内生菌提高植物抗旱性和耐盐性分子机制研究进展
2024-02-02宋雪付楚涵李家红孙雪铜韦银珠肖汇川李韦瑶秦立刚
宋雪 付楚涵 李家红 孙雪铜 韦银珠 肖汇川 李韦瑶 秦立刚

doi:10.11733/j.issn.1007-0435.2024.01.002
引用格式:
宋 雪, 付楚涵, 李家红,等.内生菌提高植物抗旱性和耐盐性分子机制研究进展[J].草地学报,2024,32(1):13-24
SONG Xue, FU Chu-han, LI Jia-hong,et al.Research Progress on Molecular Mechanism of Endophytes Improving theDrought Resistance and Salt Tolerance of Plant[J].Acta Agrestia Sinica,2024,32(1):13-24
摘要:植物-内生菌共生体在缓解植物的非生物和生物胁迫方面发挥着重要作用。在干旱和盐胁迫下,内生菌可以通过调控植物光合作用、激素浓度、渗透调节物质含量、抗氧化酶活性以及相关基因表达等来保证植物正常生长和发育,从而增强植物抗逆性。近年来,植物促生菌(Plant growth promoting bacteria,PGPB)接种剂也被广泛研究应用。本文综述了植物内生菌的多样性、共生内生菌和PGPB在干旱和盐胁迫下对植物基因的调控,为内生菌提高植物耐旱性和耐盐性的分子机制的深入研究提供参考。
关键词:植物内生菌;干旱胁迫;盐碱胁迫;基因调控;PGPB
中图分类号:Q945.78 文献标识码:A 文章编号:1007-0435(2024)01-0013-12
Research Progress on Molecular Mechanism of Endophytes Improving
the Drought Resistance and Salt Tolerance of Plant
SONG Xue, FU Chu-han, LI Jia-hong, SUN Xue-tong, WEI Yin-zhu,
XIAO Hui-chuan, LI Wei-yao, QIN Li-gang*
(College of Animal Science, Northeast Agricultural University, Harbin, Heilongjiang Province 150030, China)
Abstract:Plant-endophyte symbioses play an important role in alleviating abiotic and biotic stresses to plants. Under drought and salt stresses,endophytic bacteria can enhance the resistance of plant to the stresses by regulating plant photosynthesis,concentration of hormones,content of osmoregulatory substances,activity of antioxidant enzyme,and expression of genes to ensure a normal growth and development of plant. In recent years,plant growth-promoting bacteria (PGPB) inoculants have also been widely studied and applied. In this paper,we reviewed the diversity of endophytic bacteria,the regulation of plant genes by plant symbiotic endophytes and PGPB under drought and salt stresses,and provided a reference for the in-depth study of the molecular mechanism of endophytic bacteria to improve the tolerance of plant to drought and salt stresses.
Key words:Plant endophytes;Drought stress;Salinity stress;Gene regulation;PGPB
收稿日期:2023-05-17;修回日期:2023-09-15
基金项目:国家自然科学基金(32271770);黑龙江省优秀青年基金(YQ2023C013)资助
作者简介:
宋雪(2000-),女,满族,辽宁本溪人,硕士研究生,主要从事草地植物资源利用研究,E-mail:songxue2023@163.com;*通信作者Author for correspondence,E-mail:qinligang@neau.edu.cn
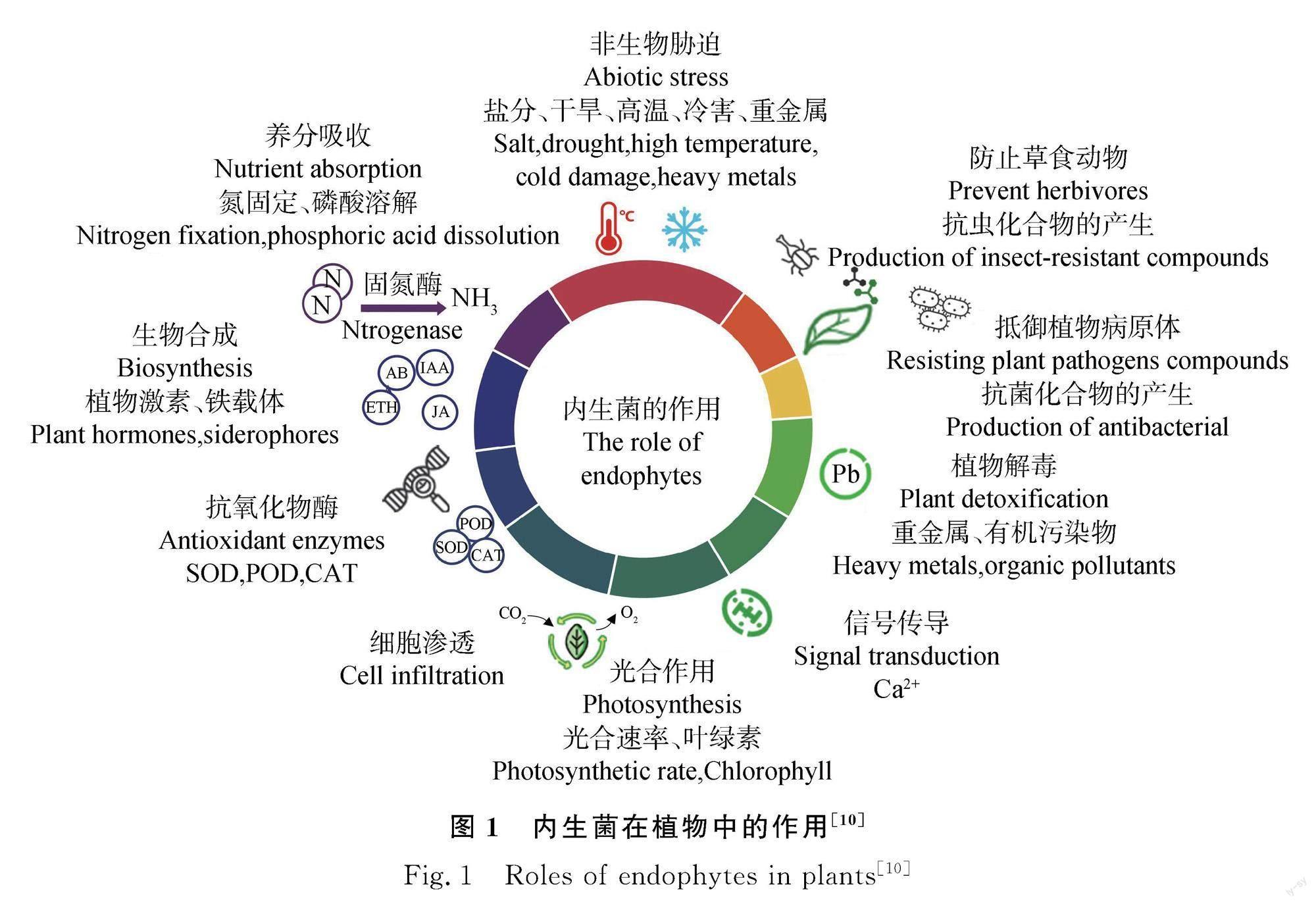
植物內生菌是指在植物生命周期内存在于植物体中的非致病性的微生物[1],最早发现于1898年[2]。目前已被发现的内生菌有200多个属,约100多万个种[3-4]。从图1中可以看出,内生菌不会对宿主产生不利影响,在植物病害控制、次生代谢物合成、植物生长调节和抗逆性等方面发挥着重要作用[5]。内生菌与宿主植物存在长期的共生关系。一方面,内生菌通过吸收水分养分[6-9],诱导产生激素、铁载体[10]和抗菌次生代谢物[11],调节脯氨酸含量,提高抗氧化酶活性[12-14]等一系列措施促进植物生长,提高植物抗逆性和抗病性;另一方面,植物通过木质化影响内生细菌和真菌的发育过程和多样性并改变内生菌的代谢功能[15],加速内生菌在宿主植物体内定植[16]。此外,内生菌及其代谢物也可作为生物活性化合物的来源,用于新型抗生素的发明、抗癌药物和替代药物的研究[17]。
干旱和盐碱是影响植物生长发育的重要环境因素,影响植物渗透调节水平和信号转导,造成植物氧化损伤和膜质过氧化,进而产生毒害作用[18-20]。用育种和基因工程等技术培育耐旱耐盐植物是应对干旱和盐碱问题的主要手段之一,但缺点是耗时费力,且容易受到多种因素限制。利用内生菌提高植物耐旱性和耐盐性也是有效解决手段之一,且利于生态和农业可持续发展[21]。植物促生菌(Plant growth promoting bacteria,PGPB)接种也可以在环境胁迫下通过刺激植物分泌生长调节物质或诱导激素合成进而提高抗逆性[22-23]。因此,合理利用内生菌来提高植物的生产力和抗性前景非常广阔,可作为应对气候变化和粮食生产带来的挑战的一种新策略。在此背景下,了解干旱和盐胁迫下内生菌与植物的相互作用的分子机制至关重要。本文概述了植物内生菌多样性,干旱和盐碱胁迫下内生菌对植物基因的调控研究,以期为内生菌分子机制研究提供参考。
1 植物内生菌多样性
1.1 种类多样性
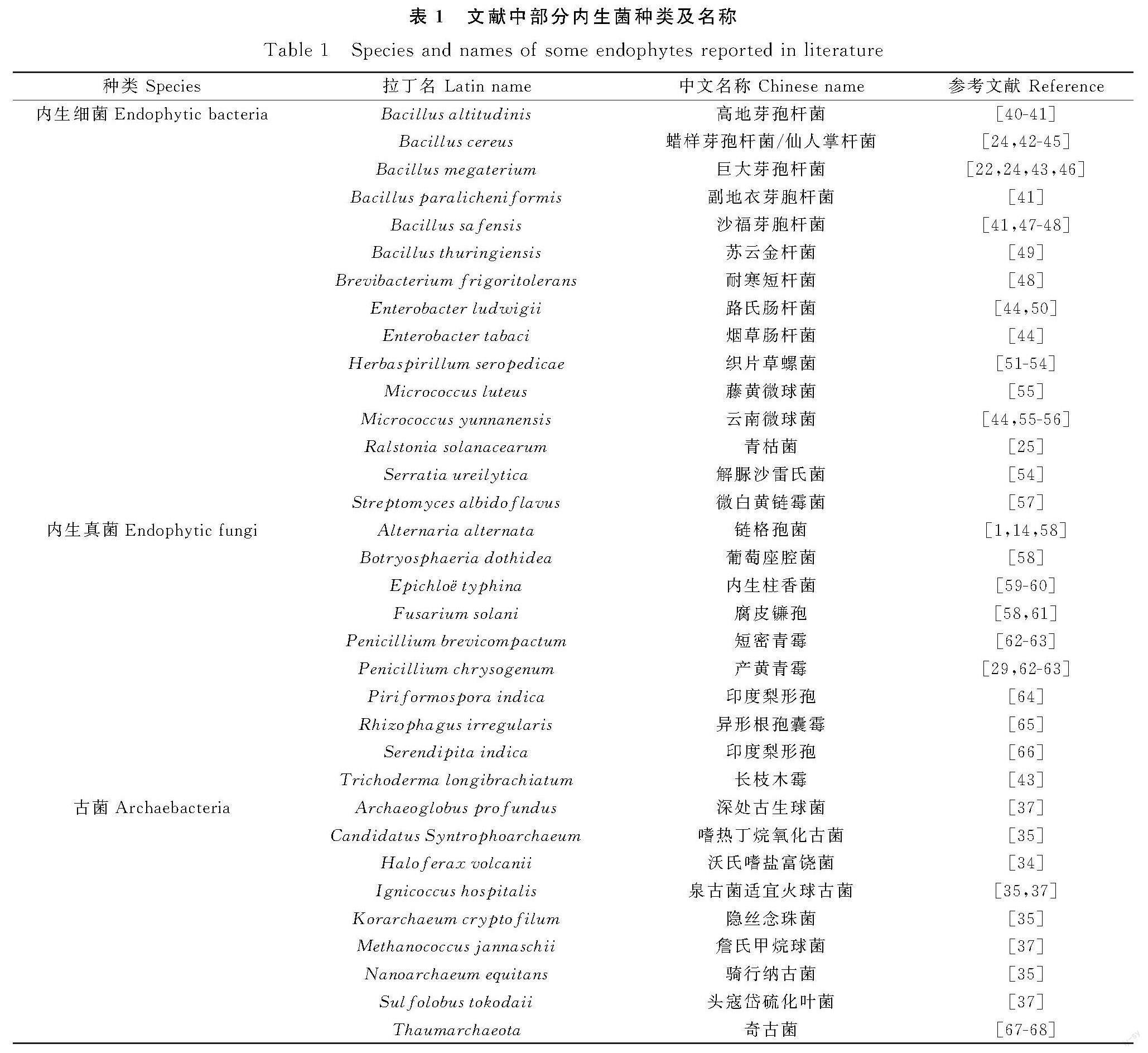
植物内生菌种类繁多,主要分属于细菌、真菌、古菌和卵菌[24]。表1中列出了文献中提到的部分内生菌。植物内生细菌主要存在于植物根系,通过植物促进[25]、生物施肥[26]和生物控制[27]三种相互关联的机制促进植物发育。芽孢杆菌(Bacillus)、肠杆菌(Enterobacter)、节杆菌(Arthrobacter)、偶氮杆菌(Azotobacter)、异肽菌(Isolptericola)、链霉菌(Streptomyces)和假单胞菌(Pseudomonas)等属的细菌可以提高植物对高温、干旱和盐碱的抗逆性[28-29]。植物内生真菌主要是存在于宿主植物的茎和叶内,是生物活性代谢物的天然来源[30],主要包括子囊菌、担子菌、接合菌、卵菌、有丝分裂孢子真菌及其无孢菌类等多个真菌类群[31]。其中镰刀菌属(Fusarium)是最主要的内生真菌之一,约有70种。镰刀菌属在遗传学上存在较大差异,是次生代谢物的丰富来源,能产生100多种具抗菌、抗氧化、抗寄生虫和免疫调节等多种生物活性的有着特殊结构的化合物[32]。植物内生古菌主要存在植物根和根际,根和根际可以提供缺氧的微生态位[33],内生古菌与宿主植物的关系比细菌更近,代表了生命的第三个领域,是分子生物学与生物技术应用的宝贵的模型系统和来源[34]。目前,研究已经发现的27个古菌门中,广古菌门(Euryarchaeota)、德潘超门(Dpann superphylum)、泉古菌门(Crenarchaeota)、奇古菌门(Thaumarchaeota)、深古菌门(Bathyarchaeota)、阿斯加德超门(Asgard superphylum)这6种具有培养代表性[35],已经建立遗传系统的古菌有产甲烷菌[36]、嗜盐菌、嗜热欧古菌和嗜热古菌[37]。植物内生卵菌是植物内生菌中的一类植物病原菌,寄生在植物体各個部位,会导致作物损产失收[38]。目前已经在植物上发现了近30种卵菌亚种,常见的物种有苎麻疫霉(Phytophthora boehmeriae),烟草疫霉菌(Phytophthora nicotianae)和终极腐霉(Pythium ultimum)[39]。
1.2 分布多样性
