURA S, IMAI-SUMIDA M, TANAKA Y, DAHIYA R. Interaction and cross-talk between non-coding RNAs. Cellular and Molecular Life Sciences, 2018, 75(3): 467-484.
[13] MA N N, TIE C R, YU B, ZHANG W, WAN J. Identifying lncRNA-miRNA-mRNA networks to investigate Alzheimer’s disease pathogenesis and therapy strategy. Aging, 2020, 12(3): 2897-2920.
[14] REN J Y, JIANG C J, ZHANG H, SHI X L, AI X, LI R Y, DONG J L, WANG J, ZHAO X H, YU H Q. LncRNA-mediated ceRNA networks provide novel potential biomarkers for peanut drought tolerance. Physiologia Plantarum, 2022, 174(1): e13610.
[15] TUERSONG T, LI L L, ABULAITI Z, FENG S M. Comprehensive analysis of the aberrantly expressed lncRNA‑associated ceRNA network in breast cancer. Molecular Medicine Reports, 2019, 19(6): 4697-4710.
[16] 冉宏标, 赵丽玲, 王会, 柴志欣, 王吉坤, 王嘉博, 武志娟, 钟金城. LncFAM200B对牦牛肌内前体脂肪细胞脂质沉积的影响. 中国农业科学, 2022, 55(13): 2654-2666. doi: 10.3864/j.issn.0578-1752.2022. 13.014.
RAN H B, ZHAO L L, WANG H, CHAI Z X, WANG J K, WANG J B, WU Z J, ZHONG J C. Effects of lnc FAM200B on the lipid deposition in intramuscular preadipocytes of yak. Scientia Agricultura Sinica, 2022, 55(13): 2654-2666. doi: 10.3864/j.issn.0578-1752.2022.13.014 (in Chinese)
[17] 王会, 柴志欣, 朱江江, 钟金城, 张成福, 信金伟. 牦牛Linc24063的克隆鉴定及其与miRNAs表达水平的相关性分析. 中国农业科学, 2019, 52(14): 2538-2547. doi: 10.3864/j.issn.0578-1752.2019. 14.012.
WANG H, CHAI Z X, ZHU J J, ZHONG J C, ZHANG C F, XIN J W. Cloning and identification of long-chain non-coding RNA Linc24063 and its correlation with the expression level of miRNAs in yak. Scientia Agricultura Sinica, 2019, 52(14): 2538-2547. doi: 10.3864/ j.issn.0578-1752.2019.14.012. (in Chinese)
[18] HAN X H, PAN Y Y, FAN J F, WANG M, WANG L B, WANG J L, AFEDO S Y, ZHAO L, WANG Y Y, ZHAO T, ZHANG T X, ZHANG R, CUI Y, YU S J. LncRNA MEG3 regulates ASK1/JNK axis-mediated apoptosis and autophagy via sponging miR-23a in granulosa cells of yak tertiary follicles [J]. Cellular Signalling, 2023, 107: 110680.
[19] LI M H, NIU M H, FENG Y Q, ZHANG S E, TANG S W, WANG J J, CAO H G, SHEN W. Establishment of lncRNA-mRNA network in bovine oocyte between germinal vesicle and metaphase II stage. Gene, 2021, 791: 145716.
[20] LIU A J, LIU M H, LI Y X, CHEN X Y, ZHANG L M, TIAN S J. Differential expression and prediction of function of lncRNAs in the ovaries of low and high fecundity Hanper sheep. Reproduction in Domestic Animals, 2021, 56(4): 604-620.
[21] YAO W, PAN Z X, DU X, ZHANG J B, LIU H L, LI Q F. NORHA, a novel follicular atresia-related lncRNA, promotes porcine granulosa cell apoptosis via the miR-183-96-182 cluster and FoxO1 axis. Journal of Animal Science and Biotechnology, 2021, 12(1): 103.
[22] YAO Y L, MENG Z Y, LI W C, XU Y F, WANG Y L, SUOLANG S Z, XI G Y, CAO L, GUO M. Profiling and functional analysis of long non-coding RNAs in yak healthy and atretic follicles. Animal Reproduction, 2022, 19(3): e20210131.
[23] 牛家强, 王玉恒, 索朗斯珠, 强巴央宗, 徐业芬, 郭敏, 程玲华, 杨士承. 牦牛Smad 4基因3’UTR区双荧光素酶载体构建及与bta-miR-146a的靶向验证. 畜牧兽医学报, 2018, 49(7): 1366-1376.
