Anti-obesity effects of fucoidan from Sargassum thunbergii in adipocytes and high fat diet induced obese mice through inhibiting adipogenic specif ic transcription factor
2024-01-24HyoGeunLeeJywrhnFengqiYngNghwththLiyngeKyungMoSongYunSngChoiSeungHongLeeYouJinJeonMinCheolKng
Hyo-Geun Lee, H.H.A.C.K. Jywrhn, Fengqi Yng, D.P. Nghwthth, N.M. Liynge,Kyung-Mo Song, Yun-Sng Choi, Seung-Hong Lee, You-Jin Jeon,, Min-Cheol Kng,
a Department of Marine Life Sciences, Jeju National University, Jeju 63243, Republic of Korea
b Marine Science Institute, Jeju National University, Jeju 63333, Republic of Korea
c Research Group of Food Processing, Korea Food Research Institute, Wanju 55365, Republic of Korea
d Department of Pharmoceutical Engineering, Soonchunhyang University, Asan 31538, Republic of Korea
Keywords:Sargassum thunbergii Fucoidan Anti-obesity Anti-steatosis
ABSTRACT The prevalence of obesity has increased and is a health concern worldwide. Due to the concerns regarding synthetic anti-obesity treatments, nowadays natural products become a trend. Previous studies proved that there is a potential to use marine algae as anti-obesity agents. Therefore, in this study, the lipid inhibitory effect of crude polysaccharide of amyloglucosidase-assisted hydrolysate from Sargassum thunbergii (STAC)and its fucoidan fractions (STAFs) on 3T3-L1 cells and high-fat diet (HFD)-induced obese mice were investigated. According to the results, the STAF3, showed the highest xylose content and exhibited signif icant inhibitory effects on lipid accumulation by downregulating adipogenic and lipogenic proteins in 3T3-L1 cells.Furthermore, oral supplementation with STAC significantly declined gain in body weight and fat weight,and serum lipid contents in an HFD-induced obesity mouse model. Structural and chemical characterizations demonstrated that purified STAF3 has consistent surface morphology and small particle size, with similar structural characteristics as commercial fucoidan. Together, these results indicate that STAC and purified STAF3 from Sargassum thunbergia is a potent source to develop as ananti-obesity agents or functional food products to counter obesity.
1. Introduction
Cases of obesity and obesity-related issues have increased worldwide during the past few decades. Moreover, global obesity rates among children and adolescents have increased rapidly[1].Obesity is a group of complicated metabolic diseases characterized by increased body weight, high lipid content, and an inclination towards the secretion of detrimental hormones from adipose tissues. Excessive accumulation of body fat in overweight or obese conditions exerts negative effects and its highly increased the incidence of metabolic diseases such as type 2 diabetes, cardiovascular disease, non-alcoholic fatty liver disease, neurodegenerative disease[2]. Therefore, a reduction in the amount of food ingested, lipid accumulation, and lipogenesis or intake of antiobesity agents may help reduce obesity. Currently,synthetic anti-obesity agents such as orlistat and sibutramine are used to treat obesity. However, continuous use of these synthetic antiobesity agents may precipitate undesirable side effects. The most frequently reported side effect of sibutramine is the gastrointestinal disturbance, leading to steatorrhea with excessive flatulence and a number of severe, adverse, hepatic disorders (hepatic failure, necrosis,and cholestatic hepatitis)[3]. Accordingly, the development of new,natural anti-obesity agents against obesity and its complications is warranted.
Adipogenesis is a cellular differentiation process that occurs in adipocytes. During the adipogenic process, the morphologic characteristics of preadipocytes are altered and preadipocytes are finally transformed into mature adipocytes that store lipids. The process of adipogenesis is regulated by adipogenic and lipogenic protein expression as described previously[4]. Therefore, adipogenesis and lipogenesis are markedly affected by adipogenic and lipogenic proteins. Moreover, sterol regulatory element-binding protein 1(SREBP-1), a lipogenic protein, plays an important role in lipid accumulation in adipocytes. Additionally, previous studies have shown that fatty acid binding protein 4 (FABP4), a lipid transport protein, regulated by adipogenic peroxisome proliferator-activated receptor γ (PPAR-γ) levels[5]and lipogenic SREBP-1, induces high PPAR-γ expression, which facilitates lipid biosynthesis during adipocyte differentiation[6]. These proteins regulate lipid storage and synthesis in adipocytes by interacting with each other[7]. Therefore,dual targeting of adipogenic and lipogenic proteins is an effective strategy that may be used to reduce lipid accumulation responsible for obesity.