内生菌广泛存在于水陆以及藻类各种植物中,具有群落多样性。不同植物共生内生菌种也各有不同[69]。担子菌目(Babacinales)印度梨形孢(Piriformospora indica)可与蕨类植物、苔藓植物、裸子植物、被子植物等各种植物建立共生关系[64],泛菌属(Pantoea)主要共生在水稻(Oryza sativa L.)种子中,提高宿主耐盐碱能力[70]。芦苇(Phragmites australis)和虎尾草(Chloris virgata)中内生菌的优势菌为变形杆菌(Proteobacteria)、放线菌(Actinobacteria)、厚壁菌(Firmicutes)、拟杆菌(Bacteroidetes)和柔膜菌(Tenericutes)[43]。新西兰菠菜(Tetragonia tetragonioides (Pall.) Kuntze)叶子和根部存在农杆菌(Agrobacterium)、寡养单胞菌(Stenotrophomonas)、芽孢杆菌、短杆菌(Brevibacterium)、假单胞菌、链霉菌、假杆菌(Pseudarthrobacter)、拉乌尔菌(Raoultella)、短小杆菌(Curtobacterium)和泛菌[61]。香柱菌属真菌内生菌Epichloё coenophiala常共生在高羊茅(Festuca arundinacea Schreb.)中[71],黑麦草中能分离出Epichloё festucae var. lolii[72]。豆科植物中也会共生许多根瘤菌[50],如豌豆根瘤菌(Rhizobium leguminosarum)[73]、羽扇豆慢生根瘤菌(Bradyrhizobium lupini)、锦鸡儿中慢生根瘤菌(Mesorhizobium caraganae)、百脉根中慢生根瘤菌(Mesorhizobium loti)等。鹰嘴豆(Cicer arietinum L.)根中内生菌包括变形菌门、厚壁菌门和放线菌门[74]。内生真菌Phomopsis Liquidambaris可与花生、水稻和拟南芥建立共生关系,并促进这些植物生长[75-76]。
同一植物不同组织中内生菌群落也具有多样性[55]。内生菌在植物的根、叶、茎、花及种子等组织中均有存在[51],丰度和多样性由其生态位决定。植物根部内生细菌多样性要高于其他器官组织,内生真菌多样性则在叶片,尤其是老叶中最高[77]。内生菌分布还与植物的株龄有关[78],如黄管秦艽(Gentiana officinalis H. Smith)不同年份根系样品的优势细菌门为变形杆菌,相对丰度为50.76%~72.32%,一年龄根系样本的优势属是原小单孢菌属(Promicromonospora),三年龄根样本的优势属是假单胞菌属,五年龄根样本的优势属是分枝杆菌属(Mycobacterium)[79]。
1.3 传播途径多样性
内生菌的传播途径主要是水平传播和垂直传播。水平传播途径是指从土壤到根的转移。内生菌先通过根表皮进入根内部,然后在根毛和侧根分布[80]。也有些内生菌如重氮营养葡糖酸醋杆菌(Gluconacetobacter diazotrophicus)可通过叶序层入侵植物[52]。垂直传播(即种子介导的遗传力)是指内生菌在种子萌发的过程中定植到植物内部,再到发育器官中,实现跨代传播[53,81]。在加拿大野黑麦(Elymus canadensis)中,内生真菌Epichloё canadensis能垂直传播并发生稳定的遗传变异[82]。Epichloё coenophiala通过高羊茅的花序原基和卵母细胞垂直传播,其高度表达的相关应激基因还可能具有促进垂直传播的作用,因为内生菌转录组的转移开始于宿主早期花发育[59]。靠种子传播的内生菌同时具有影响种子萌发和幼苗生长的潜力[56,83]。
2 内生菌提高植物抗旱性分子机制
干旱胁迫会影响植物生长过程和耐旱相关的基因表达,内生菌也通过调节根系生长、植物激素、代谢过程和抗旱相关基因的表达来增强宿主植物对干旱胁迫的耐受性[8,84]。内生菌通过调节植物细胞渗透、代谢水平及光合作用等相关基因的表达,影响植物生理生化水平,提高植物的耐旱性。而PGPB接种剂则通过影响植物内源激素和代谢产物的产生及抗氧化剂的积累相关的基因表达,进而提高植物的抗旱性(图2)。
2.1 植物共生内生菌响应干旱的基因表达
干旱条件下内生菌群落会发生变化,但不受植物宿主的耐旱水平的影响[91]。
Epichloё属是一种共生在冷季型禾草中的常见内生真菌。Epichloё属及其寄主植物通过协调胁迫反应或单独激活胁迫反应机制,共同作用实现植物-内生菌互相保护[92-93]。Epichloё能显著提高宿主植物的光合速率和生物量,使抗旱基因c51525.graph_c1,c47798.graph_c0和c64087.graph_c0表达水平上调[62]。研究发现,种子传播的内生真菌Epichloё coenophiala通过提高与干旱胁迫耐受性相关的代谢产物含量和编码脱水蛋白和热休克蛋白/蛋白伴侣的基因表达[90],上调参与氧化应激反应、氧自由基解毒、碳水化合物代谢、热休克和细胞转运途径的基因表达来响应应激,进而提高冷季型草坪草高羊茅的耐旱性[60]。
产黄青霉(Penicillium chrysogenum)和短密青霉(Penicillium brevicompactum)是南漆姑(Colobanthus quitensis)根中的优势内生真菌,它们可以调控扩展蛋白基因表达,使扩展蛋白表面产生一个开放的凹槽,从而降低干旱胁迫下南漆姑的氧化应激水平、提高糖和脯氨酸含量、增强CqNCED1,CqABCG25和CqRD22等耐旱基因的表达[85,94]。
许多研究发现内生细菌通过调节宿主植物体内渗透调节物质含量和抗氧化能力来帮助植物抵御干旱条件。比如,根际内生细菌Ochrobactrum sp. EB-165,Microbacterium sp. EB-65,Enterobacter sp. EB-14 和Enterobacter cloacae strain EB-48可以提高脯氨酸积累、细胞渗透调节、相对含水量和细胞膜稳定性指数,同时促进干旱响应基因sbP5CS2和sbP5CS1的上调[67],进而促进植物生长[87]。
2.2 体外培养内生菌响应干旱的基因表达
PGPB接种剂会影响根系内生细菌群落,提高干旱胁迫下植物产量和光合能力[95]。
芽孢杆菌属(Bacillus)是一种常见的植物促生细菌,分布广泛且种类繁多,被广泛用于工业、农业、医学等领域,可以通过调节植物的渗透作用、植物激素水平及代谢以提高植物耐旱性。研究发现,珍珠粟(Pennisetum glaucum L.)内最普遍的耐渗透性内生菌是Bacillus[96]。Bacillus subtilis Dcl1具有耐旱性,基因组测序表明IAA,H2S、乙酰丙酮、丁二醇、鞭毛和铁载体产生的基因与Bacillus subtilis Dcl1的磷酸鹽溶解和生物膜形成有关。此外,甘氨酸甜菜碱、谷氨酸和海藻糖基因的鉴定进一步证明Bacillus subtilis Dcl1具有耐旱特性[97]。内生枯草芽孢杆菌(Bacillus subtilis)可以提高小麦(Triticum aestivum L.)幼苗中TaCTR1基因的表达水平[98],促进小麦内源水杨酸(Salicylic acid,SA)积累,增加SA依赖性防御途径的标记PR-1基因转录物的相对表达水平,改善植物生长并增强耐旱性[99]。短小芽孢杆菌(Bacillus pumilus)会影响乌拉尔甘草(Glycyrrhiza uralensis Fisch.)代谢,提高其根中总黄酮、总多糖和甘草酸的含量,增加甘草酸合成关键酶基因HMGR,SQS和β-AS的表达,通过调节抗氧化剂的积累来改善干旱胁迫下的乌拉尔甘草生长[100]。也有研究发现Bacillus属菌在干旱下会影响植物的抗氧化能力以抵御干旱。解淀粉芽孢杆菌(Bacillus amyloliquefaciens)在干旱、盐碱和重金属胁迫下可以提高辣椒中叶绿素、水杨酸、糖、氨基酸和脯氨酸含量,降低脂质代谢、脱落酸、蛋白质、过氧化氢含量和抗氧化酶活性,还会导致XTH基因表达增强,降低WRKY2,BI-1,PTI1和重链结合蛋白(heavy-chain binding protein,BiP)基因的表达来维持辣椒生长[88]。芥菜(Brassica juncea L.)接种芽孢杆菌后转录因子DREB2和DREB1-2的表达显著上调,淀粉积累减少、H2O2酶活性增强、脂质过氧化降低[49]。
PGPB种类繁多,除Bacillus外,类芽孢杆菌属(Paenibacillus)、节杆菌属(Arthrobacter)等细菌也可以用作提高植物耐旱性的接种剂。Paenibacillus sp. strain B2和Arthrobacter spp. strain AA通过上调小麦防御和细胞渗透、活性氧、茉莉酸、苯基丙酸和植物抗毒素等基因表达,提高小麦的抗病性和耐旱性[101]。干旱胁迫下接种内生菌腐败希瓦氏菌(Shewanella putrefaciens)和都柏林克洛诺斯杆菌(Cronobacter dublinensis)使珍珠粟内IAA,ABA和GA含量显著升高,植物激素生物合成基因SbNCED,SbGA20oX和SbYUC及编码干旱响应基因SbAP2,SbNAC1和PgDREB2A的表达水平增强,提高了珍珠粟的抗旱性[102]。
此外,接种青霉菌属(Penicilium)、拟盾壳霉属(Paraconiothyrium)等真菌和链霉菌属(Streptomyces)等放线菌也能在干旱条件下促进植物生长。干旱胁迫下在豌豆(Pisum sativum L.)种子中接种Penicilium SMCD2206,Paraconiothyrium SMCD2210和Streptomyces sp. SMCD2215可以促进种子萌发、降低植物根部ROS积累水平并下调叶片中脯氨酸、超氧化物歧化酶(SOD)和锰超氧化物歧化酶(MnSOD)基因表达[42]。
3 内生菌提高植物耐盐性分子机制