NIU J Q, WANG Y H, SUOLANGSIZHU, QIANGBAYANGZONG, XU Y F, GUO M, CHENG L H, YANG S C. Construction of yak smad 4 gene 3’UTR dual-luciferase reporter vector and its targeting validation to bta-miR-146a. Chinese Journal of Animal and Veterinary Sciences, 2018, 49(7): 1366-1376. (in Chinese)
[24] RODGERS R J, IRVING-RODGERS H F. Morphological classification of bovine ovarian follicles. Reproduction, 2010, 139(2): 309-318.
[25] 王玉恒, 索朗斯珠, 强巴央宗, 徐业芬, 牛家强, 姚一龙, 王英杰. 西藏林芝地区牦牛卵泡颗粒细胞体外分离培养和形态观察. 畜牧与兽医, 2018, 50(5): 12-14.
WANG Y H, SUOLANGSIZHU, QIANGBAYANGZONG, XU Y F, NIU J Q, YAO Y L, WANG Y J. Isolated culture and morphology of follicle granulosa cells in yak in the Nyingchi region of Tibet. Animal Husbandry & Veterinary Medicine, 2018, 50(5): 12-14. (in Chinese)
[26] 王运路, 孟朝轶, 姚一龙, 郭敏, 牛家强, 索朗斯珠, 徐业芬. 牦牛“海绵吸附”bta-miR-146a的lncRNA筛选及靶向验证. 中国畜牧兽医, 2023, 50(3): 859-869.
WANG Y L, MENG Z Y, YAO Y L, GUO M, NIU J Q, SUOLANGSIZHU, XU Y F. Screening and targeting verification of lncRNA of “sponge adsorption” bta-miR-146a in yak. China Animal Husbandry & Veterinary Medicine, 2023, 50(3): 859-869. (in Chinese)
[27] WANG M M, WANG Y, YAO W, DU X, LI Q F. Lnc2300 is a cis-acting long noncoding RNA of CYP11A1 in ovarian granulosa cells. Journal of Cellular Physiology, 2022, 237(11): 4238-4250.
[28] PAN Y, YANG S F, CHENG J R, LV Q, XING Q H, ZHANG R M, LIANG J Y, SHI D S, DENG Y F. Whole-transcriptome analysis of LncRNAs mediated ceRNA regulation in granulosa cells isolated from healthy and atresia follicles of Chinese buffalo. Frontiers in Veterinary Science, 2021, 8: 680182.
[29] SUN L, ZHANG P J, LU W F. lncRNA MALAT1 regulates mouse granulosa cell apoptosis and 17β-estradiol synthesis via regulating miR-205/CREB1 axis. BioMed Research International, 2021, 2021: 6671814.
[30] DERYNCK R, ZHANG Y E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature, 2003, 425(6958): 577-584.
[31] YU C, ZHANG Y L, FAN H Y. Selective Smad4 knockout in ovarian preovulatory follicles results in multiple defects in ovulation. Molecular Endocrinology, 2013, 27(6): 966-978.
[32] MA X P, YI H S. BMP15 regulates FSHR through TGF-β receptor II and SMAD4 signaling in prepubertal ovary of Rongchang pigs. Research in Veterinary Science, 2022, 143: 66-73.
[33] LI X Y, DU X, YAO W, PAN Z X, LI Q F. TGF-β/SMAD4 signaling pathway activates the HAS2-HA system to regulate granulosa cell state. Journal of Cellular Physiology, 2020, 235(3): 2260-2272.
[34] 陈露露, 王会, 王吉坤, 王嘉博, 柴志欣, 陈智华, 钟金城. 藏黄牛与宣汉黄牛心脏miRNA表达谱比较. 中国农业科学, 2020, 53(8): 1677-1687. doi: 10.3864/j.issn.0578-1752.2020.08.016.
