黑色素瘤循环肿瘤细胞检测的金属卤化物钙钛矿外泌体复合探针检测新策略
2024-02-20陈志山徐朋飞张绍安
陈志山, 徐朋飞, 张绍安, 李 杨
(广州医科大学 生物医学工程学院, 广东 广州 511436)
1 Introduction
Melanoma derived from epidermal improperly proliferating melanocytes is featured by a high metastatic tendency and high mortality rates, and now become a highly malignant tumor[1-2].Conventional melanoma diagnosis starts from abnormalities of biochemical indicators.Recently, the use of liquid biopsy has provided new opportunities to rapidly identify melanoma tumor progression, since liquid biopsy can complement tumor biopsy to effectively obtain pathological information by enabling contributing biomarkers[3-4].Circulating tumor cells(CTCs) released by the primary tumor or metastasis become one of the popular biomarkers, and are extensively investigated in liquid biopsy, due to the large heterogeneity deriving from the transport from the primary location to other, distant and susceptible tissues, as well as the formation of micro- and/or macro-metastases[5-6].However, in clinical diagnosis, the development of CTCs detection for liquid biopsy of melanoma is still limited, as the lack of accuracy and sensitivity[7-9].
Actually, for accurate and sensitive CTCs detection of melanoma in liquid biopsy, the alternative strategy should possess standard targeted recognition capability and sensitively positive fluorescence feedback[10-11].whereas, the existing route just depends on the epithelial cell adhesion molecule(EpCAM) to achieve CTCs enrichment, and employs organic probes as signal indicators to feedback results[12-13].EpCAM is a transmembrane glycoprotein that has received more attention as a “universal” targeted molecule of tumors for the increase of cell concentration in sample[14-15].Numerous studies have expounded that EpCAM can be used for CTC recognition when it is strongly expressed in tumors, yet it isn't useful in EpCAM-negative or low-expression tumors, such as melanoma[16-18].Accordingly, such EpCAM-triggered CTCs detection leads to an inferior accuracy and sensitivity for melanoma in liquid biopsy.On the other hand, the sensitivity also involves with the brightness of the used luminescent probes in CTCs detection strategy.Since the commercial products of liquid biopsy are essentially based on the luminescence technique, photoluminescence quantum yields(PLQYs) of probes are crucial[5].Nevertheless, the PLQYs of fluorescent dyes employed as commercial probes are still dissatisfactory(Fig.2(h)); meanwhile, these products not only require the specific storage conditions, but also cannot be preserved for a long time based on factors such as activity, further affecting the accuracy and sensitivity[19-21].
To address these issues, we construct a new strategy of CTCs detection for liquid biopsy of melanoma by employing high-PLQYs metal halide perovskite(MHP) quantum dots(QDs) and strongly targeted-enrichment exosome as signal indicators and recognition analytes, respectively.Exosomes are vesicles surrounded by plasma membrane and released by cells into microenvironment; tumor-derived exosomes are derived from tumor cells and have a phospholipid configuration that promotes intercellular communication[22-23].As a result, tumorderived exosomes have a certain ability to target parent tumor cells, that is, “homing” properties, and to deliver exosome-encapsulated analytes to tumor sites[24-26].Furthermore, MHP QDs as the emerging star optical materials have already shown great potential in optoelectronic applications due to their higher PLQY than fluorescent dyes.Becoming a superior photoluminescence(PL) indicator compared to conventional fluorescent dyes in biosensing, MHP QDs exhibit enhanced sensitivity and improved temporal-spatial resolution[27-29].Recent developments of MHP QDs also further push the envelope on fighting hygroscopicity, toxicity, photo and thermal instability, thus activating a gratifying anticipation as optical probe of liquid biopsy[30-35].
2 Experiment
2.1 Materials
Cesium bromide(CsBr, Macklin, 99.999%),lead bromide(PbBr2, Macklin, 99.999%), Oleic acid(OA, Aladdin, 90%), Oleylamine(OAm, Aladdin,99%), N, N-Dimethylformamide(DMF, Aladdin,99.5%), Tetramethoxysilane(TMOS, Energy Chemical, 99%), Toluene(DAMAO, 99.95%) were used directly without further purification.Phosphoethanolamine-N- [methoxy(polyethylene glycol)-2000],(mPEG-DSPE, MW 2 000 u) was bought from Tansh-Tech.For cell culture, phosphate buffer(PBS), dulbecco's modified eagle medium(DMEM), penicillin streptomycin and fetal bovine serum(FBS) were bought from Gibco.The B16 cell lines were purchased from Sigma-Aldrich(ECACC, 93021013 and 85111505).Antibody against TSG101(ab125011)was purchased from Abcam.