Sargassumthunbergiiis a common brown alga, found in the intertidal zone. It is widely distributed in East Asia (China,Korea, and Japan). Previous studies have described the respective effects of the bioactive components found inS. thunbergii, such as polysaccharides, lipids, and phenolic compounds[8]. Among them,sulfated polysaccharide fromSargassumspecies show hypolipidemic and antiobesity activities[9-10]. However, there is a lack of adequate knowledge on the structural characteristics and antiobesity activity of the compounds and fewer reports on their lipid inhibitory activity followed by chemical and molecular characteristic changes of polysaccharide fromS. thunbergii. Thus, the objective of this study was to investigate the potent antiobesity effects of crude polysaccharide of amyloglucosidase (AMG)-assisted hydrolysate fromS. thunbergii(STAC) and the purified fucoidan fractions of AMG-assisted hydrolysate fromS. thunbergii(STAFs)in vitroandin vivo.
2. Materials and methods
2.1 Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), bovine serum (BS), phosphate-buffered saline (PBS,pH 7.4), and penicillin-streptomycin (PS) were purchased from GIBCO-BRL (Grand Island, NY, USA). Cell differentiation reagents,including isobutyl-1-methylxanthine (IBMX), dexamethasone,and insulin were purchased from Sigma Chemical Co. (St. Louis,MO, USA). Serum lipid profiles (triglyceride and cholesterol) were analyzed using a colorimetric kit purchased from Abcam (Cambridge,MA, USA). All chemicals and reagents used were of analytical grade and obtained from commercial sources.
2.2 Preparation of enzyme-assisted hydrolysate and its crude polysaccharide from S. thunbergii
S. thunbergiispecimens were collected from the coastline of Jeju Island, South Korea, in March 2017. The collected specimens were carefully washed using tap water to remove the surrounding salt,sand, and epiphytes attached to the surface. The washed specimens were frozen in a deep freezer and maintained at –80 °C. The frozen specimens were freeze-dried and homogenized with a grinder prior to enzyme extraction. Enzyme-assisted extraction was conducted using the method described by Wijesinghe et al.[11]with slight modifications. Briefly, driedS. thunbergii(100 g) was mixed with 1 L distilled water and 100 μL AMG. Then, the mixture was extracted in a shaker for 24 h under optimal extraction conditions (50 °C, pH 4.5).Next, the mixture was placed in a 100 °C water bath to inactivate enzyme activity, and pH was adjusted to a final value of 7.00. The mixture was then clarified via centrifugation (12 000 r/min, 10 min,4 °C) to remove any residue. The supernatant was filtered through Whatman No. 4 filter paper and was subjected to precipitation with double volumes of 99.5% ethanol overnight at 4 °C to collect a crude polysaccharide. The precipitate was collected by centrifugation and referred to as STAC. Finally, STAC was lyophilized for further purification steps.
2.3 Purification of fucoidans from STAC using DEAEs ionexchange chromatography
Isolation and purification of fucoidans and sulfated polysaccharide were performed using methods described by Kang et al.[12]. To purify the fucoidan fractions, STAC was dissolved in distilled water and passed through a 0.45 μm pore size syringe filter. The filtrate was then applied to DEAE-cellulose, ion-exchange chromatography.The DEAE separation was performed with gradient elution of NaCl solution at a flow rate of 2 mL/min. Initially, the DEAE column was equilibrated using 50 mmol/L sodium acetate. Next, separation was performed with a linear gradient up to 2.0 mol/L NaCl (0, 50, 100,200, 400, 800, 1 000, and 2 000 mmol/L) after sample loading. The four polysaccharide fractions were identified using DEAE-cellulose and ion-exchange chromatography. The purified polysaccharide fractions were applied to dialysis membranes (Spectra/Por USA) to nullify the effect of sodium chloride on toxicity. Dialysis steps were repeated several times until conductivity reached the value of distilled water.