盐胁迫会限制植物生长发育,影响作物生产和产量[65]。光合作用、气孔导度和激素平衡等植物生理参数的变化可以作为盐胁迫对植物影响的衡量指标。植物-内生菌共生提高了植物的光合速率、光系统II量子效率和RWC,使编码参与根中Na+/K+稳态的膜转运蛋白的基因上调[103]。PGPB也可以缓解盐分对植物的危害[43,103]。植物共生内生菌能影响植物代谢水平、光合作用、抗氧化酶活性、信号转导等基因表达,提高植物对盐胁迫的耐受性。接种PGPB可以影响植物生物合成、内源激素、光合作用、抗氧化酶活性及渗透等相关基因表达,从而减轻盐胁迫对植物的影响(图3)。
3.1 植物共生内生菌响应盐分的基因表达
内生菌能显著提高水稻幼苗的耐盐碱性,通过影响生物合成、能量代谢、酶活性、光合作用、ROS清除系统和激素信号传导等促进其生长[58]。内生真菌在盐胁迫阶段能有效提高植物对盐胁迫逆境耐受能力。
Epichloё内生真菌与醉马草共生体研究是我国禾草内生真菌研究领域的一个重要方向。Epichloё gansuensis作为种子內生真菌,可以与醉马草建立共生关系并赋予其耐盐性,在基因水平上通过影响根中的基因表达调节氨基酸代谢、碳水化合物代谢、TCA循环、二次代谢和脂质代谢的多种途径;在转录水平上影响了醉马草根中胞吐、糖酵解、果糖代谢和钾离子转运等生物过程,并改变了磷酸肌醇代谢、半乳糖代谢、淀粉和蔗糖代谢等代谢途径[40,93]。
Fusarium菌属是生产上较难防治的一种病害菌属,可以侵染多种植物,但研究发现Fusarium菌属可以促进水稻在盐胁迫下的生长,调控参与非生物和生物胁迫耐受、参与信号感知的富含亮氨酸的重复蛋白、受体样激酶等和转导过程中Ca2+和钙调素结合蛋白、转录因子、二次代谢和氧化应激清除的蛋白质有关基因的编码。基因OsIFR,OsWRKY1,OsCAM,OsbHLH和OsORD的转录水平在无内生菌处理的幼苗的根中下调,但在盐胁迫和镰刀菌的存在下上调[57]。
Bacillus具有优良的耐盐特性,可缓解盐胁迫对植物造成的损伤。Bacillus属可以调控植物根中参与细胞运动、Na1转运和固氮及磷酸盐溶解等促生长功能基因的表达从而提高植物耐盐性[107]。高地芽孢杆菌(Bacillus altitudinis)WR10具有高耐盐性,可以上调H+-ATP酶基因表达,减少盐胁迫植物中Na+的积累,并提高K+,P和Ca2+的摄取,在转录水平上提高小麦根中与谷胱甘肽(Glutathione,GSH)生物合成相关的L-抗坏血酸过氧化物酶(Ascorbate peroxidase,APX)、GSH合成酶活性,上调苯丙醇生物合成基因CYP73A,4CL和CAD及脯氨酸脱氢酶基因,下调GSH代谢基因以增加APX活性和GSH水平,降低脯氨酸含量和H2O2水平[68]。
诺卡氏菌(Nocardosis)和Enterobacter常用于临床研究,但也有研究表明这两种细菌可以提高植物耐盐性。Arthrobacter和Nocardosis在盐胁迫下可以上调编码叶绿素a还原酶、肽蛋氨酸(R)-S-氧化物还原酶和K+摄取的基因,参与类胡萝卜素生物合成、苯丙氨酸代谢、苯丙烷类生物合成、甘油脂代谢和氮代谢等途径从而提高植物耐盐性[105]。Enterobacter sp. SA187与拟南芥在盐胁迫下相互作用,改变细菌的碳与能量代谢,上调各种营养物质和代谢产物转运蛋白以及整个硫途径的基因,抑制盐诱导的活性氧物质积累以及LSU突变体的超敏反应,减轻盐胁迫对植物的不良影响[48]。
3.2 体外培养内生菌响应盐分的基因表达
植物根际促生菌(Plant growth-promoting rhizobacteria,PGPR)是一类已被证明能促进植物生长和产量的微生物,被广泛用于多种农业作物以促进植物生长并保护其免受各种胁迫条件的影响[54,108-109]。
Bacillus通过调节离子平衡及渗透调节物质、植物激素和光合色素含量和代谢水平缓解盐胁迫对植物的影响。巨大芽孢杆菌(Bacillus megaterium)ZS-3菌株改善了在重度盐胁迫下拟南芥的生长情况,显著提高拟南芥的生物量、叶绿素含量和类胡萝卜素含量,调节盐胁迫下植物体内渗透物质的含量,上调NHX1和AVP1基因的表达来分离囊泡中的Na+,同时通过下调HKT1基因表达来限制Na+的摄取,激活水杨酸相关基因NPR1和PR1及茉莉酸/乙烯信号通路关键基因AOS,LOX2,PDF1.2和ERF1,从而诱导植物的耐盐性[66]。研究发现,沙福芽胞杆菌(Bacillus safensis)BTL5、海内氏芽孢杆菌(Bacillus haynesii)GTR8、副地衣芽胞杆菌(Bacillus paralicheniformis)GTR11和Bacillus altitudinis GTS16可以降低番茄细胞程序性死亡、增加叶绿素含量、减少活性氧(ROS)积累,调节LKT1,NHX1,SOS1,LePIP2,SlERF16和SlWRKY39等非生物胁迫响应基因的表达进而调节Na+/K+平衡和水稳态,减轻盐胁迫对番茄的影响[45]。此外,耐寒短杆菌(Brevibacterium frigoritolerans)W19和Bacillus safensis BTL5上调SOD1,CATa,NHX1和PAL1这四个耐盐基因的表达,改善了植物在盐胁迫下的生长和发育[86]。盐胁迫下,蜡状芽孢杆菌(Bacillus cereus)显著增加了乌拉尔甘草幼苗的根长和侧根数、上调苯丙醇的生物合成和MVA途径相关的HMGR,β-AS,CHS,LUS,UGAT,CYP72A154,CYP88D6和SE基因的表达水平,增加了甘草酸和甘草次酸的含量[63]。
关于接种其他内生细菌提高植物耐盐性的研究也有很多,例如,从盐生植物地中海滨藜(Atriplex halimus L.)和灰绿针草(Lygeum spartum L.)分离出的内生细菌接种到番茄中会影响与渗透感应、渗透调节和渗透保护的互补机制相关的基因和多种酶抗氧化过程潜在相关的各种基因的表达,减少盐诱导的ROS过度产生,降低盐胁迫对番茄植株的影响[46]。原发节杆菌(Arthrobacter protophormiae,SA3)和纳氏双球菌(Dietzia natronolinaea,STR1)可以提高小麦IAA含量、降低ABA/ACC、调节乙烯信号通路的调节成分CTR1和DREB2转录因子的表达,改善小麦作物耐盐性[98]。在盐胁迫下,接种微白黄链霉菌(Streptomyces albidoflavus)OsiLf-可以降低水稻植株内源ABA含量,增加GSH和脯氨酸和可溶性糖含量,提高光合作用效率和SOD,POD和CAT酶活性,上调光合作用相关基因(OsALAD,OsPSY3,OsatpE)、离子转运相关基因(sSOS1,OsNHX1,OsHKT5)、黄素单加氧酶基因(OsYUCCA1)和生长素外排载体(OsPIN1)基因表达水平,增强了水稻耐盐性,从而提高盐碱条件下的水稻产量[44,89]。
接种内生真菌也可以缓解盐分对植物的胁迫作用。在盐胁迫下接种有益DSE真菌T010后的蓝莓幼苗生长旺盛,根内抗氧化酶活性增强[110],转录激活剂VabZIP12结合G-Box 1和G-Box 2基序后过表达,增加转基因拟南芥中酶促抗氧化剂活性并上调相关基因以增强耐盐性[41]。接种Penicillium breviccompactum和Penicillium chrysogenum可以提高番茄和生菜在盐胁迫条件下的营养素和Na+含量、净光合作用、水分利用效率、产量和存活率,同时上调液泡NHX1 Na+/H+反转运蛋白的表达,提高番茄和生菜的耐盐性[106]。印度梨形孢(Serendipita indica)调控转运蛋白基因SiENA5的表达,降低了拟南芥植物的Na+含量[111]。
4 小结与展望
在干旱和盐胁迫下,内生菌可以调控植物的转录水平、激素及生物合成、抗氧化系统、细胞代谢、信号转导、渗透和光合作用等多种相关基因的表达,使植物积累IAA,ABA,SA等植物激素及脯氨酸等代谢物,抗氧化酶活性提高,植物光合速率加快,生物量增多,从而促进植物生长,提高植物抗逆性。近年来,植物内生菌研究受到国内外学者的广泛关注。尽管对内生菌提高植物的耐旱性和耐盐性的研究已有很多,但具体的分子机制尚有待进一步研究。因此,未来可在以下方面进行进入研究:
1)植物内生菌种类繁多,目前还有许多菌种未被发现,阐明内生菌的多样性有助于了解这些生物活性细菌在寄主植物微生态系统中的功能和潜在作用[112]。从尚未被研究的植物中分离和鉴定内生微生物,可以发现新的物种。
2)同时研究植物共生内生菌和内生菌接种剂对植物的抗逆性的影响,信息互补,可以更全面的了解内生菌的多样性及生物技術潜力。
3)研究已经证实内生菌能够促进植物生长、提高植物对非生物胁迫的耐受性和对生物胁迫的抵抗力,为识别最适合特定环境条件的微生物,还需要深入研究植物-内生菌这种共生模式及其相互作用的分子和生化基础,开发新的生物接种剂从而应用到农业生产中。
4)植物内生菌对植物的影响在人工实验室、温室和田间试验中有所不同,因此,有必要开展田间试验,真正了解微生物在农业系统中的作用。
5)用组学技术研究内生菌之间的协同或拮抗作用和内生菌与植物协同或拮抗作用也有利于内生菌生物接种剂的开发,实现农业可持续发展。
参考文献
[1]PETRINI O. Fungal endophytes of tree leaves[C]//ANDREWS J H,HIRANO S S. Microbial Ecology of Leaves.New York:Springer-Verlag New York Inc.,1991:179-197
[2]BARMAN D,BHATTACHARJEE K. Endophytic bacteria associated with medicinal plants:The treasure trove of antimicrobial compounds[C]//EGAMBERDIEVA D,TIEZZI A. Medically Important Plant Biomes:Source of Secondary Metabolites. Singapore:Springer Singapore,2019:153-187