2.2 Synthesis of CsPbBr3@SiO2
CsPbBr3@SiO2were preparedviaone-pot route.0.147 2 g PbBr2, 0.086 8 g CsBr, 0.6 mL OAm and 1.8 mL OA were added into 10 mL DMF.Then the mixture was stirred at 60 ℃ for 1 h to obtain a clear solution.Ammonia solution(40 µL, 2.8%) was added into 2 mL of the precursor solution.After this, 0.2 mL of the precursor solution was rapidly added to 10 mL of dry toluene containing 5 µL of TMOS under vigorous agitation(1 500 r/min).After 10 s, the shaking rate was set to 150 r/min and maintained for 120 min.The final products were collected by centrifuging to 10 000 r/min for 5 min and dried at 60 ℃.
2.3 Fabrication of CsPbBr3@SiO2@PEG
The obtained powders were disposed simply by ultrasonic, thus being uniformly dispersed in water.Then mPEG-DSPE was adder into the solution and treated with ultrasound for several minutes.The products were collected by centrifugation and washed for several times with water to remove the residual reactants.
2.4 Isolation and Protein Analysis of Exosomes
In brief, exosomes were isolated by classical differential ultracentrifugation[36].The cells were discarded from the collected B16 cells culture medium by centrifugation at a low speed of 500 ×g, the supernatant was collected and further centrifugated at a speed of 16 500 ×g.Then the cell debris and bullae were removed from the collected supernatant by filtration through a 0.22 µm sterile filter.After final 110 000 ×gcentrifugation, concentrated exosomes were collected, washed with PBS once, and then stored at 4 ℃.The protein components of purified exosomes were determined by BCA protein concentration kit followed the instructions.The surface marker of exosomes was identified by western blot,Anti-TSG101 was used as primary antibodies.
2.5 Preparation of PSPE
The above obtained nanomaterials were mixed with a certain amount of MEX, then the mixture was extruded through a polyester porous membrane in a micro extruder for 15 times.After extruding, the mixture was centrifuged at 10 000 r/min for 5 min and the supernatant was removed.The precipitate was then redispersed in PBS and washed three times with PBS.The obtained product was stored at 4 ℃.
2.6 Cell Culture
B16 cells were routinely cultured in DMEM medium with 10% FBS, and 1% penicillin/streptomycin(10 000 U/mL) in an incubator(5% CO2, 37 ℃).Once cells reached 80% confluency, they were trypsinized(Trypsin, Thermo Fisher Scientific) and the cell suspension was used for the experiment.
2.7 In Vitro Cytotoxicity Assay
CsPbBr3QDs, CsPbBr3@SiO2, and CsPbBr3@SiO2@PEG were dispersed in fresh culture medium for following experiment.Then B16 cells were seeded in a 96-well plate at a density of 1 × 104cells per well overnight and then incubated with the pre-prepared reagent at different concentrations for 24 hours.Subsequently, the cells were treated with 100µL of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazoliu bromide(MTT) reagent for 2 h.Before detection, 150 µL DMSO was added into each well and the absorbance at 490 nm was measured using a microplate reader.
2.8 Cellular Uptake of PSPE
In this study, B16 cells were used as the target cells.The B16 cell suspension was loaded into a cell culture dish(1×104) and then incubated at 37 ℃ for 24 h.PSPE was introduced into the culture medium(without FBS) of B16 cells and co-cultured for 30 min.Subsequently, the culture medium was removed, the cells were fixed with 4% paraformaldehyde for 15 minutes.After fixing, the cells were washed three times with PBS.Lastly, the cells were imagedviaa Zeiss laser confocal microscope(LSM980).
2.9 Targeting Efficiency of PSPE
The B16 cells(1×106cells) incubated with PSPE(0.25 µg/mL) for different durations with gentle shaking at 37 ℃.After incubation, the sample underwent centrifugation at 1 500 r/min for 3 min.Next, the supernatant was discarded, and the sample was washed three times with PBS.Finally, the number of labeled cells was analyzed using flow cytometry.