2.4 Proximate composition analysis
The chemical compositions of STAC and the STAFs were analyzed following the AOAC method[13]. The total polysaccharide content was determined via the phenol-sulfuric acid method described by Nielsen[14]. The polyphenol content was determined using the Folin-Ciocalteu method[15]. Sulfate contents determined by the barium chloride gelatin method from Dodgson et al.[16]with slight modifications. Protein levels determined by BCA protein assay kit(Bio-Rad, USA) followed by manufacturers protocol.
2.5 Multi angle light scattering (MALS) analysis
To determine the averaged molecular weights of STAC and its STAFs, the MALS analysis performed using the DAWN Heleos II(Wyatt Technology, CA, USA) system connected with an Optilab T-rEX refractive index detector (MALS-RI Wyatt Technology, Santa Barbara, CA, USA). In brief, each sample was dissolved in 500 mmol/L NaCl, and syringe filtered. The filtrate was placed into a separation column. The separation column temperature was set at 25 °C and it was separated via gradient 500 mmol/L NaCl elution at a 0.5 mL/min flow rate for 50 min. The MALS results were analyzed with ASTRA 6 software (Wyatt Technologies, Santa Barbara, CA, USA).
2.6 Fourier transform infrared (FTIR) analysis
The structural properties of STAC and STAF3 were analyzed using an FTIR spectrometer (Thermo scientific, NicoletTM6700,MA, USA). The analytic samples intended for analysis were mixed and ground with potassium bromide (KBr). The mixtures were then pressed to form a disk-shaped FTIR pellet that was applied to the FTIR spectrometer. FTIR spectra were observed under a wave number of 500–2 000 cm–1.
2.7 Nuclear magnetic resonance (NMR) analysis
The1H NMR spectrum of STAF3 was obtained using the JEOL JNM-ECX400 NMR spectrometer operated at 400 MHz (JEOL,Tokyo, Japan). The STAF3 was dissolved in deuterium oxide and dried several times for deuterium exchange. Then, the dried STAF3 was re-dissolved in deuterated methanol for NMR analysis. A proton NMR spectrum was obtained with 64 scans.
2.8 Cytotoxicity
Cytotoxic effects were determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT) assay. The 3T3-L1 cells were seeded into 96-well plates at a concentration of 1 × 105cells/well and subjected to subsequent treatment and incubation with different concentrations of samples for 48 h. Thereafter, MTT solution (2 mg/mL) was added to the cell culture medium and incubated for an additional 2–3 h. Formazan crystals, which are produced by viable cells, were dissolved in DMSO and detected using a Synergy HT Multi-Detection microplate reader (BioTek Instruments, Winooski, VT, USA). Cell viability was calculated by measuring the relative formazan crystal content. Crystal violet content was measured using a microplate reader at 540 nm.
2.9 Cell culture and cell differentiation
The 3T3-L1 cells were obtained from the American Type Culture Collection (ATCC, CL-173, Rockville, MD, USA) and cultured in DMEM supplemented with 10% of BS and 1% PS under optimal cell culture conditions (37 °C, 5% CO2, humidified). The 3T3-L1 cells were plated in 12-well plates (1 × 105cells/well). After 2 days, at 90%–100% confluence, adipogenic differentiation was induced in MDI solution [insulin (5 μg/mL), IBMX (0.5 mmol/L), and dexamethasone (0.25 μmol/L)] for 2 days. The adipocyte culture medium was replaced with fresh DMEM at every two-day interval,and the tested samples were also treated at 2-day intervals for 8 days.
2.10 Quantification of lipid accumulation by Oil Red O(ORO) staining
ORO staining was used to determine potential lipid inhibitory effects of the purified polysaccharides. The 3T3-L1 cells were seeded at a concentration of 1 × 105cells/wells in a 12-well plate for 48 h,subjected to differentiation as per previously described methods[17],fixed with 10% formalin for 1–2 h, and washed twice with a 60%isopropanol solution. Then, the plates were dried at 25 °C. After drying, the cells were stained with the ORO solution for 1–2 h.Thereafter, the ORO solution was removed, and cells were washed several times using distilled water. Images of stained lipid droplets were observed under the Lionheart™ FX Automated Microscope(BioTek Instruments, Inc., Winooski, VT, USA).