CHEN L L, WANG H, WANG J K, WANG J B, CHAI Z X, CHEN Z H, ZHONG J C. Comparative analysis of miRNA expression profiles in the hearts of Tibetan cattle and Xuanhan cattle. Scientia Agricultura Sinica, 2020, 53(8): 1677-1687. doi: 10.3864/j.issn.0578-1752.2020. 08.016. (in Chinese)
[35] 陈慧芳, 黄绮亮, 胡智超, 潘晓婷, 吴志胜, 白银山. 外泌体microRNA在猪成熟和闭锁卵泡中的表达差异及功能分析. 中国农业科学, 2021, 54(21): 4664-4676. doi: 10.3864/j.issn.0578-1752.2021.21.005.
CHEN H F, HUANG Q L, HU Z C, PAN X T, WU Z S, BAI Y S. Expression differences and functional analysis of exosomes microRNA in porcine mature and atretic follicles. Scientia Agricultura Sinica, 2021, 54(21): 4664-4676. doi: 10.3864/j.issn.0578-1752.2021. 21.005.(in Chinese)
[36] YUAN H, LU J, XIAO S Y, HAN X Y, SONG X T, QI M Y, LIU G S, YANG C X, YAO Y C. miRNA expression analysis of the sheep follicle during the prerecruitment, dominant, and mature stages of development under FSH stimulation. Theriogenology, 2022, 181: 161-169.
[37] 刘雪宁. 流体剪切应力通过调控miR-146a-5p/SMAD4信号轴抑制MC3T3-E1细胞凋亡[D]. 兰州: 兰州大学, 2022.
LIU X N. Fluid shear stress inhibits apoptosis of MC3T3-E1 cells by regulating the signal axis of miR-146a-5p/SMAD4[D]. Lanzhou: Lanzhou University, 2022. (in Chinese)
[38] SHAO J H, DING Z R, PENG J H, ZHOU R, LI L X, QIAN Q R, CHEN Y. miR-146a-5p promotes IL-1β-induced chondrocyte apoptosis through the TRAF6-mediated NF-kB pathway. Inflammation Research, 2020, 69(6): 619-630.
[39] ZHANG W W, SHAO M M, HE X J, WANG B J, LI Y C, GUO X Y. Overexpression of microRNA-146 protects against oxygen-glucose deprivation/recovery-induced cardiomyocyte apoptosis by inhibiting the NF-κB/TNF-α signaling pathway. Molecular Medicine Reports, 2018, 17(1): 1913-1918.
[40] HE F P, LIU Y H, LI T, MA Q L, ZHANG Y M, HE P Q, XIONG C Y. microRNA-146 attenuates lipopolysaccharide induced ovarian dysfunction by inhibiting the TLR4/NF- κB signaling pathway. Bioengineered, 2022, 13(5): 11611-11623.
[41] TAY Y, RINN J, PANDOLFI P P. The multilayered complexity of ceRNA crosstalk and competition. Nature, 2014, 505(7483): 344-352.
[42] DU X, LIU L, LI Q Q, ZHANG L F, PAN Z X, LI Q F. NORFA, long intergenic noncoding RNA, maintains sow fertility by inhibiting granulosa cell death. Communications Biology, 2020, 3(1): 131.
[43] WU Y, XIAO H W, PI J S, ZHANG H, PAN A L, PU Y J, LIANG Z H, SHEN J, DU J P, HUANG T. LncRNA lnc_13814 promotes the cells apoptosis in granulosa cells of duck by acting as apla-miR-145-4 sponge. Cell Cycle, 2021, 20(9): 927-942.