2.10 Measurement and Characterization
Transmission electron microscopy(TEM) images and elemental mapping were acquired by an FEI Talos F200S microscope.XRD data were measured by a Bruker D8 Advance, Cu Kα radiation(λ=0.154 06 nm), and the operating voltage and current were measured at 50 kV and 40 mA, respectively.The Fourier transform infrared(FTIR) were taken using a Thermo Scientific Nicolet IS50 FTIR spectrometer.The PL spectrum was measured by the Fluorolog-3 fluorescence spectrometer(HORIBA Instruments Incorporated).Confocal laser scanning microscope(CLSM, Zeiss, LSM980) was employed for cell imaging.
3 Results and Discussion
As shown in Fig.1, we first synthesized CsPb-Br3@SiO2viaone-pot route with some modifications[37-38].Then, amorphous silicon was coated onto the surface of CsPbBr3QDs, forming CsPbBr3@SiO2nanoparticles(NPs) with core-shell structure.Subsequently, to further improve the hydrophilicity and dispersibility of CsPbBr3@SiO2, the obtained NPs were loaded into the micelles constituted by PEGgrafted phospholipid(mPEG-DSPE) and formed CsPbBr3@SiO2@PEG.Finally, the exosomes derived from melanoma cells were selected to endow CsPbBr3@SiO2@PEG with ability to target melanoma cells because of their “homing” ability.The as-prepared CsPbBr3@SiO2@PEG and melanoma-derived exosome(MEX) were encapsulated together to form CsPbBr3@SiO2@PEG@EV(PSPE) by being squeezed through a filter membrane physically in an extruder.In a word, exosome-triggered MHP-mediated CTCs detection would show enormous potential in accurate melanoma CTCs diagnosis of liquid biopsy.

Fig.1 A schematic description of the production of exosome-triggered metal halide perovskite-mediated for melanoma CTCs detection
CsPbBr3QDs were synthesized through a onestep method at room temperature.CsPbBr3QDs exhibited a cubic shape with a mean length of 20 nm in the TEM images(Fig.2(a)), and the corresponding elemental mapping images demonstrated a uniform distribution of Cs, Pb, and Br elements.
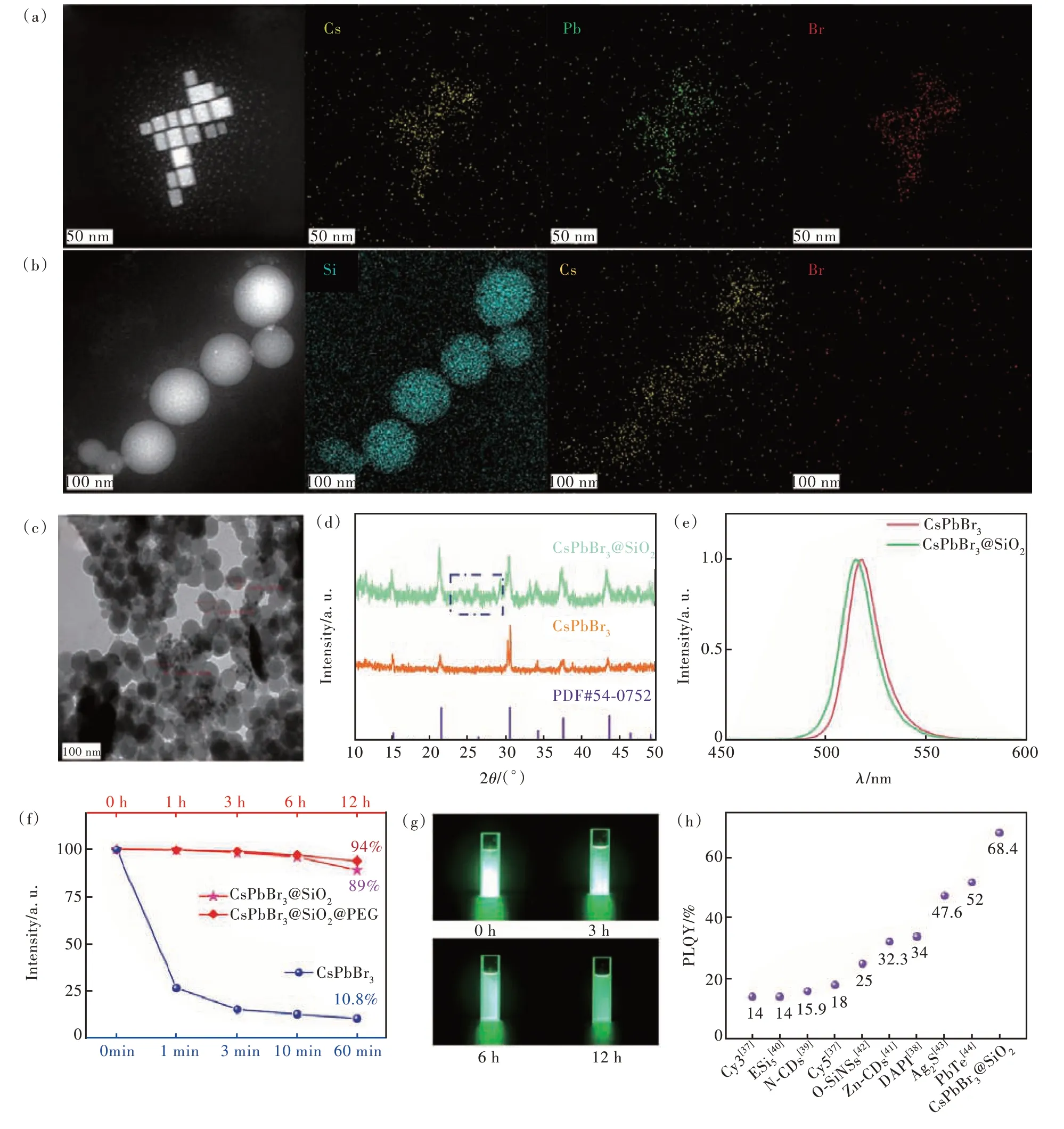