[3]CHEN G,ZHANG X Y,ZHAO T. Endophytes of terrestrial plants:A potential source of bioactive secondary metabolites[J]. Journal of Food and Nutrition Research,2020,8(7):362-377
[4]STROBEL G A. Endophytes as sources of bioactive products[J]. Microbes and Infection,2003,5(6):535-544
[5]OUKALA N,AISSAT K,PASTOR V. Bacterial endophytes:The hidden actor in plant immune responses against biotic stress[J]. Plants-Basel,2021,10(5):1012
[6]HEWITT K G,POPAY A J,HOFMANN R W,et al. Epichloё a lifeline for temperate grasses under combined drought and insect pressure[J]. Grass Research,2021,1(1):1-12
[7]LANGRIDGE P,REYNOLDS M. Breeding for drought and heat tolerance in wheat[J]. Theoretical and Applied Genetics,2021,134:1753-1769
[8]DE VRIES F T,GRIFFITHS R I,KNIGHT C G,et al. Harnessing rhizosphere microbiomes for drought-resilient crop production[J]. Science,2020,368(6488):270-274
[9]OLDROYD G E D,DOWNIE J A. Coordinating nodule morphogenesis with rhizobial infection in legumes[J]. Annual Review of Plant Biology,2008,59:519-546
[10]PAPIK J,FOLKMANOVA M,POLIVKOVA-MAJOROVA M M,et al. The invisible life inside plants:Deciphering the riddles of endophytic bacterial diversity[J]. Biotechnology Advances,2020,44:107614
[11]DINI-ADREOTE F. Endophytes:The second layer of plant defense[J]. Trends in Plant Science,2020,25(4):319-322
[12]MOGHADDAM M S H,SAFAIE N,SOLTANI J,et al. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops[J]. Plant Physiology and Biochemistry,2021,160:225-238
[13]MORSY M,CLECKLER B,ARMUELLES-MILICAN H. Fungal endophytes promote tomato growth and enhance drought and salt tolerance[J]. Plants-Basel,2020,9(7):877
[14]金忠民,李春月,刘本松,等. 菌株JB12影响铅镉胁迫下菊苣黄酮合成的轉录组分析[J]. 草地学报,2023,31(6):1648-1655
[15]KANG P,FANG X,HU J,et al. Branch lignification of the desert plant Nitraria tangutorum altered the structure and function of endophytic microorganisms[J]. Agronomy,2022,13(1):90
[16]BATSTONE R T,OBRIEN A M,HARRISON T L,et al. Experimental evolution makes microbes more cooperative with their local host genotype[J]. Science,2020,370(6515):476-478
[17]MANIAS D,VERMA A,SONI D K. Microbial Endophytes[M]. United Kingdom:Woodhead Publishing,2020:1-14
[18]ZHU J K. Abiotic stress signaling and responses in plants[J]. Cell,2016,167(2):313-324
[19]ZHU J K. Salt and drought stress signal transduction in plants[J]. Annual Review of Plant Biology,2002,53(1):247-273
[20]AMOAH J N,KO C S,YOON J S,et al. Effect of drought acclimation on oxidative stress and transcript expression in wheat (Triticum aestivum L.)[J]. Journal of Plant Interactions,2019,14(1):492-505
[21]OLESKA E,MALEK W,WOJCIK M,et al. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions:A methodical review[J]. Science of the Total Environment,2020,743:140682
[22]GLICK B R. Plant growth-promoting bacteria:mechanisms and applications[J]. Scientifica,2012,2012(5):963401
[23]CABOT C,SIBOLE J V,BARCELO J,et al. Lessons from crop plants struggling with salinity[J]. Plant Science,2014,226:2-13
[24]ARAUJO W L,MACCHEROI JR W,AGUILAR-VILDOSO C I,et al. Variability and interactions between endophytic bacteria and fungi isolated from leaf tissues of citrus rootstocks[J]. Canadian Journal of Microbiology,2001,47(3):229-236
[25]VISHWAKARMA K,KUMAR N,SHANDILYA C,et al. Unravelling the role of endophytes in micronutrient uptake and enhanced crop productivity[J]. Symbiotic Soil Microorganisms:Biology and Applications,2021,60:63-85
[26]BARRERA M C,JAKOBS-SCHOENWANDT D,GOMEZ M I,et al. Formulating bacterial endophyte:Pre-conditioning of cells and the encapsulation in amidated pectin beads[J]. Biotechnology Reports,2020,26:e00463
[27]RANA K L,KOUR D,KAUR T,et al. Endophytic microbes:biodiversity,plant growth-promoting mechanisms and potential applications for agricultural sustainability[J]. Antonie Van Leeuwenhoek,2020,113:1075-1107
[28]RANA K L,KOUR D,KAUR T,et al. Endophytic microbes from diverse wheat genotypes and their potential biotechnological applications in plant growth promotion and nutrient uptake[J]. Proceedings of the National Academy of Sciences,India Section B:Biological Sciences,2020,90:969-979