2.11 Animals
Fifty male C57BL/6J mice, weighing (20 ± 2) g, were obtained from the Jung Ang Lab Animal Inc (Seoul, Korea). All mice were housed and acclimated under controlled environmental conditions(temperatures: 20–22 °C; humidity: 55%; and a 12-h light/12-h dark cycle). After one week, the mice were divided into 5 experimental groups (n= 10 per group) as follows: the chow diet group (CD);the high-fat diet group (HFD); the high-fat diet-induced STAC(250 mg/kg)-treated group (STAC); the high-fat diet-inducedGarcinia cambogiaextract (165 mg/kg)-treated group (GCE); and the high-fat diet-induced orlistat (10 mg/kg)-treated group (ORL).All experiments were performed in accordance with the experimental animal guidelines of Jeju National University Animal Center and were approved by the Institutional Animal Care and Use Committee(IACUC) of Jeju National University, Jeju Special Self-Governing Province, Korea (approval number: 2018–0048).
2.12 Histological analysis
Hematoxylin & eosin (H&E) staining was used for the histological analysis of mouse liver and white adipose tissues[18]. The collected tissues were fixed in 4% formaldehyde solution for 24 h. The fixed tissues were embedded in low-melting point paraffin wax and sliced into 4 μm-thick sections. The sliced paraffin-embedded sections were stained with H&E, and the histologic appearance of mouse tissues was observed under the LionheartTMFX automated microscope(BioTek Instruments, Inc.,Winooski, VT, USA). Histological images were processed using the ImageJ software.
2.13 Analysis of adipogenic and lipogenic gene expression via real time polymerase chain reaction (RT-qPCR)
Adipogenic and lipogenic gene expressions were determined with RT-qPCR (Thermal Cycler Dice Real Time System, Takara, Japan).To obtain mRNA, 30–40 mg of white adipose tissues and 1 mL of the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) were added in bead tubes and mRNA was extracted using the TacoTMpreb bead beater (Taco, Taichung, Taiwan). Thereafter, cDNA was synthesized from 1 μg of the extracted mRNA, DEPC water, and cDNA reverse transcription kit (Takara, Shiga, Japan). Quantitative polymerase chain reaction was conducted using 10 μL of PCR mixture containing 3 μL of cDNA, 5 μL of SYBR green (SYBR), 0.4 μL of target primers, and 1.2 μL of RNase-free water under optimal conditions(enzyme activation: 95 °C, 10 s; denaturation: 40 cycles, 95 °C, 5 s;annealing/extension: 58 °C, 10 s; melting curve: 72 °C, 10 s).
2.14 Statistical analysis
All the measurements are expressed as the means ± standard deviation (SD), and the three independent experiments were conducted. The statistical analysis was performed with statistical package for the Graph Prism (Version 6.0; GraphPad Software Inc.,San Diego, CA, USA). The significant differences were analyzed using one-way ANOVA and Dunnett’s multiple comparison test in Graph Prism 6.0 software.
3. Results
3.1 Purification and structural characterization of STAC and STAF3
Enzyme-assisted hydrolyzation was adopted to purify the fucoidans fromS. thunbergii. Four types STAFs were obtained from the STAC using DEAE ion-exchange chromatography. The DEAE chromatogram presented in Fig. 1A. The structural properties of the purified fucoidans were determined by MALS analysis, FTIR spectroscopy, SEM, and NMR analysis. The averaged molecular weight of STAFs were measured with MALS. Following the MALS results, the averaged molecular weight of STAF1, STAF2,STAF3, and STAF4 were 4.751 × 106, 1.895 × 106, 2.095 × 106, and 6.224 × 105g/mol, respectively (Fig. 1B). As indicated in Fig. 1C,the structural characteristics of STAC and STAF3 were determined via FTIR spectroscopy. The IR spectra of STAC and STAF3 showed intense FTIR peaks at 1 035 (polysaccharide glycosidic C–O–C bridge bond), 1 135 (polysaccharide glycosidic C–O bond), and 1 616 cm–1(carboxylate O–C bond). Furthermore, a relatively broad and low-intensity FTIR peak was observed at 1 120–1 220 cm–1(sulfate groups S=O). The strong peak observed at 1 650 cm–1demonstrated that C=O bond in glucans.