Fig.2 TEM images and corresponding elemental mapping images of CsPbBr3 QDs(a) and CsPbBr3@SiO2(b).(c) TEM image of CsPbBr3@SiO2.(d)XRD patterns of CsPbBr3 QDs and CsPbBr3@SiO2.(e)Emission spectra of CsPbBr3 QDs and CsPb-Br3@SiO2.(f)Time-dependent PL intensity variation of CsPbBr3, CsPbBr3@SiO2, CsPbBr3@SiO2@PEG in aqueous solution.(g)Optical photograph of CsPbBr3@SiO2 under 365 nm UV light irradiation.(h)PLQYs comparison of different fluorescent materials[41-48]
In the TEM images of CsPbBr3@SiO2(Fig.2(b)),CsPbBr3@SiO2NPs were all monodispersed nanospheres.The distribution of the Si element further confirmed the successfully coating of SiO2on the surface of CsPbBr3QDs.Fig.2(c)presented a larger view of CsPbBr3@SiO2, which exhibited a mean diameter of 60 nm.X-ray diffraction(XRD)patterns were utilized to confirm the formations of CsPbBr3QDs and CsPbBr3@SiO2.In Fig.2(d), XRD diffraction peaks of CsPbBr3QDs matched well with the standard card(PDF No.54-0752).Additionally, a small and broad shoulder diffraction peak was observed at 2θ= 26.2°, indicating the presence of low crystallinity amorphous silicon on the surface of CsPbBr3@SiO2[33,39-40].
Fig.2(e) showed the PL spectra of CsPbBr3QDs and CsPbBr3@SiO2.The PL spectrum of CsPb-Br3@SiO2exhibited a slight shift compared to that of CsPbBr3QDs, which was attributed to the restriction imposed by the SiO2shell[37].Moreover, the SiO2shell greatly enhanced the stability of CsPb-Br3@SiO2.As shown in Fig.2(f), the PL intensity of CsPbBr3QDs in water decreased to 26.6% within 1 min and to 10.8% within 60 min.However, CsPb-Br3@SiO2exhibited excellent dispersity and retained stable green luminescence for 12 h(Fig.2(g)), indicating the SiO2shell effectively enhances the stability of CsPbBr3@SiO2.The PL intensity of CsPb-Br3@SiO2remained at 89% even after 12 h of dispersion in water, as depicted in Fig.2(f), demonstrating excellent stability.Additionally, the confinement of the CsPbBr3core and the presence of the SiO2shell might synergistically enhance the photoluminescence quantum yield(PLQY) of CsPbBr3@SiO2to 68.4%(Fig.2(h)).