[29]KHALIL A M A,HASSAN S E D,ALSHARIF S M,et al. Isolation and characterization of fungal endophytes isolated from medicinal plant Ephedra pachyclada as plant growth-promoting[J]. Biomolecules,2021,11(2):140
[30]ZHANG J,ZHU Y,SI J,et al. Metabolites of medicine food homology-derived endophytic fungi and their activities[J]. Current Research in Food Science,2022,5:1882-1896
[31]ZHENG R Y,JIANG H. Rhizomucor endophyticus sp. nov.,an endophytic zygomycetes from higher plants[J]. Mycotaxon,1995,56:455-466
[32]AHMED A M,MAHMOUD B K,MILLAN-AGUINAGA N,et al. The endophytic Fusarium strains:a treasure trove of natural products[J]. RSC Advances,2023,13(2):1339-1369
[33]MOISSL-EICHINGER C,PAUSAN M,TAFFNER J,et al. Archaea are interactive components of complex microbiomes[J]. Trends in Microbiology,2018,26(1):70-85
[34]DE LISE F,IACONO R,MORACCI M,et al. Archaea as a model system for molecular biology and biotechnology[J]. Biomolecules,2023,13(1):114
[35]BAKER B J,DE ANDA V,SEITZ K W,et al. Diversity,ecology and evolution of Archaea [J]. Nature Microbiology, 2020,5(7):887-900
[36]EME L,SPAG A,LOMBARD J,et al. Archaea and the origin of eukaryotes[J]. Nature Reviews Microbiology,2017,15(12):711-723
[37]STRAUB C T,COUNTS J A,NGUYEN D M N,et al. Biotechnology of extremely thermophilic archaea[J]. FEMS Microbiology Reviews,2018,42(5):543-578
[38]CHEN F,QI Y,JIANG B,et al. Metalaxyl-resistant mutant strains of Phytophthora boehmeriae are as aggressive and fit as their metalaxyl-sensitive wild-type parents[J]. Tropical Plant Pathology,2023:48,128-138
[39]WANG T,GAO C,CHENG Y,et al. Molecular diagnostics and detection of oomycetes on fiber crops[J]. Plants-Basel,2020,9(6):769
[40]WANG C,HUANG R,WANG J,et al. Comprehensive analysis of transcriptome and metabolome elucidates the molecular regulatory mechanism of salt resistance in roots of Achnatherum inebrians mediated by Epichloё gansuensis[J]. Journal of Fungi,2022,8(10):1092
[41]QU D,WU F,ZHAO X,et al. A bZIP transcription factor VabZIP12 from blueberry induced by dark septate endocyte improving the salt tolerance of transgenic Arabidopsis[J]. Plant Science,2022,315:111135
[42]KUMARI V,VUJANOVIC V. Transgenerational benefits of endophytes on resilience and antioxidant genes expressions in pea (Pisum sativum L.) under osmotic stress[J]. Acta Physiologiae Plantarum,2020,42:1-11
[43]KASHYAP B K,ARA R,SINGH A,et al. Halotolerant PGPR bacteria:Amelioration for salinity stress[J]. Microbial Interventions in Agriculture and Environment,2019,28:509-530
[44]NIU S,GAO Y,ZI H,et al. The osmolyte-producing endophyte Streptomyces albidoflavus OsiLf-2 induces drought and salt tolerance in rice via a multi-level mechanism[J]. The Crop Journal,2022,10(2):375-386
[45]SAHU P K,SINGH S,SINGH U B,et al. Inter-genera colonization of Ocimum tenuiflorum endophytes in tomato and their complementary effects on Na+/K+ balance,oxidative stress regulation,and root architecture under elevated soil salinity[J]. Frontiers in Microbiology,2021,12:744733
[46]DIF G,BELAOUNI H A,YEKKOUR A,et al. Performance of halotolerant bacteria associated with Sahara-inhabiting halophytes Atriplex halimus L. and Lygeum spartum L. ameliorate tomato plant growth and tolerance to saline stress:from selective isolation to genomic analysis of potential determinants[J]. World Journal of Microbiology and Biotechnology,2022,38(1):16
[47]WU T,LI X,XU J,et al. Diversity and functional characteristics of endophytic bacteria from two grass species growing on an oil-contaminated site in the Yellow River Delta,China[J]. Science of The Total Environment,2021,767:144340
[48]ANDRES-BARRAO C,ALZUBAIDY H,JALAL R,et al. Coordinated bacterial and plant sulfur metabolism in Enterobacter sp. SA187-induced plant salt stress tolerance[J]. Proceedings of the National Academy of Sciences,2021,118(46):e2107417118
[49]BADEPPA S,PAUL S,THAKUR J K,et al. Antioxidant,physiological and biochemical responses of drought susceptible and drought tolerant mustard (Brassica juncea L) genotypes to rhizobacterial inoculation under water deficit stress[J]. Plant Physiology and Biochemistry,2019,143:19-28