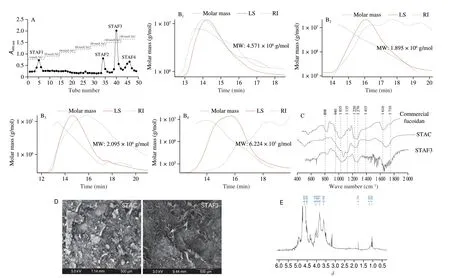
Fig. 1 Purification of fucoidan fractions from STAC using DEAE, ion-exchange chromatography and the structural characterization of STAFs via MALS, FTIR spectroscopy, SEM, and proton NMR analysis. (A) DEAE chromatograms; (B) MALS profiles of STAF1-STAF4; (C) FTIR-ATR spectra;(D) SEM image; (E) 1H NMR spectrum.
The surface morphologies of STAC and STAF3 were examined using FE-SEM (Fig. 1D). STAC showed a non-consistent particle size and mostly displayed large-sized flattened particles with rough surfaces. However, the SEM image of STAF3 showed a significant difference in the size and shape of particles compared with that of STAC. The particle size of STAF3 was smaller than that of STAC and showed formation of a thread-like structure. The structural characteristics of purified STAF3 were analyzed using NMR spectroscopy (Fig. 1E). Chemical shifts of the polysaccharide in the proton NMR spectrum were assigned on the basis of previous studies[19]. The1H NMR spectrum of STAF3 indicated a peak that corresponded to fucoidan from brown seaweed. Additionally, the peaks atδ1.0–1.2 represented methyl groups of fucose units[20]. The1H NMR signals observed atδ1.7–2.0 were attributable to alcoholic protons[21]. The intense signals ranging fromδ3.5 toδ4.0 were attributed to protons of sugar residues such as O-6 ofD-galactose[22].The strong signal observed at approximatelyδ4.6 indicated the NMR solvent and deuterium oxide.
3.2 Chemical and monosaccharide composition of STAC and STAFs
The chemical and monosaccharide compositions of the STAC and its purified STAFs are summarized in Table 1. The phenolsulfuric acid assay as well as the barium chloride assay revealed high levels of carbohydrate and sulfate contents in STAF1 (carbohydrate:(38.71 ± 1.49)%; sulfate: (31.72 ± 1.47)%) and STAF4 (carbohydrate:(38.98 ± 1.36)%; sulfate: (31.22 ± 0.38)%), respectively.Comparatively low levels of polysaccharides and sulfates were observed in STAF2 and STAF3. Relatively high levels of proteins were found in STAF2 ((23.82 ± 3.49)%) and STAF3 ((23.10 ± 2.87)%).The monosaccharide composition of STAC and STAFs are demonstrate that the STAFs were composed of six monosaccharides,namely fucose, rhamnose, arabinose, galactose, glucose, and xylose.Most STAFs contained high levels of fucose and galactose. High levels of fucose (41.13%) and galactose (35.39%) were observed in STAF2. STAF3 was mainly composed of xylose (31.59%).
3.3 Lipid inhibitory effect of STAC and STAFs in 3T3-L1 adipocytes
ORO staining was used to investigate the potential lipid inhibitory effects of STAC and STAFs in 3T3-L1 cells. In Fig. 2A, microscopic images of 3T3-L1 cells showed high levels of lipid accumulation in the control group. However, lipid accumulation was significantly suppressed by treatment with STAC and STAFs. In Figs. 2B and C,cell viability and ORO contents showed that STAC and STAFs did not exert toxic effects on 3T3-L1 cells and effectively reduced intracellular lipid accumulation compared with that control group.Among the purified polysaccharides, STAF3 showed the highest lipid inhibitory activity in 3T3-L1 cells. STAF3 treatment significantly reduced ORO contents at the concentrations of 200 μg/mL((0.33 ± 0.01)-fold) respectively. These results indicated that STAC suppressed lipid accumulation. Additionally, among its purified STAFs, STAF3 exerted excellent lipid inhibitory effects on 3T3-L1 cells. Adipocyte differentiation leading to adipogenesis, and lipogenesis is regulated by adipose-specific proteins, including adipogenic and lipogenic proteins. Western blot results indicated that adipogenic PPAR-γ and lipogenic SREBP-1 protein expressions were markedly inhibited by STAF3 treatment. High concentrations of STAF3 (200 μg/mL) significantly decreased the expression levels of adipogenic PPAR-γ (0.22 ± 0.01-fold), FABP4 (0.41 ± 0.01-fold) and lipogenic SREBP-1 (0.11 ± 0.00-fold) proteins compared with those in the non-treated control group (Figs. 2D and 2E). Obtained findings indicated that STAF3 regulate lipid accumulation by downregulating adipogenic and lipogenic proteins in 3T3-L1 adipocytes.