Here, CsPbBr3@SiO2NPs are anticipated to serve as luminescent probes for liquid biopsy.The cytotoxicity tests were performed on B16 mouse melanoma cells using the MTT assay.Fig.3(a) and(b) show that the cell viabilities of B16 cells decreased to below 70% after a 24-h incubation with CsPbBr3QDs and CsPbBr3@SiO2.To mitigate the cytotoxicity, CsPbBr3@SiO2was encapsulated with mPEG-DSPE, a compound comprising a hydrophilic head and a hydrophobic tail, through self-assembly[49].After being mixed with mPEG-DSPE and subjected to ultrasound for several minutes, CsPb-Br3@SiO2@PEG composites were formed.Fig.3(c)demonstrates the improved viability of B16 cells, attributed to the protection provided by mPEG-DSPE.The results reveal that the viabilities of B16 cells reached nearly 100% after co-incubation with CsPb-Br3@SiO2@PEG for 24 h, with a corresponding concentration increase from 0.15 mg/mL to 1.25 mg/mL.Thus, CsPbBr3@SiO2@PEG demonstrated excellent biocompatibility.Furthermore, the encapsulation of mPEG-DSPE does not affect the stability of CsPbBr3@SiO2@PEG(Fig.2(f)).

Fig.3 Cytotoxicity analyses of CsPbBr3 QDs(a), CsPbBr3@SiO2(b), and CsPbBr3@SiO2@PEG(c).(R7) SEM(d) and TEM(e) and corresponding elemental mapping images of CsPbBr3@SiO2@PEG.(f)FTIR spectra of CsPbBr3 QDs, CsPb-Br3@SiO2 and CsPbBr3@SiO2@PEG.(g)XRD pattern of CsPbBr3@SiO2@PEG.(h)Hydrodynamic diameter distribution of CsPbBr3@SiO2@PEG
The scanning electron microscope(SEM) and TEM were utilized to characterize the morphology of CsPbBr3@SiO2@PEG NPs.Fig.3(d) and (e) display the monodisperse spheric morphology of CsPb-Br3@SiO2@PEG NPs.The elemental mapping revealed the distribution of N and P elements, which originates from mPEG-DSPE, confirming the successful coating of mPEG-DSPE on the surface of CsPbBr3@SiO2.The above results were further confirmed by the FTIR spectra.
The FTIR results were consistent with the above results.As depicted in Fig.3(f), the characteristic peak at 1 095 cm-1was attributed to the Si—O—Si vibration of the amorphous SiO2, which results from the formation of more cross-linked SiO2networks through the hydrolysis process of TMOS[50].A strong ridge was observed at 2 925 cm-1, which was attributed to —CH2CH2O— in the PEG portion of mPEG-DSPE[51].The successful encapsulation of CsPbBr3@SiO2@PEG was further confirmed by XRD patterns.In Fig.3(g), the characteristic diffraction peaks at 11.9°, 18.9° and 23.1° were attributed to the mPEG-DSPE, demonstrating the existence of mPEG-DSPE on the surface of CsPbBr3@SiO2@PEG.Subsequently, the hydrodynamic size of the CsPb-Br3@SiO2@PEG in PBS was evaluatedviathe dynamic light scattering(DLS) technique.As indicated in Fig.3(h), the CsPbBr3@SiO2@PEG exhibited an average diameter of ∼70 nm.
As known to us, tumor-derived exosomes have attracted more and more attention due to their unique features such as minimal immunogenicity and toxicity, efficient cargo loading, preferred tumor homing,etc[52-56].In order to endow the potential application of CsPbBr3@SiO2@PEG for liquid biopsy, melanomaderived exosome(MEX) was selected as recognition analyte for its unique biological characteristics.
As shown in Fig.4(a), MEX was extracted from the collected culture medium of B16 cellsviaclassical differential ultracentrifugation[57].Then, the obtained exosomes were used to load CsPbBr3@SiO2@PEG and form melanoma cells targeted nanoprobes PSPE by the physical extrusion method[58].