lncRNA-MSTRG.7889.1 Competitively Binds to bta-miR-146a Targeting4 to Regulate Apoptosis of Yak Granulosa Cells

1College of Animal Sciences, Tibet Agriculture and Animal Husbandry College, Nyingchi 860000, Tibet;2Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences, Shenzhen 518000, Guangdong;3College of Animal Science and Technology, China Agricultural University, Beijing 100193
【background】 Apoptosis of granulosa cells (GCs) is an important cause of follicular atresia, and competing endogenous RNA mechanism has been proven to be involved in regulating the apoptosis process of GCs. The previous transcriptome sequencing study of yak ovary showed that lncRNA-MSTRG.7889.1 had binding sites with bta-miR-146a, while bta-miR-146a had a targeting relationship with4. 【Objective】The aim of this experiment was to explore the molecular regulatory mechanisms of ceRNA that affected the apoptosis of follicular GCs in yak, so as to lay a foundation for revealing the mystery of the reproductive regulation of the yak.【Method】Healthy and atretic follicles of adult female yaks were collected and isolated, and the expressions of lncRNA-MSTRG.7889.1, bta-miR-146a and4 in follicles were detected by RT-qPCR. Healthy and atretic follicles were sliced, and GCs apoptosis in healthy and atretic follicles was detected by TUNEL. The localizations of lncRNA-MSTRG.7889.1, bta-miR-146a and4 in follicles were analyzed by fluorescence in situ hybridization. The yak GCs were isolated and cultured, and transfected with4 overexpression vector and bta-miR-146a mimics, respectively. The apoptosis rate of GCs cells was detected by flow cytometry, and the expressions of pro-apoptotic proteins CASPASE3 and BAX and anti-apoptotic proteins BCL-2 were detected by Western blot. After co-transfecting4 overexpression vector and bta-miR-146a mimics with GCs, it was investigated whether bta-miR-146a targeting4 affects GCs apoptosis in yaks. lncRNA-MSTRG.7889.1 overexpression lentiviral vector was used to infect GCs, and the effect of lncRNA-MSTRG.7889.1 on GCs apoptosis was detected by flow cytometry and Western blot. lncRNA-MSTRG.7889.1 vector and bta-miR-146a mimics were co-transfected with GCs to further analyze whether lncRNA-MSTRG.7889.1 competitive binding of bta-miR-146a targeting4 affected the apoptosis of GCs in yaks. 【Result】The expression of lncRNA-MSTRG.7889.1 andgene mRNA in healthy yak follicles was significantly higher than that in atresia follicles (<0.01), while the expression of bta-miR-146a was opposite (<0.01). TUNEL test results showed that the fluorescence intensity of GCs in atretic follicles was significantly higher than that in healthy follicles (<0.01), indicating that the apoptosis of GCs in atretic follicles was significantly higher than that in healthy follicles. The results of fluorescence in situ hybridization showed that4, bta-miR-146a and lncRNA-MSTRG.7889.1 were co-expressed in the follicles of yaks, and their expressions in healthy and atretic follicles were basically consistent with the results of RT-qPCR, indicating that ceRNA mechanism might be involved in the development of healthy follicles and follicle atresia in yaks. Overexpression of4 in yaks GCs significantly decreased the apoptosis rate of GCs (<0.01), and the expressions of CASPASE3 and BAX protein were significantly decreased (<0.01), while the expression of BCL-2 was significantly increased (<0.01). Overexpression of bta-miR-146a significantly increased the apoptosis rate of GCs (<0.01), increased the expressions of CASPASE3 and BAX proteins (<0.01), and decreased the expression of BCL-2 (<0.01). bta-miR-146a targeted inhibition of4 expression (<0.01);4 and bta-miR-146a were co-transfected into GCs, and the results showed that bta-miR-146a targeted inhibition of4 reduced the former's promoting effect on GCs apoptosis (<0.01). Overexpression of lncRNA-MSTRG.7889.1 significantly decreased the apoptosis rate of GCs in yaks (<0.01), the expressions of CASPASE3 and BAX protein were significantly decreased (<0.01), and the expression of BCL-2 was significantly increased (<0.01). lncRNA-MSTRG.7889.1 significantly inhibited the expression of bta-miR-146a (<0.01). lncRNA-MSTRG.7889.1 and bta-miR-146a were co-transfected into GCs, and lncRNA-MSTRG.7889.1 targeting bta-miR-146a decreased the promotion effect of the latter on GCs apoptosis (<0.01), and the results of RT-qPCR and Western blot showed that lncRNA-MSTRG7889.1 competitively bound bta-miR-146a to promote the expression of4 gene at mRNA and protein levels (<0.01). 【Conclusion】 lncRNA-MSTRG.7889.1 competitively binds to bta-miR-146a, promotes the expression of4 and inhibits the apoptosis of yak GCs.
lncRNA; bta-miR-146a; apoptosis; granulosa cells; yak

2023-06-27;
2023-09-10
国家自然科学基金(31960661)、西藏自治区科技厅区域科技协同创新专项(QYXTZX-NQ2022 -04)、国家肉牛牦牛产业体系项目(CARS-37)
孟朝轶,E-mail:392472987@qq.com。王运路,E-mail:3225254770@qq.com。孟朝轶和王运路为同等贡献作者。通信作者徐业芬,E-mail:xzlzxyf@163.com
(责任编辑 林鉴非)