Fig. 2 Screening of the potential lipid inhibitory effect of STAC and STAF3 in 3T3-L1 cells. (A) Microscopic images; (B) Cell viability; (C) ORO content;(D) Western blotting results of adipogenic proteins (PPAR-γ, FABP4) and lipogenic protein (SREBP-1); (E) Quantification of bands of Western blotting of PPAR-γ,FABP4 and SREBP-1. GAPDH was used as internal control. Bands from Western blots were analyzed using the ImageJ software. All data are expressed as mean ± SD,n = 3 for each group, and significant differences were determined at *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.000 1 compared to the control group.
3.4 Effect of STAC on body and fat weight and serum lipid biochemistry
To determine whether STAC ameliorated obesity, we investigated body weight, body weight gain, and white adipose tissue weight of HFD-induced obese mice. Body weight and body weight gain were monitored for 6 weeks, and fat weight was measured following sacrifice. Data on body weight, white adipose tissue weight and serum lipid profiles are presented in Fig. 3. Our findings showed that body weight steadily increased with HFD during the monitoring period of 6 weeks, and that body weight of the HFD group was higher than that of the CD group. In the control HFD group, body weight increased from 21 g to 36 g during these 6 weeks. However, HFD-induced increase in body weight was significantly lowered by STAC treatment. The body weight of the STAC-administered group at week 6 was (33.35 ± 2.31) g(Fig. 3A). Furthermore, we measured the total fat weight to clarify whether the reduction in body weight caused by STAC originated from a decrease in adipose tissue weight in the HFD-fed obese mice. White adipose tissue weight was notably lower in the STACtreated group ((1.05 ± 0.02) g) compared with that in the HFD group((1.66 ± 0.14) g) at 6 weeks (Fig. 3B). Our findings substantiated those of a previous anti-obesity study conducted by Kim et al.[22]who investigated purified polysaccharides from marine brown seaweeds.Our findings suggested that STAC effectively reduced body weight and fat weight in HFD-fed obese mice.
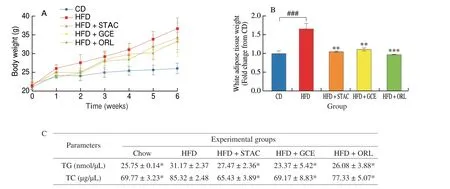
Fig. 3 STAC ameliorates obesity in HFD-induced obese mice. (A) Body weight; (B) White adipose tissue weight. Data are expressed as mean ± SD (n = 10).Significant differences were identified at *P < 0.05, **P < 0.01, ***P < 0.001 compared to the HFD group, and ###P < 0.001 compared to the CD group.
3.5 Effect of STAC on serum lipid biochemistry
Excessive high fat consumption may cause dyslipidemia,which leads to decreased LDL cholesterol and increased HDL cholesterol levels, including elevated serum triglyceride levels[23].To investigate the effect of STAC on the serum lipid profile, mouse sera were analyzed using commercial kits. The serum triglyceride(TG), and total cholesterol (TC) levels are summarized in Table 2.The HFD group showed significantly increased serum TG,and TC levels compared with those of the CD diet group.However, oral administration of STAC markedly reduced TG((27.47 ± 2.36) nmol/μL), and TC ((65.43 ± 3.89) μg/μL) in the sera. Therefore, our findings suggest that STAC may regulate hyperlipidemia in obese mice fed with HFD.

Table 2 Effect of STAC on serum TG, and TG levels in HFD-induced obese mice.