[50]AHMAD M,NASEER I,HUSSAIN A,et al. Appraising endophyte-plant symbiosis for improved growth,nodulation,nitrogen fixation and abiotic stress tolerance:An experimental investigation with chickpea (Cicer arietinum L.)[J]. Agronomy,2019,9(10):621
[51]TAULE C,VAZ-JAURI P,BATTISTONI F. Insights into the early stages of plant-endophytic bacteria interaction[J]. World Journal of Microbiology and Biotechnology,2021,37:1-9
[52]COMPAT S,CLEMET C,SESSITSCH A. Plant growth-promoting bacteria in the rhizo-and endosphere of plants:their role,colonization,mechanisms involved and prospects for utilization[J]. Soil Biology and Biochemistry,2010,42(5):669-678
[53]FRANK A C,SALDIERNA G J P,SHAY J E. Transmission of bacterial endophytes[J]. Microorganisms,2017,5(4):70
[54]NADEEM S M,AHMAD M,ZAHIR Z A,et al. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments[J]. Biotechnology advances,2014,32(2):429-448
[55]CHIELLINI C,DE LEO M,LONGO V,et al. Characterization of the endophytic bacterial community of Bituminaria bituminosa plant grown in vitro and its interaction with the plant extract[J]. Frontiers in Plant Science,2022,13:1076573
[56]TYC O,PUTRA R,GOLS R,et al. The ecological role of bacterial seed endophytes associated with wild cabbage in the United Kingdom[J]. Microbiology Open,2020,9(1):e00954
[57]SAMPAGI-RAMAIAH M H,DEY P,JAMAGI S,et al. An endophyte from salt-adapted Pokkali rice confers salt-tolerance to a salt-sensitive rice variety and targets a unique pattern of genes in its new host[J]. Scientific Reports,2020,10(1):1-14
[58]REN X,SHAN Y,LI X,et al. Application of RNA sequencing to understand the benefits of endophytes in the salt-alkaline resistance of rice seedlings[J]. Environmental and Experimental Botany,2022,196:104820
[59]NAGABHYRU P,DINKINS R D,SCHARDL C L. Transcriptomics of Epichloё-grass symbioses in host vegetative and reproductive stages[J]. Molecular Plant-Microbe Interactions,2019,32(2):194-207
[60]LEUCHTMANN A,BACON C W,SCHARDL C L,et al. Nomenclatural realignment of Neotyphodium species with genus Epichloё[J]. Mycologia,2014,106(2):202-215
[61]EGAMBERDIEVA D,ALIMOV J,SHURIGIN V,et al. Diversity and plant growth-promoting ability of endophytic,halotolerant bacteria associated with Tetragonia tetragonioides (Pall.) Kuntze[J]. Plants-Basel,2021,11(1):49
[62]ZHONG R,BASTIAS D A,ZHANG X,et al. Vertically transmitted Epichloё systemic endophyte enhances drought tolerance of Achnatherum inebrians host plants through promoting photosynthesis and biomass accumulation[J]. Journal of Fungi,2022,8(5):512
[63]ZHANG Y,LANG D,ZHANG W,et al. Bacillus cereus enhanced medicinal ingredient biosynthesis in Glycyrrhiza uralensis Fisch. under different conditions based on the transcriptome and polymerase chain reaction analysis[J]. Frontiers in Plant Science,2022,13:858000
[64]KHALID M,RAHMAN S,HUANG D. Molecular mechanism underlying Piriformospora indica-mediated plant improvement/protection for sustainable agriculture[J]. Acta Biochimica et Biophysica Sinica,2019,51(3):229-242
[65]YOUSEFIRAD S,SOLTANLOO H,RAMEZANPOUR S S,et al. The RNA-seq transcriptomic analysis reveals genes mediating salt tolerance through rapid triggering of ion transporters in a mutant barley[J]. Plos One,2020,15(3):e0229513
[66]SHI L N,LU L X,YE J R,et al. The endophytic strain ZS-3 enhances salt tolerance in Arabidopsis thaliana by regulating photosynthesis,osmotic stress,and ion homeostasis and inducing systemic tolerance[J]. Frontiers in Plant Science,2022,13:820837
[67]GOVINDASAMY V,GEORGE P,KUMAR M,et al. Multi-trait PGP rhizobacterial endophytes alleviate drought stress in a senescent genotype of sorghum [Sorghum bicolor (L.) Moench][J]. 3 Biotech,2020,10(1):1-14
[68]YUE Z,CHEN Y,WANG Y,et al. Halotolerant Bacillus altitudinis WR10 improves salt tolerance in wheat via a multi-level mechanism[J]. Frontiers in Plant Science,2022,13:2502
[69]LI F,HE X,SUN Y,et al. Distinct endophytes are used by diverse plants for adaptation to karst regions[J]. Scientific Reports,2019,9(1):1-9
[70]WANG Z,ZHU Y,LI N,et al. High-throughput sequencing-based analysis of the composition and diversity of endophytic bacterial community in seeds of saline-alkali tolerant rice[J]. Microbiological Research,2021,250:126794
[71]DALE J C M,NEWMAN J A. A First Draft of the Core Fungal Microbiome of Schedonorus arundinaceus with and without Its Fungal Mutualist Epichloё coenophiala[J]. Journal of Fungi,2022,8(10):1026
[72]GEDDES-MCALISTER J,SUKUMARAN A,PATCHETT A,et al. Examining the impacts of CO2 concentration and genetic compatibility on perennial ryegrass—Epichloё festucae var lolii interactions[J]. Journal of Fungi,2020,6(4):360
[73]熊海琳,毛培春,田小霞,等. 接種根瘤菌对林下红三叶草产量与品质及土壤特性影响[J]. 草地学报,2023,31(11):3561-3568
[74]BRIGIDO C,SINGH S,MENENDEZ E,et al. Diversity and functionality of culturable endophytic bacterial communities in chickpea plants[J]. Plants-Basel,2019,8(2):42
[75]YANG B,WANG X M,MA H Y,et al. Fungal endophyte Phomopsis liquidambari affects nitrogen transformation processes and related microorganisms in the rice rhizosphere[J]. Frontiers in microbiology,2015,6:982
[76]ZHANG W,SUN K,SHI R H,et al. Auxin signalling of Arachis hypogaea activated by colonization of mutualistic fungus Phomopsis liquidambari enhances nodulation and N2-fixation[J]. Plant Cell and Environment,2018,41(9):2093-2108
[77]LIN H,LIU C,PENG Z,et al. Distribution pattern of endophytic bacteria and fungi in tea plants[J]. Plant Microbiome:Diversity,Functions,and Applications,2022,13:872034
[78]CHEN D W,WANG Y H,SHI W J,et al. Analysis of endophyte diversity of Rheum palmatum among different tissues and ages[J]. Archives of Microbiology,2023,205(1):14
[79]HOU Q Z,CHEN D W,WANG Y,et al. Analysis of endophyte diversity of Gentiana officinalis among different tissue types and ages and their association with four medicinal secondary metabolites[J]. PeerJ,2022,10:e13949
[80]COMPANT S,REITER B,SESSITSCH A,et al. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN[J]. Applied and Environmental Microbiology,2005,71(4):1685-1693
[81]NELSON E B. The seed microbiome:origins,interactions,and impacts[J]. Plant and Soil,2018,422:7-34
[82]SULLIVAN T J,ROBERTS H,BULTMAN T L. Genetic Covariation Between the Vertically Transmitted Endophyte Epichloё canadensis and Its Host Canada Wildrye[J]. Microbial Ecology,2023,86(3):1686-1695
[83]SHADE A,JACQUES M A,BARRET M. Ecological patterns of seed microbiome diversity,transmission,and assembly[J]. Current Opinion in Microbiology,2017,37:15-22
[84]LI X,HE C,HE X,et al. Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development[J]. Plant and Soil,2019,439:259-272
[85]HEREME R,MORALES-NAVARRO S,BALLESTEROS G,et al. Fungal endophytes exert positive effects on Colobanthus quitensis under water stress but neutral under a projected climate change scenario in Antarctica[J]. Frontiers in Microbiology,2020,11:264
[86]GUPTA A,TIWARI R,SHUKLA R,et al. Salinity alleviator bacteria in rice (Oryza sativa L.),their colonization efficacy,and synergism with melatonin[J]. Frontiers in Plant Science,2022,13:1060287
[87]GOVINDASAMY V,RAINA S K,GEORGE P,et al. Functional and phylogenetic diversity of cultivable rhizobacterial endophytes of sorghum[Sorghum bicolor (L.) Moench][J]. Antonie van Leeuwenhoek,2017,110:925-943
[88]KAZEROONI E A,MAHARACHCHIKUMURA S S N,ADHIKARI A,et al. Rhizospheric Bacillus amyloliquefaciens protects Capsicum annuum cv. Geumsugangsan from multiple abiotic stresses via multifarious plant growth-promoting attributes[J]. Frontiers in Plant Science,2021,12:669693
[89]KHAN M A,ASAF S,KHAN A L,et al. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants[J]. Plant Biology,2020,22(5):850-862
[90]DINKINS R D,NAGAHYRU P,YOUNG C A,et al. Transcriptome analysis and differential expression in tall fescue harboring different endophyte strains in response to water deficit[J]. The Plant Genome,2019,12(2):180071
[91]PANKE-BUISSE K,CHENG L,GAN H,et al. Root fungal endophytes and microbial extracellular enzyme activities show patterned responses in tall fescues under drought conditions[J]. Agronomy,2020,10(8):1076
[92]NAGABHYRU P,DINKINS R D,SCHARDL C L. Transcriptome analysis of Epichloё strains in tall fescue in response to drought stress[J]. Mycologia,2022,114(4):697-712
[93]WANG J,HOU W,CHRISTENSEN M J,et al. Role of Epichloё endophytes in improving host grass resistance ability and soil properties[J]. Journal of Agricultural and Food Chemistry,2020,68(26):6944-6955
[94]MORALES-QUINTANA L,BARRERA A,HEREME R,et al. Molecular and structural characterization of expansins modulated by fungal endophytes in the Antarctic Colobanthus quitensis (Kunth) Bartl. exposed to drought stress[J]. Plant Physiology and Biochemistry,2021,168:465-476
[95]YAGHOUBI K M,CRECCHIO C,VERBRUGGEN E. Shifts in the rhizosphere and endosphere colonizing bacterial communities under drought and salinity stress as affected by a biofertilizer consortium[J]. Microbial Ecology,2022,84(2):483-495
[96]MANJUNATHA B S,PAUL S,AGGARWAL C,et al. Diversity and tissue preference of osmotolerant bacterial endophytes associated with pearl millet genotypes having differential drought susceptibilities[J]. Microbial Ecology,2019,77(3):676-688
[97]JAYAKUMAR A,NAIR I C,RADHAKRISHNAN E K. Environmental adaptations of an extremely plant beneficial Bacillus subtilis Dcl1 identified through the genomic and metabolomic analysis[J]. Microbial Ecology,2021,81:687-702
[98]BARAWAL D,BHARTI N,PADEY S S,et al. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression[J]. Physiologia Plantarum,2017,161(4):502-514
[99]LASTOCHKINA O,IVANOV S,PETROVA S,et al. Role of Endogenous Salicylic Acid as a Hormonal Intermediate in the Bacterial Endophyte Bacillus subtilis-Induced Protection of Wheat Genotypes Contrasting in Drought Susceptibility under Dehydration[J]. Plants-Basel,2022,11(23):3365
[100]XIE Z,CHU Y,ZHANG W,et al. Bacillus pumilus alleviates drought stress and increases metabolite accumulation in Glycyrrhiza uralensis Fisch[J]. Environmental and Experimental Botany,2019,158:99-106
[101]SAMAIN E,ERNENWEIN C,AUSSEAC T,et al. Effective and durable systemic wheat-induced resistance by a plant-growth-promoting rhizobacteria consortium of Paenibacillus sp. strain B2 and Arthrobacter spp. strain AA against Zymoseptoria tritici and drought stress[J]. Physiological and Molecular Plant Pathology,2022,119:101830
[102]MANJUNATHA B S,NIVETHA N,KRISHNA G K,et al. Plant growth-promoting rhizobacteria Shewanella putrefaciens and Cronobacter dublinensis enhance drought tolerance of pearl millet by modulating hormones and stress-responsive genes[J]. Physiologia Plantarum,2022,174(2):e13676
[103]CHEN J,ZHANG H,ZHANG X,et al. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis,water status,and K+/Na+ homeostasis[J]. Frontiers in Plant Science,2017,10(8):1739
[104]BAKHSHANDEH E,GHOLAMHOSSEINI M,YAGHOUBIAN Y,et al. Plant growth promoting microorganisms can improve germination,seedling growth and potassium uptake of soybean under drought and salt stress[J]. Plant Growth Regulation,2020,90:123-136
[105]DONG Z Y,RAO M P N,WANG H F,et al. Transcriptomic analysis of two endophytes involved in enhancing salt stress ability of Arabidopsis thaliana[J]. Science of the Total Environment,2019,686:107-117
[106]MOLINA-MONTENEGRO M A,ACUNA R I S,TORRES D C,et al. Antarctic root endophytes improve physiological performance and yield in crops under salt stress by enhanced energy production and Na+ sequestration[J]. Scientific Reports,2020,10(1):1-10
[107]ZHENG Y,XU Z,LIU H,et al. Patterns in the microbial community of salt-tolerant plants and the functional genes associated with salt stress alleviation[J]. Microbiology Spectrum,2021,9(2):e0076721
[108]张银翠,姚拓,赵桂琴,等. 耐盐促生菌筛选鉴定及对盐胁迫燕麦生长的影响[J]. 草地学报,2021,29(12):2645-2652
[109]BARAWAL D,BHARTI N,MAJI D,et al. 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation[J]. Plant Physiology and Biochemistry,2012,58:227-235
[110]WU F L,LI Y,TIAN W,et al. A novel dark septate fungal endophyte positively affected blueberry growth and changed the expression of plant genes involved in phytohormone and flavonoid biosynthesis[J]. Tree Physiology,2020,40(8):1080-1094
[111]LANZA M,HARO R,CONCHILLO L B,et al. The endophyte Serendipita indica reduces the sodium content of Arabidopsis plants exposed to salt stress:Fungal ENA ATPases are expressed and regulated at high pH and during plant co-cultivation in salinity[J]. Environmental Microbiology,2019,21(9):3364-3378
[112]XU W,WANG F,ZHANG M,et al. Diversity of cultivable endophytic bacteria in mulberry and their potential for antimicrobial and plant growth-promoting activities[J]. Microbiological Research,2019,229:126328
(責任编辑 闵芝智)