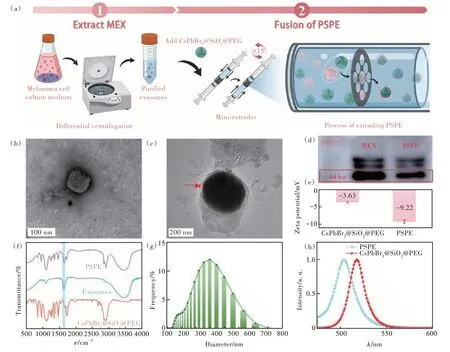
Fig.4 (a)Schematic diagrams of the extracting process of MEX and the extrusion process of PSPE.TEM images of MEX(b) and PSPE(c).(d)Western blot image of MEX and PSPE.Zeta potential analysis(e), and FTIR spectra(f) of CsPbBr3@SiO2@PEG and PSPE.(g)Hydrodynamic diameter distribution of PSPE.(h)PL spectrum of CsPbBr3@SiO2@PEG and PSPE
Micromorphology analysisviaTEM was performed on MEX.In Fig.4(b), a typical exosome structure was observed with a diameter of ~50 nm.The successful extraction of MEX was confirmed by the detection of exosomal-specific protein TSG101 through western blot experiments, as illustrated in Fig.4(d)[59].The as-prepared MEX was blended with CsPbBr3@SiO2@PEG and subsequently extruded to generate exosome membrane-cloaked PSPE.After extruding, the exosome layer can be observed in the TEM image of PSPE(Fig.4(c)).Furthermore, as shown in Fig.4(d), TSG101 was found in PSPEviawestern blotting, illustrating the successful coating of MEX on the surface of PSPE.Zeta potential change from -3.63 mV to -9.62 mV in the PSPE after modification with MEX(Fig.4(e)) and the presence of characteristic protein absorption peaks around 1 634 cm-1(C=O)(Fig.4(f)) further confirmed the extrusion self-assembly method[60].In addition, the hydrodynamic diameter of the PSPE increased to ~240 nm(Fig.4(g)).Notably, compared to CsPbBr3@SiO2@PEG, PSPE exhibited a slight blue shift in its PL emission peak at 504 nm.
Although all cells exhibit nonspecific uptake,which affects the specificity and sensitivity of detection, tumor-derived exosomes can specifically target parental tumor cells without further modification[54,61].The proteins and complex lipids on the surface of tumor-derived exosomes, as well as the conservation of tropism between parent and recipient cells contribute to the targeting ability of exosomes[62-64].
To verify the capability of PSPE as a fluorescent probe, we introduced PSPE into the culture medium of melanoma B16 cells and co-cultured them.Then, as shown in Fig.5(a), we conducted CLSMzstack scanning from thez-axis range of 0 to 35 µm,with 5 µm-step intervals, to verify the PSPE incorporation within the cells.The merged images of brightfield images and PSPE fluorescence in dark-field images taken at different depthsvia z-stack scanning were presented in Fig.5(b).Although cell morphology is visible throughout the range ofz= 5-30 µm,the fluorescence of PSPE is observable in the range ofz= 10-25 µm.Subsequently, we constructed a three-dimensional model of PSPE fluorescence from the dark-field images.The stereoscopic fluorescence model in Fig.5(c) confirms the uptake of PSPE by the B16 cells.These results suggest that PSPE nanoprobes were internalized within the cells and can be utilized as effective nanoprobes for detecting melanoma cells.
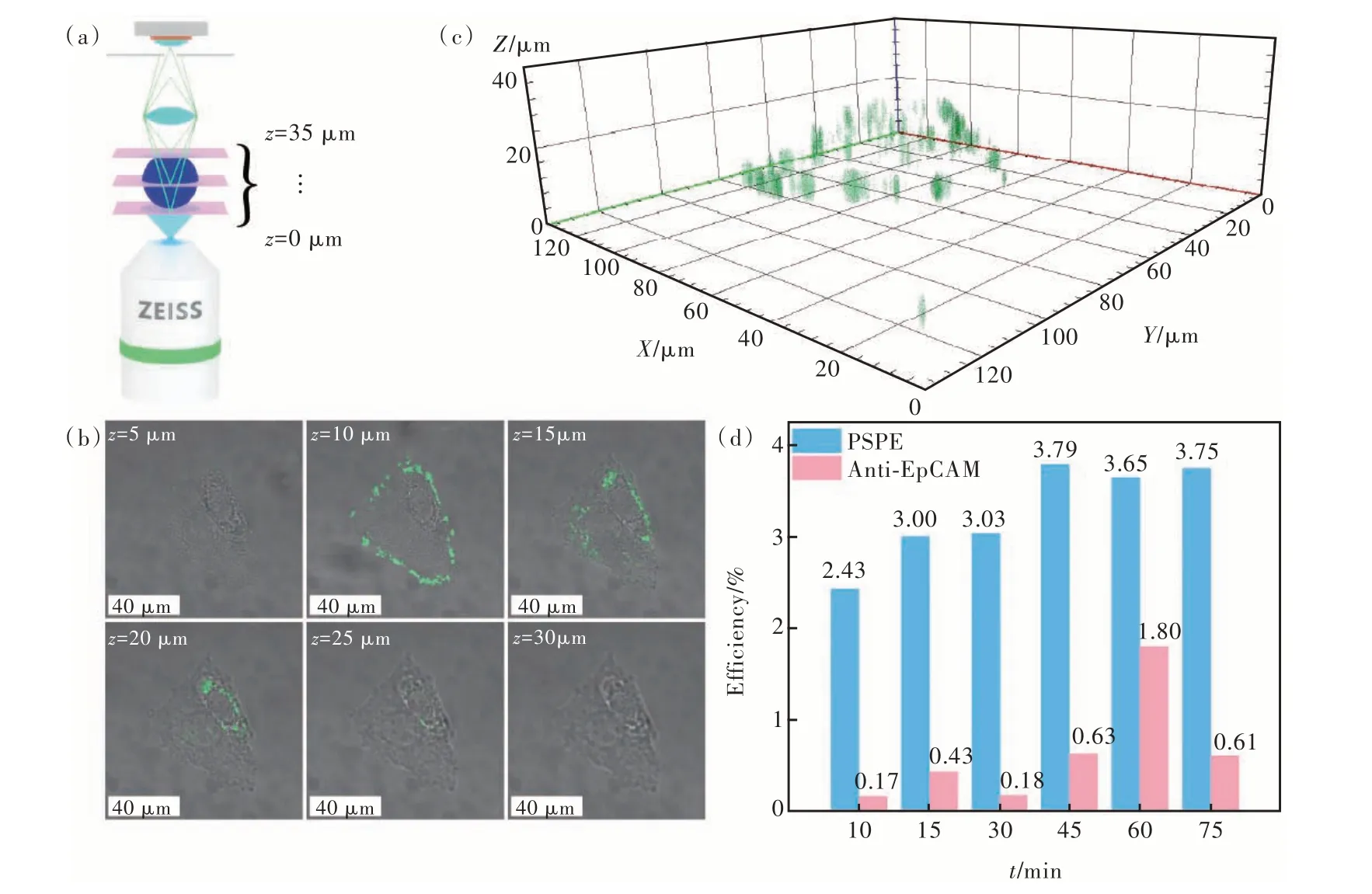
Fig.5 (a)Schematic diagrams of confocal laser scanning microscope z-stack scanning setup.(b)Merged fluorescence with bright-field images from z-stack scanning at the same region.The images are taken with a 5 µm-step.(c)Three-dimensional model image of PSPE fluorescence based on the z-stack images.(d)Flow cytometry evaluating the efficiency of PSPE labeling melanoma cells
Flow cytometry was utilized to evaluate the efficiency of PSPE in labeling B16 cells.Fig.5(d) displays the low targeting efficiency of the commercialized Anti-EpCAM antibody in targeting B16 cells.However, after being co-cultured with PSPE for different durations, the efficiency of labeling reached its peak at 40 min before reaching a plateau.In contrast to commercial Anti-EpCAM antibody, the detection sensitivity of our reagent materials was raised up by an order of magnitude.It indicated that exosomes could promote nanomaterials to target parent tumor cells.For melanoma cells, the labeling efficiency of PSPE was almost ten-fold higher than that of the Anti-EpCAM antibodies, demonstrating that MEX exhibited considerable potential in detecting melanoma CTCs and could significantly improve the internalization of CTCs.
4 Conclusion
We have developed a new nanoscale probe for detecting CTCs in melanoma.This nanoprobe is composed of high PLQY perovskite quantum dots as signal generators and melanoma-derived exosomes as biosensing elements.Due to their poor stability,pure CsPbBr3QDs are difficult to apply in the field of biomedicine.By encapsulating them with SiO2and mPEG-DSPE, we significantly improved their stability, PLQY, and biological compatibility, eventually leading to the successful development of CsPb-Br3@SiO2@PEG.After loading with melanoma-derived exosomes that possess the homing ability,CsPbBr3@SiO2@PEG is endowed with biological targeting capability, forming PSPE nanoprobe.Finally,we performed proof-of-concept experiments to show the cellular uptake and the melanoma cells labeling.The results suggest that the PSPE nanoprobe may find applications in detecting melanoma CTCs.
Response Letter is available for this paper at:http://cjl.lightpublishing.cn/thesisDetails#10.37188/CJL.20230237.