3.6 Effect of STAC on histological changes in the liver and white adipose tissues
H&E staining was performed on liver and white adipose tissues to establish the inhibitory effect of STAC on lipid accumulation in liver and white adipose tissues. Histological images of mouse liver and white adipose tissues are shown in Fig. 4A. Hepatic cells arranged in a dense and orderly manner were observed in the CD group. The hepatic cells of the HFD group showed irregular shapes and high levels of lipid accumulation. However, hepatic lipid accumulation was noticeably improved by STAC supplementation. Quantitative hepatic lipid accumulation in each group is shown in Fig. 4B. Next,we evaluated whether STAC supplementation lowered intracellular lipid accumulation not only in hepatocytes but also in white adipose tissues. As shown in Fig. 4C, the histological appearance of white adipose tissues revealed that HFD significantly increased lipid accumulation. However, a dramatic reduction in intracellular lipid accumulation was observed in the STAC-administered group. Taken together, these results indicated that STAC reduces the risk of liver steatosis and adipocyte hypertrophy.
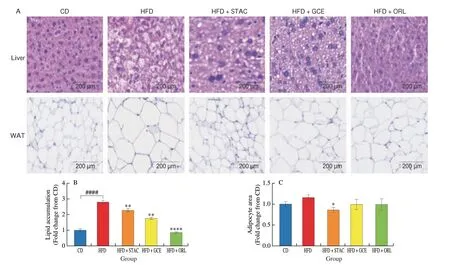
Fig. 4 Effect of STAC on histopathology of the liver and white adipose tissues from HFD-induced obese mice. (A) Histologic analysis of liver and white adipose tissues; (B) Quantification of intracellular lipid accumulation in hepatic tissues; (C) Measurement of white adipocyte area. ImageJ software was utilized to quantify the area of white adipocyte and lipid droplet in hepatic tissues. Results are expressed as mean ± SD (n = 3); *P < 0.05, **P < 0.01, ***P < 0.001, and****P < 0.000 1 compared to the HFD group, and ####P < 0.000 1 compared to the CD group.
3.7 Inhibitory effect of STAC on adipogenic and lipogenic gene expressions in white adipose tissues
The RT-qPCR was adopted to determine the adipogenic PPAR-γ and lipogenic SREBP-1 gene expression in white adipose tissues of HFD induced obesity mice. As indicated in Fig. 5,PPAR-γandSREBP-1expressions were significantly increased in HFD group compared with CD group. However, the oral administration of STAC significantly lowered adipogenicPPAR-γand lipogenicSREBP-1gene expressions in white adipose tissues. Concisely these results indicated that STAC effectively reduced adipogenesis and lipogenesis by modulation of the major adipogenic and lipogenic gene expressions,PPAR-γandSREBP-1, in HFD induced obesity mice.
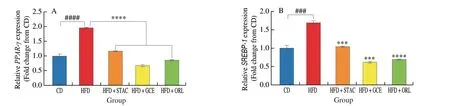
Fig. 5 Effect of STAC on adipogenic and lipogenic gene expressions in white adipose tissues. Quantitative mRNA expression of (A) PPAR-γ and (B) SREBP-1.The mRNA levels are expressed as mean ± SD (n = 3); ***P < 0.001, and ****P < 0.000 1 compared to the HFD group, and ###P < 0.001, and ####P < 0.000 1 compared to the CD group.
4. Discussion
Current study evaluated the physical and structural characteristics of STAFs via MALS, FTIR, SEM and1H NMR analysis. MALS results demonstrated fucoidan fractions with molecular weight in the range of 6.224 × 105–4.751 × 106g/mol in the STAFs. The structural characteristics of polysaccharide molecules analyzed via FTIR spectroscopy demonstrated that STAF3 showed similar functional groups and commonly sulfate groups (S=O) detected at 1 120–1 220 cm–1wave number. This observation is similar to that reported in a previous study conducted to explore polysaccharides derived from brown seaweeds as described by Fernando et al.[24]. SEM images showed that the morphological surface shapes of STAF3 were more consistent and the particle size of STAF3 was smaller than that of STAC. This result indicated that the physical characteristics of STAF3 were changed during DEAE separation steps. Moreover,1H NMR analysis of the purified polysaccharides indicated that NMR peaks corresponded to those representing the structural properties of fucoidan.
Four polysaccharides were derived from STAC via DEAEcellulose ion-exchange chromatography. According to chemical analyses, the chemical and monosaccharide compositions of these purified polysaccharides were different. The chemical analysis revealed that high levels of polysaccharides and sulfates were present in STAF1 and STAF4 fractions. In contrast, STAF2 and STAF3 had relatively low polysaccharide and sulfate contents. However, the protein contents of STAF2 and STAF3 were high. The polyphenol content in all polysaccharide fractions was low. Monosaccharide analyses showed that the purified polysaccharides were composed of 6 monosaccharides (fucose, rhamnose, arabinose, galactose, glucose, and xylose). Generally, all polysaccharide fractions contained high levels of fucose and galactose. Notably, STAF3 contained the highest level of xylose (31.59%) compared to the other polysaccharide fractions.
Potential lipid inhibitory effects of purified polysaccharides were evaluated using ORO analysis. ORO results demonstrated that lipid accumulation in polysaccharide and crude polysaccharidetreated groups was significantly reduced, and that lipid inhibitory activities were higher following polysaccharide purification. Among the groups tested, the STAF3-treated group showed the highest lipid inhibitory activity. Furthermore, Western blot analysis demonstrated that the expression of PPAR-γ, FABP4, and SREBP-1 proteins were dramatically reduced in the presence of STAF3. These results were consistent with those reported by Lee et al.[25], who investigated the correlation between lipid inhibitory effects and adipose-specific proteins in 3T3-L1 cells.
Previous studies have indicated thatD-xylose supplementation down regulate the adipogenesis and lipogenesis and it reduced body weight, white adipose tissue weight, blood glucose levels, and improved liver steatosis and the blood lipid profiles of mouse HFDinduced obesity models[26]. Taken together, it can be implied that high levels of xylose may potentially lead to anti-hyperglycemic and anti-adipogenic activities. Based on the above-mentioned results, we suggest that potential lipid inhibitory activity of STAF3 originated from its monosaccharide composition, specifically from the high amount of xylose.
To investigate the anti-obesity effects of STACin vivo, we analyzed its effects on HFD-induced obese mice. Mice with HFDinduced obesity showed symptoms that were similar to those observed in obese humans. During the 6 weeks of experimental period, body weight and serum lipid levels were significantly increased. Previous reports have indicated that HFD remarkably affects increases in body weight as well as progression of obesity via various biological mechanisms[27]. However, administration of polysaccharide for 6 weeks significantly reduced body weight, fat weight, serum TG,and cholesterol contents in the HFD-induced obese mice. In addition,histological analyses indicated that the STAC reduced excessive lipid accumulation in liver and white adipose tissues, and it could reduce the risk of adipocyte hypertrophy and fatty liver.
To elucidate the molecular mechanisms responsible for the lipid accumulation in white adipose tissues, adipogenic and lipogenic mRNA levels were analyzed by RT-qPCR. The results revealed that purified polysaccharide considerably suppressed adipogenic and lipogenic mRNA expression in white adipose tissues. Thus, we propose that STAC treatment may affect body weight, fat weight, and serum lipid profiles by regulating adipogenic and lipogenic protein expression in white adipose tissues.
5. Conclusions
In conclusion, the current study clearly demonstrated the antiobesity effects of STAC and STAFsin vitroandin vivo. The purified STAFs considerably suppressed lipid accumulation and dramatically downregulated the expression levels of adipogenic PPAR-γ and FABP4 and lipogenic SREBP-1 proteins in 3T3-L1 cells. Additionally, STAC supplementation controlled HFD-induced increase in body weight and fat weight in a mouse obesity model.Furthermore, STAC successively improved HFD-induced TG and TC levels and effectively reduced the expression of key regulators of adipogenesis and lipogenesis in white adipose tissues. Therefore, STAC and its STAFs are valuable sources of anti-obesity agents that may be utilized to alleviate hyperlipidemia and dyslipidemia in obesity.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The “Basic Science Research Program” extended its support via the National Research Foundation of Korea (NRF), which is sponsored through the Ministry of Education (2018R1C1B6004780)and this research was supported by Main Research Program(E0211200-03) of the Korea Food Research Institute (KFRI) funded by the Ministry of Science and ICT.
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango