Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
2024-01-24EnningZhouQiangqiangLiDanZhuGangChenLimingWu
Enning Zhou, Qiangqiang Li,, Dan Zhu, Gang Chen, Liming Wu
a Institute of Apicultural Research, Chinese Academy of Agricultural Sciences, Beijing 100093, China
b Department of Food Science, University of Otago, Dunedin 9016, New Zealand
c School of Food and Health, Beijing Technology and Business University, Beijing 100048, China
Keywords: Food allergens Protein structural characterization Immunogenicity evaluation Food processing modification
ABSTRACT Food allergens are mainly naturally-occurring proteins with immunoglobulin E (IgE)-binding epitopes.Understanding the structural and immunogenic characteristics of allergenic proteins is essential in assessing whether and how food processing techniques reduce allergenicity. We here discuss the impacts of food processing technologies on the modification of physicochemical, structural, and immunogenic properties of allergenic proteins. Detection techniques for characterizing changes in these properties of food allergens are summarized. Food processing helps to reduce allergenicity by aggregating or denaturing proteins, which masks, modifies, or destroys antigenic epitopes, whereas, it cannot eliminate allergenicity completely, and sometimes even improves allergenicity by exposing new epitopes. Moreover, most food processing techniques have been tested on purif ied food allergens rather than food products due to potential interference of other food components. We provide guidance for further development of processing operations that can decrease the allergenicity of allergenic food proteins without negatively impacting the nutritional profile.
1. Introduction
Food allergy is a stress response that occurs when the immune system reacts adversely to specific food component. In humans, food allergies can be divided into immunoglobulin E (IgE)-mediated and non-IgE-mediated types. The clinical symptoms caused by an IgEmediated food allergy include diarrhea, vomiting, rash, angioedema,and bronchospasm, which usually appear within a few minutes to several hours after eating allergenic foods[1]. Non-IgE-mediated anaphylaxis is usually chronic and relatively difficult to cure[2].Generally, IgE-mediated anaphylaxis plays a dominant role in allergic responses to food. Briefly, IgE antibodies are produced when the body is first exposed to a food allergen. IgE then binds to the surface of mast cells and basophils to cause sensitization. When the same allergen enters the body again, it binds to the specific IgE, triggering degranulation of mast cells and basophils; this releases many allergic mediators such as histamine, 5-hydroxytryptamine, leukotriene,prostaglandin, and eosinophile chemokines, which induce clinical symptoms[3-4]. Food allergies reportedly affect up to 10% of the global population, meaning it is an intractable problem of global concern[5].
The most common allergens found in foods are proteins or glycoproteins. The World Health Organization/International Union of Immunological Societies (WHO/IUIS) nomenclature database lists a total of 959 known allergenic proteins from animals, plants, fungi,and bacteria[6]. These have been assigned to 151 protein families according to the Allergen Families (AllFam) database, although 83 allergens remain unclassified[7]. Homologs within a protein family have conserved structures and biologically active sites that can aid in determining whether proteins have allergenicity. More than 160 foods have been identified as causing food allergies to date. Wheat,eggs, peanuts, soy, milk, nuts, crustaceans, and fish are the eight most common allergenic foods, causing more than 90% of food allergies[8].Individual countries have published different guidelines for avoiding allergic reactions to foods, such as precautionary labelling for allergen content, but there is a wide disparity between developed and developing countries in allergen labelling legislation.
To minimize food allergies, it is essential to reduce the allergenicity of allergenic food proteins while preventing the loss of other nutrients. This could be accomplished by applying appropriate food processing techniques (including thermal and non-thermal treatments) and controlling the intensity of relevant processing parameters. However, food processing cannot completely eliminate allergenicity, and sometimes instead increases it. Most research in this area has involved the use of food processing techniques on purified food allergens rather than food products. This is due to the interference of other food components (e.g., lipids, sugars, minerals,and other proteins) affects the effect of processing on reducing allergenicity[9-10]. For instance, peanut allergens could undergo nonenzymatic Maillard reactions with sugars in the food matrix during roasting treatment, inducing the increase of their allergenicity[11].Therefore, the modification of allergens based on whole food to reduce the allergenicity of food is still one of the difficulties to be solved in the future.
Understanding the physicochemical, structural, and immunogenic characteristics of allergenic proteins is necessary to evaluate changes in allergenicity as a result of food processing and to provide guidance for the optimization of food processing conditions. Protein structural characterization is essential for recognizing the linear and conformational epitopes of allergens, which are correlated with allergenicity. The amino acid residues within allergenic proteins that can bind to IgE antibody are regarded as IgE-binding epitopes.Food processing can cause these epitopes to be exposed, masked, or destroyed through alteration, aggregation, or denaturation of protein structures. These changes could either increase or decrease the allergenicity of allergenic food proteins.
A broad range of characterizations have been conducted on allergenic food proteins to date, including explorations of the dityrosine, sulfhydryl, and free amino acids contents and of the linear and spatial conformation properties. Protein structural changes caused by food processing may influence gastrointestinal transport and the digestive properties of some allergens, leading to potential alterations in allergenicity[12]. It is essential to evaluate and analyze changes in allergenicity using bothin vitroandin vivoimmunological and biological methods. Such analyses on the physiochemical, structural,and immunogenic properties of allergenic food proteins will allow further guidance to be developed for food processing techniques to reduce allergenicity.
A survey of the literature was conducted by searching publications with the keywords ‘food’ and ‘allergen’ from 2007 to May 2022.There has been an increase in research on this topic in recent years(Fig. 1A). This indicated that there have been heightened concerns around nutrition and demands for food safety. Previous review papers published from 2007 to mid-2022 mainly focused on advances in food processing for alteration of allergenicity within specific food categories, such as soybean, peanut, milk, and seafood (Fig. 1B).Food allergies and food processing modifications have attracted increasing attention worldwide. Additionally, previous publications reviewed the pathogenesis, diagnosis, and treatment of food allergy[13]and the impact of food processing (including thermal and non-thermal techniques) on conformational and allergenicity changes in allergenic proteins[14-18]. We here provide an update, reviewing the impacts of food processing operations on the physicochemical and immunogenic properties of allergenic proteins. Furthermore, we summarize the detection techniques and methods applied to characterize the physicochemical, structural, and immunogenic changes of food allergens. We provide a scientific basis for further development of processing techniques to decrease the allergenicity of allergenic food proteins without impacting the functional attributes of other nutritional components.
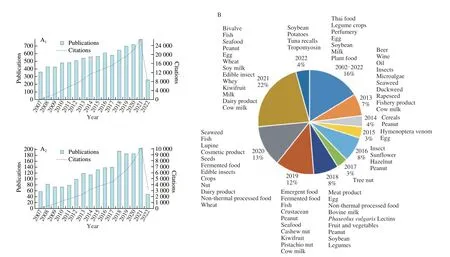
Fig. 1 Number of publications and citations identified in the literature survey (2007 to mid-2022) on Web of Science with ‘food’ AND ‘allergen’ in the topic (A).The specific foods studied in the retrieved articles published from 2007 to mid-2022 (B).
2. Effects of food processing on allergenic proteins
The linear and conformational epitopes of proteins that have IgEbinding capacity directly affect their allergenicity in foods. Effective food processing techniques change the linear and conformational epitopes of allergenic proteins to alleviate allergenicity[7]. Notably,with some processing methods, allergen content may not be reduced,but instead the detectability of the allergen is decreased due to aggregation or structural changes. Most food processing technologies cannot make the allergens completely non-allergenic. On the contrary,some processing techniques may even improve allergenicity. Current food processing methods include thermal and non-thermal treatments(Table 1). Each method has costs and benefits, exerting different impacts on allergenic proteins with different properties.

Table 1 Effects of food processing on allergenic protein properties.
2.1 Effect of thermal treatment on allergenic proteins in food
Commercially produced food is typically heat-treated to combat spoilage, keeping it fresh and prolonging its shelf life. In general,traditional thermal treatments include steaming, boiling, frying, and baking; non-traditional thermal treatments include microwaving and ohmic heating. Changes in the protein structure largely depend on the type of protein and its thermal stability[13]. Thermal treatment can change the protein structure by modifying secondary and tertiary structures, causing the formation or fracture of covalent or noncovalent bonds between molecules, or inducing protein aggregation,which can mask protein epitopes and change the immunogenic characteristics.
2.1.1 Traditional thermal treatment
Traditional methods such as steaming, boiling, frying, and baking kill some microorganisms, inactivate enzymes, and can improve the color, flavor, taste, and storage tolerance of foods. Additionally,proper cooking can destroy allergenic proteins by changing the spatial structure or masking antigenic epitopes to reduce or remove their allergenicity. Studies have demonstrated that treating glutenin at an appropriate temperature can expose some functional groups and reduce its allergenicity[19]. At high temperatures, food allergens may further aggregate or be destroyed over time, reducing their allergenicity. Peanuts are one of the eight major allergenic foods, and can induce breathing difficulties, skin itching, vomiting, and even death. It has previously been shown that treatment with boiling water causes peanut protein aggregation. Enzyme-linked immunosorbent assay (ELISA) showed that the IgE-binding capacity of peanut proteins decreased by eight times after boiling for 2 h and decreased by 19 times after 12 h, indicating that the allergenicity of peanut allergens can be effectively diminished by adjusting the temperature and time of heat treatment[20].
Although boiling can reduce peanut allergenicity, other studies have found that roasting increases the allergenicity of peanut proteins;it was hypothesized that roasting exposed the antigenic epitopes of peanut allergens and increased their allergenicity[21]. Some studies found that roasting increased the activity of the peanut allergen Ara h 2 and made it more difficult to digest, thus increasing allergenicity[22].In addition to boiling and roasting, other heat treatments such as microwave heating and baking have different effects on food allergens. For instance, Jiang et al.[23]explored the impacts of thermal processing methods on the allergenicity of walnut allergen Jug r 1.Walnut was subjected to different thermal treatments including boiling in water for 20 min, heating in a microwave at 500 W for 15 min, and baking at 160 ºC for 15 min. Boiling resulted in a reduction of Jug r 1 binding ability to specific IgE antibody in the serum of allergic patients by ~50%; microwaving was associated with an increase of ~30%, and baking was associated with no significant change. Liu et al.[24]found that the IgE-binding capacity of major allergen tropomyosin in shrimp (Litopenaeus vannamei)was increased after boiling treatment. Similarly, Besler et al.[25]found that shrimp allergen tropomyosin can remain active after boiling treatment because it is a heat-resistant protein. Additionally,Dhakal et al.[26]found that the allergenicity of almond milk could not be significantly reduced by heat treatment, and Jacob et al.[27]found that heating did not reduce the allergenicity of the carrot allergen Dau c 1. These findings indicate that traditional heat treatments have certain limitations; for instance, they are not suitable for heat-stable or heat-resistant food allergens. In these cases, it is necessary to use other treatments to decrease allergenicity.
2.1.2 Non-traditional thermal treatment
Microwave heating can increase the temperature of foods, change the protein conformation, and affect the allergenicity of allergen[12]. For instance, the relative content ofα-helix structure of kiwifruit allergen Act d 2 was significantly decreased, and the IgE-binding ability of Act d 2 was notably reduced after microwave treatment (2.45 GHz,75 ºC, 1–5 min)[28]. The protein secondary structure of allergen tropomyosin ofL. vannameiwas also changed and its allergenicity was significantly decreased after microwave treatment[29]. Although many studies have demonstrated the potential of microwave treatment to reduce allergenicity of allergens, microwave treatment still has limitations. For example, microwave treatment cannot reduce the allergenicity of wheat gliadin due to its stable protein structures[30].Moreover, when dealing with the high viscosity or granular foods,microwave treatment generates uneven heat which can cause overheating and degradation of thermal-sensitive components, and influencing the food quality.
Ohmic heating is a novel food processing technology in which an electric field of a specific intensity is applied to each end of an electrically conductive food material to heat the food[31]. Ohmic heating with a high heat transfer rate can effectively reduce the degradation of thermal-sensitive food components with large particles[32]. The applied electric field used in ohmic heating exerts thermal and electrical effects on the food system, and researchers have applied this method to lower the allergenicity of allergenic food proteins. For instance, Pereira et al.[33]studied the thermal and electrical effects of ohmic heating on the structural and immunogenic characteristics of soybean protein using titanium electrodes at electrical frequencies of 50–20 000 Hz and electric field intensities of 2–20 V/cm. They found that the natural fluorescence of soybean protein isolate (SPI) changed significantly when treated with 50 Hz at 95 ºC, and there was a 36% reduction in the allergenicity of the soybean allergen Gly m TI. Soybean protein conformation was altered, which may be related to the release of metal ions such as Fe/Ni from the electrode at 50–500 Hz. The decreased allergenicity of Gly m TI could be attributed to the electrical effect exerted by the electric field at 50 Hz. Pereira et al.[34]also investigated changes in the allergenicity ofβ-lactoglobulin (β-LG) after high-temperature short-time (HTST) pasteurization (90 ºC, 1 s), low-temperature long-time (LTLT) pasteurization (65 ºC, 30 min), and ohmic heating(4 V/cm, 25 kHz). They found that HTST and ohmic heating reduced the allergenicity ofβ-LG, whereas LTLT increased allergenicity.Reduction inβ-LG allergenicity may be related to aggregation, which could mask the binding sites for specific antibodies. These results indicate the effects of the processing approaches and associated parameters used to process foods. At present, there are relatively few studies on the impacts of ohmic heating on food allergens. Compared with traditional thermal processing, the electrical effect of ohmic heating may provide a novel strategy for reducing the allergenicity of food allergens.
2.2 Effects of ultrasonic treatment on allergenic food proteins
Ultrasonic waves are sound waves with very short wavelengths and frequencies higher than 20 kHz. They propagate strongly in water and accumulate energy easily. The cavitation, mechanical and thermal effects of ultrasound induce the conformational changes of allergens in food materials. Studies have reported that high-speed gradients and shear stresses during ultrasonic treatment can produce micro streams with structural effects, which can disrupt van der Waals forces and hydrogen bonds in polypeptides, leading to protein denaturation,thereby changing allergen allergenicity and affecting the interaction between allergen and antibody[35].
Ultrasonic treatment has been applied to alleviate the allergenicity of various food allergens, such as tropomyosin[36]and peanut allergens[37]. As reported by Li et al.[38], ultrasonic treatment can decrease the allergenicity of peanut allergens Ara h 1 and Ara h 2,due to the cavitation, mechanical, and thermal effects generated by ultrasonic waves interacting with food materials to change the conformations of allergen epitopes. Further, ultrasonic intensity affects the efficiency of allergenicity modification. For instance, Zhang et al.[36]used different intensity ultrasonic waves (20 kHz, 100–800 W,15 min) to study the conformational stability and allergenicity of shrimp tropomyosin and performedin vitrogastrointestinal digestion experiments with treated samples. The results demonstrated that ultrasonication degraded shrimp tropomyosin and altered the secondary structure, decreasingα-helix and increasingβ-sheet,β-turn,and random coil structures after high-intensity ultrasonic treatment.Western blot and ELISA demonstrated that the allergenicity of tropomyosin decreased significantly when the ultrasonic power reached 800 W. Additionally, ultrasonic treatment time also affects the modification of allergenicity. Dong et al.[39]treated shrimp with ultrasonication (20 kHz, 400 W) for 0, 5, 10, 15, and 20 min and found that tropomyosin allergenicity decreased with longer treatment times. Moreover, Wang et al.[40]determined that the microstructure of kiwifruit tissue and the secondary structure of kiwifruit allergen Act d 2 were significantly degraded with longer time ultrasonic treatment.This was associated with a decrease in allergenicity and a significant increase inin vitrodigestibility. Therefore, the effect of ultrasonic treatment on allergenicity modification is closely related to the ultrasonic intensity and treatment time.
In conclusion, ultrasonic treatment is more suitable than thermal treatment for heat-resistant allergens, such as tropomyosin in shrimp and crab. This may be due to interactions of the cavitation,mechanical, and thermal effects generated by ultrasonic waves with food materials, changing the conformations of allergen epitopes. To strengthen food quality control during processing, it is necessary to identify appropriate ultrasonic intensities to induce desired changes in the structural and functional properties of food proteins and allergens.
2.3 Effects of ultrahigh pressure treatment on allergenic food proteins
Ultrahigh pressure is a cold processing technology that does not significantly increase food temperature. Foods or raw materials are sealed in flexible packages and placed in an ultrahigh pressure container, after which the hydrogen bonds, ionic bonds, and noncovalent bonds are destroyed and re-formed using water or another liquid to apply pressure ranging from 100 MPa to 1 000 MPa.Ultrahigh pressure treatment can destroy tertiary and quaternary protein structures, but has fewer effects on secondary structures,and moreover, it can destroy the covalent bonds within and between allergenic proteins to change the conformation and allergenicity of allergens. For instance, Dhakal et al.[26]treated almond milk proteins with ultrahigh pressure (600 MPa, 30 °C), and used western blot and dot blot to confirm a loss of immunogenicity. Additionally,cross-reactivity is one of the major factors causing allergic rhinitis and respiratory diseases. For instance, the birch pollen Bet v 1 can induce cross-reactivity with foods such as hazelnut, cherry, peach, celery, and apple in susceptible populations; however, this effect was significantly reduced by high pressure treatment (300–600 MPa, 5 min)[41].
Ultrahigh pressure treatment cannot reduce the allergenicity of all food allergens due to differences in structural characteristics.Researchers studied the serum of patients who were allergic to apples that were treated with ultrahigh pressure. They measured the binding ability of apple allergens to specific IgE antibodies with a radioimmunoassay test (RAST) and found that ultrahigh pressure treatment below 800 MPa had little effect on the IgE-binding ability of apple allergens[42]. Similarly, Husband et al.[43]found that a single high-pressure treatment had little effect on the secondary structures and allergenicity of the apple allergens Mal d 1 and Mal d 3,whereas combining high-pressure and high-temperature treatments significantly decreased allergenicity. In contrast, Kleber et al.[44]found that the allergenicity ofβ-LG from whey protein isolate(WPI) increased along with pressure and holding time in treatments ranging 30–68 ºC at 200, 400, and 600 MPa for 0, 10, or 30 min.These findings indicate that it is important to understand the pressure sensitivity of allergens when selecting appropriate processing parameters to reduce allergenicity. A combination of different techniques, such as a combined high-pressure and high-temperature treatment, could more effectively reduce food protein allergenicity than a single treatment alone.
2.4 Effects of microbial fermentation on allergenic food proteins
Fermentation is a process by which microbial enzymes biochemically modify major food substrates, and microbes decompose organic macromolecules into small organic molecules. For instance,yeast converts sugar and starch into alcohol and converts proteins into peptides or amino acids[45]. It not only can be used to improve the nutritional characteristics of food, reduce anti-nutrients, and increase product diversity, but also can produce proteolytic enzymes to change protein structures and destroy the linear and conformational epitopes of allergens, which could effectively reduce food allergenicity[46].
Current studies have applied fermentation in reducing the allergenicity of peanuts, milk, soybeans, wheat, nuts, and fish, etc.Taking soybeans as examples, previous study demonstrated that the main allergenic proteins in legumes showed reduced allergenicity after hydrolysis into polypeptides during fermentation[46]. In addition,Song et al.[47]usedL. plantarum,Bifidobacterium lactis, andS. cerevisiaeto ferment soybean meal; ELISA and Western blot assays were then performed to detect the immune reactivity of IgE in the plasma of patients with soybean allergy, which showed that allergenicity was decreased by fermentation. Moreover, Yang et al.[48]also applied mixed strains ofB. subtilis,L. casei, and yeast to ferment soybean meal in a solid state, then evaluated the allergenicity of soybean mealin vitro. They observed decreased immune reactivity of soybean meal proteins to specific antibodies after fermentation, providing further evidence that fermentation can reduce the allergenicity of soybean meal. Our previous study also found that allergens in bee pollen were decomposed into peptides by fermentation with yeast, and their allergenicity was decreased significantly[49]. Fermentation has thus been regarded as an important tool for food development. However, some peptides could retain allergenic properties through fermentation, and partial digestion of some proteins may increase allergenicity. Therefore, it is extremely important to standardize the microbial strains and fermentation parameters used for decreasing allergenicity while maintaining food quality and safety.
2.5 Effect of cold plasma on allergenic food proteins
As a novel non-thermal food processing technology, cold plasma processing can produce particles such as nitrogen and oxygen that can collide with electrons to generate active substances such as ozone, hydroxyl radicals, and nitrogen oxides[50]. Previous studies showed that the active substances produced by cold plasma processing can alter allergens by attacking the amino acid side chains, destroying epitopes, and modifying protein structures, thereby reducing the allergenicity of some food allergens[51-52]. For instance,Venkataratnam et al.[53]used a novel large volume pin-to-plate atmospheric plasma reactor (52 kHz, 32 kV) to study the effect of cold plasma on the allergenicity of the key peanut allergens Ara h 1 and Ara h 2. ELISA and circular dichroism (CD) spectroscopy were used to detect the allergenicity and secondary structures of Ara h 1 and Ara h 2. The antigenicity of Ara h 1 and Ara h 2 gradually decreased over the treatment time, while the relative content of random coils increased. These results showed that cold plasma could alter the secondary structure of allergens and decrease immunogenicity.
Although the temperature used in cold plasma treatments is relatively low (30–60 ºC)[54], which is conducive to treatment of heat-sensitive allergens, it is also suitable for allergens with high thermal stability (such as shrimp tropomyosin). Ekezie et al.[55]used cold argon plasma jets to treat tropomyosin fromL. vannamei,then measured the spatial structure and immunogenicity. The results showed that the relative content ofα-helix structures in tropomyosin decreased, whereasβ-sheet and random coil structures increased over the treatment time. Levels of free sulfhydryl groups also decreased as the surface hydrophobicity increased due to the formation of disulfide bonds. The IgE-binding capacity of tropomyosin decreased significantly, indicating that the active substances produced by cold plasma processing may modify the spatial structure of tropomyosin and lower its allergenicity[55].
In contrast, Filho et al.[56]treated cashews with atmospheric pressure plasma jets (80 W, 50 kHz), and found that although the contents of sucrose and anacardic acids changed, the IgE-binding ability of cashew allergens did not change. This could be due to the influence of the specific cold plasma method used, or to the extraction process used for cashew degreasing. Currently, the specific mechanism by which cold plasma alleviates allergenicity of some foods is not sufficiently clear. Some researchers have proposed that active substances produced by cold plasma processing induce structural changes in food allergens that are related to allergenicity reduction.In vivoresearch to evaluate the safety of cold plasma processing in foods is extremely limited. Therefore, it is necessary to conduct further in-depth studies to clarify the mechanism(s) and processing safety.
2.6 Effects of covalent dietary polyphenol binding on allergenic food proteins
Dietary polyphenols refer to natural aromatic ring compounds with multiple hydroxyl substituents or groups that exist in plants;these are non-energy specialized metabolites with polyphenol structures[57-59]. Both free radical grafting method and alkaline method enable to covalently bind allergenic proteins to dietary polyphenols.Binding of an allergen to dietary polyphenols can enhance protein aggregation and change the structural properties of the allergen,reducing the number of binding sites for IgE, and inhibiting the secretion of allergic cytokines by helper T cells (Th cells), and suppressing the release of allergic mediators by mast cells (Fig. 2)[60-62].

Fig. 2 The sensitization mechanism of a food allergen and the desensitization mechanism of an allergen–polyphenol copolymer during an IgE-mediated allergy reaction.
Many researchers have studied the interaction between dietary polyphenols and allergenic proteins to reduce or eliminate the allergenicity of food allergens[63-65]. In the free radical grafting method,H2O2and ascorbic acid undergo a redox reaction to generate hydroxyl radicals, and the hydroxyl radicals re-oxidize the amino acids of the protein side chain. Consequently, polyphenols can covalently bind with the oxidized allergic proteins[65]. A previous study showed that epigallocatechin gallate (EGCG) or chlorogenic acid (CA) can covalently modifyβ-LG by radical grafting, which changed the spatial structure and effectively reduced the IgE-binding ability ofβ-LG[66].In the alkaline method, polyphenols can be oxidized to quinone under alkaline conditions (pH 9) in the presence of oxygen; quinone is a reactive electrophilic intermediate that can form strong and stable C-S or C-N bonds through nucleophilic addition with a sulfhydryl or amino group in a protein side chain to form covalent bonds[65].For example, the peanut allergen Ara h 1 was covalently modified by EGCG or CA after alkali treatment, resulting in the reduction of denaturation temperature and IgE-binding ability of Ara h 1.In vitrodigestion experiments showed that the digestibility of Ara h 1 improved when it was in the polyphenol copolymer form[67]. In conclusion, the free radical method is more widely used because dietary polyphenols are very active and easily oxidized. But alkali treatment may be more suitable for food processing because it is stable to temperature and pH and does not require the use of many additives[65]. However, in almost all studies, dietary polyphenols were used to directly bind purified food allergens; they have not yet been applied in food products. Further research is required to understand the applications and effects of covalent dietary polyphenol binding approaches in food matrices.
2.7 Comparison of different food processing techniques in reducing the allergenicity of allergenic food proteins
The benefits and limitations of different food processing techniques in reducing the food allergenicity are summarized in Fig. 3 and Table 2. Thermal treatments are usually used to kill microorganisms, inactivate enzymes, and/or reduce the allergenicity of some foods such as rice, nuts, or soybean. However, thermal treatments can cause denaturation of heat-sensitive food proteins,change food color and flavor, and destroy heat-sensitive nutrients[18].Thermal treatment is ineffective against some heat-resistant allergens,such as tropomyosin in shrimp and crab; furthermore, frying and roasting increase the allergenicity of peanut proteins[21]. Non-thermal treatments are more effective against heat-sensitive compounds and heat-resistant allergens and have more applications in reducing the allergenicity of various foods such as fish, shrimp, kiwifruit,wheat, milk, egg, peanut, and other nuts[16,25,29,68]. Novel non-thermal processing techniques have also emerged, including irradiation, cold plasma, covalent modification of dietary polyphenols, and primary structure modification by glycosylation, etc.[18]. For instance, some studies reported that irradiation treatment can reduce the allergenicity of OVA,α-lactalbumin (α-LA), and tropomyosin[69-71]; glycosylation modification can decrease the allergenicity of tropomyosin[72], whey allergen[73], and chickpea albumin[74]. Combining multiple food processing technologies provides a new strategy for allergenicity reduction[16]. For instance, the immunogenicity of apple allergens and ofβ-LG can be significantly reduced by a combined high-pressure and high-temperature treatment[36,38]. The immunogenicity of peanut allergens can also be significantly decreased through joint high pressure and polyphenol oxidase or ultrasound andα-chymotrypsin treatments[34]. The immunogenicity of cashew and pistachio allergens are significantly reduced by combined enzyme and heat treatments[69].Generally, combining multiple food processing techniques could help to overcome issues of allergen resistance to a single technology; this strategy may have broad applications in the food industry.

Table 2 Advantages and disadvantages of different processing technologies during modifying food allergenicity.
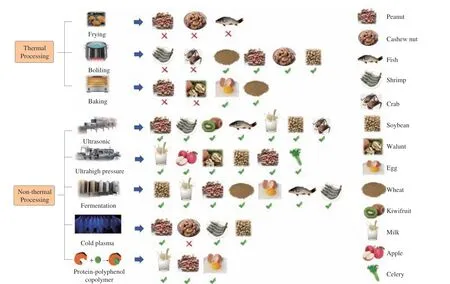
Fig. 3 Applicability of different processing techniques to various foods for allergenicity reduction. A checkmark indicates a valid effect on allergenicity reduction; an x indicates an invalid effect on allergenicity reduction.
However, most researches conducted to date have involved application of processing techniques to purified allergenic proteins.It is attributed to that the combination of metal ions, lipids, sugars,and other ligands with allergens could increase the structural stability of allergens and make it difficult to change the immunogenicity of allergens[10]. For instance, the structure of carp parvalbumin is too stable to be changed due to the binding of calcium ions, so its allergenicity is not reduced even after cooking treatment[75].Similarly, the stability ofβ-LG is enhanced after binding to lipids,making it difficult to change its allergenicity during processing[76].Also, the existence of sugars in food matrix could induce an increase in allergenicity in peanut allergens during roasting treatment[11].Thus, the applicability of these processing techniques in various food products still remains limited; issues include whether other ingredients (such as lipids, minerals, and other proteins) that exist in the complex food matrix would prevent allergenicity reduction and whether the application of processing technologies would modify or damage desirable nutrients.
3. Application of techniques for physicochemical and structural characterization of allergenic food proteins
Allergenic proteins with IgE-binding epitopes are the major inducers of food allergies. Appropriate food processing could disrupt the linear or conformational epitopes of allergenic proteins, reducing their allergenicity. This would effectively prevent food allergies at the sensitization stage or before the excitation stage. To understand the mechanisms of allergenic changes, it is necessary to analyze the relevant linear and spatial structures. Current techniques for exploring changes in the structures of allergenic proteins include analyzing dityrosine, sulfhydryl, and free amino acid contents; identifying the primary structure (i.e., the amino acid sequence) by mass spectrometry(MS); determining the secondary structure by circular dichroism spectrometry (CD) or Fourier transform infrared spectrometry (FTIR);and elucidating the tertiary structure with fluorescence spectrometry(FS) or ultraviolet absorption spectroscopy (UV). These techniques reveal the physicochemical and structural properties of allergenic proteins, allowing evaluation of the safety and quality of processed foods (Table 3).
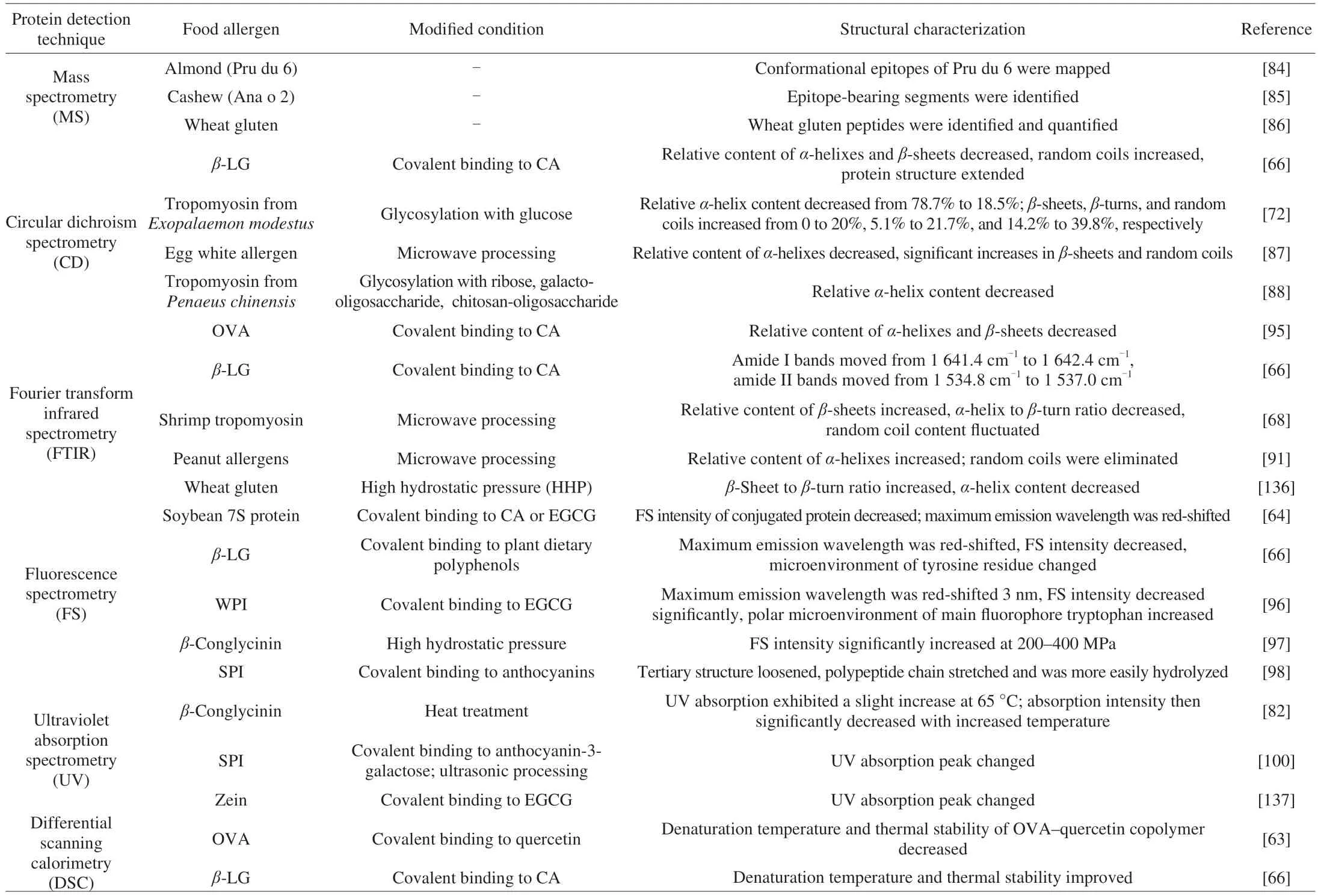
Table 3 Application of protein characterization techniques to identify changes in the physiochemical properties of allergenic proteins caused by food processing.
3.1 Analysis of dityrosine, sulfhydryl, and free amino acid contents
Changes in free amino acids or dityrosine levels reflect the degree of protein oxidation. Tyrosine is easily oxidized by reactive oxygen,producing the dityrosine formed by tyrosine free radicals and tyrosine residues. Sulfhydryl and disulfide bonds are weak secondary bonds that can maintain the tertiary structure of proteins[77]. In general,natural disulfide bonds stabilize the conformation of correctly folded proteins and minimize the stability of a denatured conformation by reducing conformational entropy[78]. The free and total sulfhydryl content can be used to assess protein unfolding and denaturation[79],with changes in those contents reflecting the degree of protein structural alteration[80]. Sulfhydryl content can be determined using 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB), which has no UV absorption at 412 nm but reacts with sulfhydryl to produce 2-nitro-5-mercaptobenzoic acid (TNB), which has strong UV absorption at 412 nm. DTNB can therefore be used for the quantitative analysis of free sulfhydryl[81]. The sulfhydryl content of allergenic food proteins can be changed by food processing, potentially affecting protein spatial structure and allergenicity. For instance, high pressure treatment (200–400 MPa) increased the sulfhydryl content ofβ-conglycinin and decreased allergenicity, which was attributed to the pressure-induced transformation of sulfhydryl groups to disulfide bonds[82]. Furthermore, the active free radicals, ions, and neutral particles produced by cold plasma treatment can oxidize susceptible free sulfhydryl groups in tropomyosin, leading to the formation of disulfide bonds and the reduction of total sulfhydryl content; this is closely related to structural changes in tropomyosin and an associated reduction in allergenicity[55]. Thus, changes in protein structure as a result of food processing can be preliminarily identified by analyzing levels of dityrosine, sulfhydryl, and free amino acids.
3.2 Characterization of allergenic protein primary structure by MS
MS is a powerful tool for identification and quantification of allergenic food proteins. In this technique, allergenic proteins are enzymatically hydrolyzed into small peptides, which are then ionized through MS. Those charged fragments are separated by mass charge ratio (m/z) after acceleration by an electric field, then converted into electrical signals that are measured and can be identified. MS can be used not only to identify the primary structure of an allergenic protein but also to predict the antigenic epitopes of identified proteins through bioinformatic analyses[83]. A hydrogen/deuterium exchange(HDX) tandem Fourier-transform ion cyclotron resonance MS system was developed and has been applied to epitope mapping of some nut allergens[84-85]. The MS-based detection has some advantages in protein characterization, such as with high resolution, enhanced data analysis packages, and extensive molecular weight of analytes. It also can simultaneously detect multiple allergenic proteins in complex samples, and is therefore recognized as a high-throughput detection technology. Moreover, it does not necessarily involve immunological methods such as the use of specific antibodies. Therefore, it can be widely used for qualification and quantification of allergenic proteins and detection of changes in amino acid sequences and antigenic epitopes of allergens in food processing[86].
3.3 Characterization of allergenic protein secondary structure by CD
CD is a physical technology that can be used to determine biomolecular structure, monitor structural changes, and characterize the secondary structure of a protein. The circular dichroism of a substance refers to the different absorptions of left-handed and righthanded circular polarized light. The CD spectrum of a protein is produced by the different absorptions of polarized light by peptide/amide bonds, aromatic amino acid residues, disulfide bonds, and other active groups. It consists of absorptions in the near-UV region(250–320 nm) and far-UV region (178–250 nm). The CD spectra in near-UV region are used to characterize the secondary structure of a protein, and the CD spectra in far-UV region reflect the circular dichroism of peptide bonds. The secondary structure of a protein is a specific conformation formed by winding or folding of the main polypeptide chain; it primarily includesα-helix,β-sheet,β-turn, and random coil structures. In those structures, peptide bonds are arranged in a highly regular manner, and the energy level transition of peptide bonds is determined by the direction of their arrangement. Therefore,different proteins produce different CD bands. For example, the CD spectrum of anα-helix has a positive characteristic peak near 192 nm and two negative characteristic shoulder peaks at 208 and 222 nm; aβ-sheet has a negative characteristic peak at 216–218 nm and a strong positive characteristic peak at 185–200 nm, whereas aβ-turn has a positive characteristic peak near 206 nm. The CD spectrum of a random coil conformation has a negative characteristic peak near 198 nm and a small and wide positive characteristic peak near 220 nm. CD data are used to calculate changes in protein secondary structure and to study alterations in protein function after food processing.
CD technology has been widely applied to characterize the secondary structural properties of allergens. For instance, Zhu et al.[87]used CD to characterize the secondary structure of egg white protein processed with ultrasound and microwave. They found that both ultrasound and microwave treatments increasedβ-sheet and decreasedα-helix structures. They concluded that the change in CD spectrum of egg white protein was due to the expansion or loosening of the protein by ultrasound and microwave treatment; this made the structure longer and more flexible, changing the secondary structure. Additionally, CD technology has been used to detect changes in the secondary structure of the shrimp allergen tropomyosin after processing. Tropomyosin is a heat-resistant allergen that cannot be degraded by heat treatment alone. However, researchers found that adding a reducing sugar to the shrimp during food processing resulted in occurrence of the Maillard reaction between the carbonyl group of the reducing sugar and the amino group of the allergenic protein; this disrupted the secondary structure of the allergen. This processing method decreased theα-helix content of tropomyosin from 78.7% to 18.5%, whereas the levels ofβ-sheets,β-turns, and random coils increased from 0 to 20%, 5.1% to 21.7%, and 14.2% to 39.8%, respectively[72]. A cellular assay showed that processed tropomyosin significantly reduced the release of allergic mediators compared with untreated tropomyosin, indicating that the allergenicity of tropomyosin was decreased. Moreover, there was a close correlation between the secondary structure and changes in allergenicity. Some researchers proposed that the allergenicity of tropomyosin was positively correlated with the change inα-helixes and negatively correlated with the change inβ-sheets, suggesting that the change in tropomyosin allergenicity can be predicted by the secondary structure[88]. Understanding the correlation between relativeα-helix/β-sheet content and the allergenicity of other proteins requires further research.
3.4 Characterization of allergenic protein secondary structure by FTIR
FTIR can be used to measure the infrared absorption wavelength and intensity of samples and to detect protein secondary structures[89].It also can be used for qualitative and quantitative protein analyses without damaging samples, and has the advantages of a fast-scanning speed and high sensitivity. In the infrared spectrum, the characteristic absorption peak of the amide I region (1 700–1 600 cm-1)is primarily generated by the C=O stretching vibration and C-N stretching vibration of the amide group, which can be used to study protein secondary structure[87,90-92]. Previous studies have shown that the infrared absorption peak is in the 1 650–1 660 cm-1range forα-helix structures; in the 1 612–1 625 cm-1and 1 625–1 640 cm-1ranges forβ-sheets; in the 1 667–1 678 cm-1and 1 660–1 668 cm-1ranges forβ-turns; and in the 1 637–1 645 cm-1range for random coils[93]. The amide I region of an original FTIR spectrum can be analyzed using the second derivative, deconvolution, or least squares method; the curve fitting method can be used for quantitative analysis of protein secondary structures[94].
FTIR has been used for detection of secondary structural changes in allergens during food processing. The covalent combination of CA withβ-LG was found to reduce the allergenicity ofβ-LG,and the secondary structure ofβ-LG was characterized with FTIR spectroscopy. After covalent bonding with CA, the amide I and amide II bands ofβ-LG moved from 1 641.4 cm-1to 1 642.4 cm-1and from 1 534.8 cm-1to 1 537.0 cm-1, respectively. This indicated a change in the secondary structure ofβ-LG[66]. Similarly, the relative contents ofα-helixes andβ-sheets were also decreased in CA–OVA copolymers compared with untreated OVA, indicating that the presence of the polyphenol could affect the spatial structure and change the allergenicity of OVA[95]. Qualitative and relative quantitative analysis of allergen secondary structures by FTIR could be used to predict changes in allergenicity. FTIR has been regarded as a robust tool for mutual verification of secondary structural changes in allergens determined with other techniques such as CD.
3.5 Characterization of allergenic protein tertiary structure by FS
Amino acids with benzene rings or conjugated double bonds in protein molecules, such as tryptophan (Trp), tyrosine (Tyr), and phenylalanine (Phe), can produce fluorescence at a certain excitation wavelength, giving the protein endogenous fluorescence. The FS spectrum shows the relationship between FS energy and corresponding wavelengths and can be used to characterize protein tertiary structure.Trp, Tyr, and Phe have different chromophores and different FS spectra; peaks appear at 348, 303, and 282 nm, respectively. There are many FS spectrum analysis technologies, including endogenous FS emission spectrum, synchronous FS spectrum, and 3D FS spectrum.Endogenous protein FS emission spectra are typically dominated by Trp residues[96]. Synchronous FS spectra can be used to study microenvironmental changes in amino acid residues by measuring changes in the emission wavelength, which reflect changes in protein structures. The characteristic FS spectrum of a Tyr residue is Δλ= 15 nm, and the characteristic FS spectrum of a Trp residue is Δλ= 60 nm. A 3D FS spectrum is a three-dimensional matrix spectrum of FS intensity, excitation wavelength, and emission wavelength. These FS spectrum technologies can be used to analyze and characterize protein tertiary structures.
FS has been used to characterize the tertiary structure of allergenic proteins. For instance, the antigenicity of the soybean allergenβ-conglycinin was found to significantly decrease after high hydrostatic pressure treatment (200–400 MPa). The FS intensity ofβ-conglycinin increased significantly at 200–400 MPa, which could be ascribed to the exposure of Phe and Tyr residues and the migration of Trp residues to the hydrophobic environment. After the high hydrostatic pressure treatment, free sulfhydryl groups and the surface hydrophobicity increased inβ-conglycinin; this indicated unfolding ofβ-conglycinin, destruction of the disulfide bond, and loosening of the structure, ultimately leading to exposure of the hydrophobic domain and changes in the antigenic epitopes[97]. Another study used 3D FS analysis to show that the FS intensity of SPI was changed by covalent binding with anthocyanin, resulting in the polypeptide chain extending and unfolding and a corresponding reduction in allergenicity[98].Similarly, FS analysis showed that the tertiary structure of WPI became more compact after covalent modification with EGCG; the maximum FS emission wavelength was red-shifted by 3 nm and the FS intensity decreased significantly. This indicated that covalent modification with EGCG reduced the exposure of Trp residues in WPI and increased the polarity of the microenvironment; this also changed the tertiary structure of EGCG-modified WPI, increasing the exposure of fluorophores in aqueous solvents[96]. FS can effectively identify microenvironmental changes in chromophore amino acid residues within allergenic food proteins, reflecting tertiary structural changes.This contributes to understanding of the mechanisms by which protein immunogenicity is altered.
3.6 Characterization of allergenic protein tertiary structure by UV spectroscopy
The aromatic ring structures of some amino acids (such as Trp and Tyr) can absorb UV light. The UV absorption spectrum can be used for structural characterization of proteins; the conformation of the protein skeleton can be detected at 200 nm, and aromatic amino acids can be detected at 280 nm. These two absorption peaks can therefore demonstrate changes in the tertiary structure of allergenic proteins during food processing[99].
A previous study applied UV spectroscopy to determine structural changes in allergens resulting from heat treatment. That study showed that the allergenicity and UV absorption ofβ-conglycinin decreased significantly after heat treatment, indicating that heat treatment disrupted the tertiary structure ofβ-conglycinin; this disruption led to the formation of protein aggregates and covering of the aromatic amino acid residues[82]. UV absorption spectra can also be used to show covalent binding between allergens and polyphenols, reflecting protein structural changes. For instance, UV spectroscopy has been used to characterize the structure of SPI–anthocyanin-3-galactose polymers and to demonstrate that the free radicals generated by ultrasonic treatment promoted formation of SPI–anthocyanin-3-galactose conjugates[100]. This demonstrates that UV spectroscopy can be used to study the interactions between proteins and other substances. A change in the maximum absorption peak indicates a modification in protein conformation caused by a change in the amino acid residue microenvironment. UV spectroscopy can be used alongside other analytic methods to effectively analyze structural changes in proteins, and should therefore be used in quality evaluation during food processing.
3.7 Characterization of allergenic protein thermal stability by differential scanning calorimetry (DSC)
DSC can provide information such as the thermal denaturation temperature (Td) and enthalpy change (ΔH) of a protein, which is valuable in characterizing thermal stability[101]. In the DSC spectrum,Tdand ΔHcan be determined using the peak value and peak area of the DSC curve of an allergenic protein.Tddirectly reflects the thermal stability of a protein, whereas ΔHcan reveal the absorbed heat of a protein by allowing calculation of the peak area. A higher thermal denaturation midpoint temperature (Tm) indicates higher thermal stability.Tmis relatively constant and is generally not affected by external factors (such as the detection environment or instrument model), so DSC is an appropriate method to evaluate the thermal stability and conformational changes of allergenic proteins.
Wu et al.[66]reduced the allergenicity ofβ-LG by covalent binding with EGCG or CA. They tested the thermal stability ofβ-LGEGCG andβ-LG-CA copolymers with DSC. The results showed that the denaturation temperatures of theβ-LG-EGCG andβ-LG-CA copolymers increased by 6.5% and 11.35%, respectively. This indicated that the spatial structure ofβ-LG was changed after covalent binding with EGCG or CA, resulting in changes in thermal stability and immunogenicity. Another study also found that the covalent combination of OVA with quercetin reduced the allergenicity of OVA. DSC detection showed that theTdand thermal stability of the OVA-quercetin copolymer was lower than that of OVA, and that this change was related to differences in the secondary and tertiary structures of OVA after covalent binding to quercetin[63]. Although changes in thermal stability differ between dietary polyphenols and food allergens, DSC can reflect changes in the structures of allergens that are closely related to their allergenicity. There have been relatively few studies focused on the thermal stability of allergens. We suggest that the thermal stability of allergens should be evaluated by DSC when selecting techniques and parameters (such as temperature) for food processing. This will be of great significance in developing food processing technologies to reduce the allergenicity of food allergens.
The applicability of the techniques discussed above to physicochemical and structural protein characterization is summarized in Table 4. MS has traditionally been used analyze protein amino acid sequences; relative levels ofα-helixes,β-sheets,β-turns, and random coils in food proteins can be determined by CD and FTIR;changes in the tertiary structure of food proteins can be predicted using FS and UV methods; and thermal stability can be analyzed by DSC. Combined utilization of these approaches can provide a comprehensive profile for use in food quality and safety control.
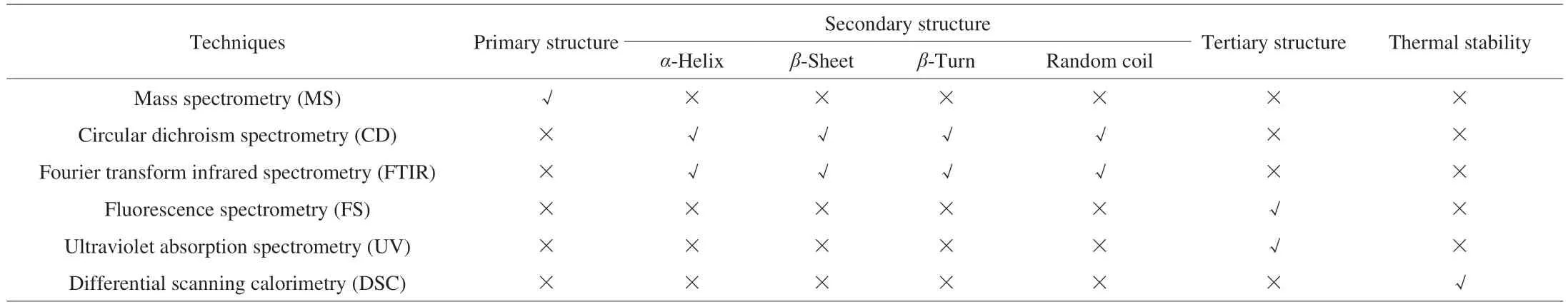
Table 4 Comparison of techniques for protein structural characterization.
4. Application of techniques for immunogenic characterization of allergenic food proteins
In addition to determining the physicochemical and structural properties of allergenic proteins, it is also essential to evaluate the associated immunogenic properties. This allows confirmation of the efficiency of an allergenicity reduction method. Many detection methods have been usedin vitroandin vivoto evaluate the immunogenicity of allergens, including immunological methods,polymerase chain reaction (PCR), biosensors, clinical human testing,and assessment of sensitized animal models.
4.1 In vitro testing
4.1.1 Food allergen detection by immunological methods
ELISA is commonly used for qualitative or relative quantitative detection of allergens in foods. It has also been used for detection of antibodies induced by food allergy in human serum. The reduction in allergen immunogenicity due to loss of a linear or conformational epitope (such as those caused by destruction or aggregation of allergenic proteins through food processing) can be revealed by ELISA[102]. Western blot involves protein separation by gel electrophoresis, transfer from the gel to a solid phase carrier,then detection of the allergenic protein with a specific antibody;this method has been widely used in measuring allergenicity of foods and for clinical diagnosis of allergic reactions[103]. Dot blot, an immunological method using a solid phase carrier without sample separation, can also be used for allergenicity detection. Compared with the western blot assay, it requires a smaller sample volume and is faster and easier to perform[104].
4.1.2 Food allergen detection by PCR
Although immunological methods have some limitations with respect to trace allergen detection, PCR can be used to measure the presence of allergens at the gene level[105]. PCR has been applied to the detection of many allergens in foods, such as the chestnut allergen Cas s 9, the buckwheat allergen Fag e 1, and celery allergens[106].Moreover, compared with immunological methods, PCR is more stable and sensitive (and therefore suitable) for the detection of allergens that are aggregated or destroyed by processing[107].
4.1.3 Food allergen detection with biosensors
Biosensors use immobilized bioactive materials as recognition elements and transform biochemical reactions into quantifiable physical or chemical signals through transducers. In the food industry,they can be applied to the detection of pathogens, additives, and allergens[108-109]. A variety of biosensors have been developed to detect food allergens. For instance, there is a disposable current-type DNA sensor for detecting the hazelnut allergen Cor a 9, a sensor based on fluorescence resonance energy transfer for rapid detection of OVA,and a current-type immune sensor for accurate detection of the shrimp allergen tropomyosin[110]. Biosensors have the advantages of high sensitivity, high specificity, and small size, and are therefore likely to be widely used for food allergen detection in the future.
4.2 In vivo testing
4.2.1 Clinical human testing
Commonly used clinical methods for allergen measurement are the skin prick test (SPT), atopic patch test (APT), intradermal test, and blood test. SPT refers to a subcutaneous introduction of allergen to detect the production of specific IgE antibody that binds to subcutaneous mast cells. SPT has high sensitivity, a low false positive rate, and high reproducibility; it causes minimal discomfort to patients and produces results within 15 min. APT involves application of patch tape with an allergen to a test site and examination of the reaction after 48–72 h. Intradermal test refers to intradermal injection of allergen; this method is more sensitive than SPT, but has a higher false positive rate and a high risk to patients.Blood tests are mainly used for patients who are suspected to have a food allergy but are unable to take a skin test. The main purpose of a blood test is to identify antibodies in the blood. Blood tests are safer and more accurate but also more expensive and time-consuming than skin tests[111]. Clinical human testing is rarely used for laboratory investigations due to ethical limitations.In vitrotests and assays based on sensitized animal models are safer and are broadly applicable in assessing food allergens.
4.2.2 Assessment of food allergen immunogenicity in animal models
Due to the safety and ethical limitations involved in human trials, sensitized animal models are necessary for the study of food allergens. Rodent models such as mouse, rat, and guinea pig are commonly used, although some studies have used non-rodent animals such as pigs or dogs. Pig and dog models have similar immune systems to humans, but they require administration of large amounts of protein and a long time period to sensitize, are expensive, and lack appropriate immunoadjuvants[112]. Rodents are widely used in assessing food allergenicity because of their small size and short reproductive cycle. The most common rodent models for food allergen evaluations are BALB/c, C3H/HeJ, A/J, C57BL/6, and KM mice; BN, SD, and Wistar rats; and Hartly guinea pigs.
When using animal models to evaluate food allergens, the selection of evaluation indicators is very important. Generally, food allergies cause an imbalance in Th1/Th2 immune responses in the body; levels of Th1 cytokines are lower than normal, whereas Th2 cytokine levels are higher. Interleukin-2 (IL-2) and interferon γ (IFN-γ)are the main cytokines produced by Th1 cells. IL-2 can stimulate Th1 cells to produce IFN-γ; IFN-γ can prevent the transformation of IgE antibody and suppress the Th2 immune response. IL-4, IL-5, and IL-13 are the main cytokines produced by Th2 cells; these promote the transformation of IgE, induce the transformation of IgG into IgG1, and stimulate mast cell proliferation, inducing systemic allergic reactions in the body[113]. Th17 cells can promote the production of IgE and IL-17A, stimulating allergic reactions[114]. Regulatory T cells(TReg cells) are another key factor in anaphylaxis; they secrete the cytokines IL-10 and/or TGF-β to suppress the immune responses of Th1, Th2, and Th17 cells, mast cells, basophils, and eosinophils, and inhibit the production of specific IgE to alleviate allergic reactions[115].Serum IgG and IgG1 levels are positively correlated with Th2 levels, and mast cell protease-1 (mMCP-1) is a key indicator of mast cell degranulation. Thus, the detection of IgG, IgG1, and mMCP-1 in serum is important in allergenic evaluation[63]. For instance,Zhang et al.[63]orally administered OVA and OVA–quercetin copolymers to BALB/c mice with cholera toxin used as an immunoadjuvant.Compared with OVA treatment, OVA–quercetin copolymer treatment reduced the levels of IgE, IgG1, IgG, histamine, and mMCP-1 in mouse serum and the Th2 cytokines IL-4, IL-5, and IL-13 in the mouse spleen. Levels of the Th1 cytokine IFN-γ were slightly increased. This suggested that OVA–quercetin copolymers can regulate the imbalance of Th1/Th2 immune responses and alleviate allergic reactions caused by OVA in BALB/c mice. Additionally,Yang et al.[113]orally administered soybean protein orLactobacillusfermented soybean protein to BALB/c mice. They found that secretion of IFN-γ, IL-2, and IL-10 increased and secretion of IL-4,IL-6, and IL-17A decreased in the fermented soybean protein group compared with the untreated soybean protein group. This indicated thatLactobacilluscould regulate the Th1/Th2 immune balance and maintain normal levels of immune cells and cytokines in BALB/c mice.
The methods of administration are very important to consider in evaluating food immunogenicity in animals. Oral administration,cutaneous exposure, intranasal administration, and intraperitoneal injection have been used to study food allergies[116]. Oral administration can mimic the effects of the human digestive tract on food allergens, but also can induce oral tolerance in experimental animals. Immunoadjuvants such as cholera toxin, aluminum hydroxide, lipopolysaccharide, andStaphylococcus aureusenterotoxin B are commonly used to enhance the immune activation of animals[117]. However, the use of immunoadjuvants may induce false positives and interfere with the assessment of food allergens. Some studies have therefore developed sensitized animal models without immunoadjuvants. Examples include the oral allergic syndrome and anaphylactic model of BALB/c mice caused by soybean glycinin andβ-conglycinin[118], the anaphylactic model of BALB/c mice caused by whey protein[119], and the anaphylactic model of BALB/c mice caused by hazelnut protein[120]. A study of the effects of milk allergens on allergenicity in C3H/HeJ mice through different administration routes suggested that cutaneous exposure could be a valid route to induce milk allergies[121]. The nasal mucosa has a thin membrane and abundant blood vessels, which can quickly absorb allergens[122].Intranasal administration can prevent the allergen from being digested by the gut or from causing liver toxicity[123]. Previous study showed that intranasal inhalation of OVA in mice exhibited more severe allergic asthma than intranasal infusion of OVA due to its greater diffusion area[124]. However, the hydrophilic macromolecules are not suitable for intranasal administration[122]. Intraperitoneal injection can induce more robust anaphylaxis responses compared with other administration routes. A previous study compared the impacts of different concentrations of OVA given through intraperitoneal injection or intranasal administration on allergic rhinitis in BALB/c mice. The anaphylactic responses of the mice were aggravated by high OVA concentrations through intranasal administration;however, severe anaphylactic responses were induced even at low concentrations of OVA through intraperitoneal injection[125]. This indicated that different administration routes could result in varied effects depending on the food allergen and animal strain being used.
The selection of a whole food, protein extract, or purified protein for administration in an animal model also significantly affects the immunogenic evaluation. Allergens may be enhanced or inhibited by the presence of food substrates including fats, carbohydrates,vitamins, minerals, contaminants, or other proteins. Wavrin et al.[126]investigated the effects of substrates on the evaluation of major milk protein allergenicity in BALB/cJ mice and found that milk,but not purifiedβ-LG, induced the production ofβ-LG-specific IgE and IgG1 after skin exposure. Similarly, intranasal exposure to milk induced more sensitive immunogenicity than purifiedβ-LG.Extraction conditions, such as temperature, pH, salt concentration,and extraction agent could affect the allergenicity of protein extracts.These factors may lead to changes in protein solubility and thus affect the immunogenicity of allergenic proteins. Previous studies showed that the addition of sodium dodecyl sulfate (SDS),β-mercaptoethanol(a reducing agent), or ethylene diamine tetraacetic acid (EDTA) in the process of protein extraction not only improved the extractability of microalbumin, but also enhanced its immunodetection ability[127].Another study demonstrated that the immune reactivity of sesame protein extracts prepared with water differed from those prepared with NaCl solutions at a range of concentrations; the immune reactivity was higher for sesame protein extracts prepared with water and with a lower concentration of NaCl solution (0.2 mol/L) than for extracts prepared with 0.6 mol/L or 1 mol/L NaCl. This could be because proteins extracted under different conditions do not have identical immunogenic properties. Another explanation is that a high concentration of salt solution caused salting out and aggregation of proteins, covering the antigenic epitopes of the protein extracts[128].It is therefore necessary to ensure the purity and quality of proteins,otherwise inaccurate results will be obtained during evaluation of protein immunogenicity in sensitized animal models.
Animal models play a vital role in the evaluation of food allergen immunogenicity, but the accuracy and validity of the results depend on the species or strains of experimental animals, administration routes,and the food allergen source and quality. It is critical to select an appropriate animal model and administration method, use high-quality allergens, and measure relevant allergy indicators to evaluate the immunogenicity of food allergens before and after processing.
5. Conclusions and prospects
Food processing can shield or destroy the linear and conformational epitopes of allergenic proteins, reducing their allergenicity. However,food processing can also produce new epitopes due to changes in protein conformation, enhancing allergenicity. Appropriate food processing techniques and parameters must be selected based on the structural and immunogenic properties of allergenic proteins to minimize allergenicity. Thermal processing, ultrasonic treatment,ultrahigh pressure treatment, microbial fermentation, cold plasma treatment, and covalent combination with dietary polyphenols are the most common processing techniques used to reduce food protein allergenicity; these typically function by masking, modifying,or destroying antigenic epitopes of allergenic food proteins.Novel techniques have been applied to alleviate the allergenicity of allergenic food proteins. These include pulsed electric field;pulsed light; primary structural modification such as glycosylation,methylation, phosphorylation, or acetylation; and enzymatic degradation to destroy the amide bonds of allergens. Most food processing techniques have been tested directly on purified food allergens, not food products, due to the potential interference of other food components, which could mask or protect allergens from being destroyed.
Combinations of processing methods have proven to be more efficient in reducing the allergenicity of food proteins by circumventing the limitations of a single technique. To select the appropriate food processing techniques and optimize processing parameters, it is important to characterize the physiochemical and structural properties of allergenic proteins by various technical means;these methods could include detection of the dityrosine, sulfhydryl,and free amino acid contents; identification of the linear and spatial structures using MS, CD, FTIR, FS, or UV spectroscopy; and thermal stability analysis by DSC. In addition, it is essential to evaluate the immunogenic properties throughbothin vitroandin vivoassays to further confirm the efficiency of allergenicity reduction by different processing methods. This review provides a scientific basis for further development of processing techniques to decrease the allergenicity of allergenic food proteins without impacting desirable nutritional and functional attributes of other food components.
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (32102605), the Agricultural Science and Technology Innovation Program under Grant (CAAS-ASTIP-2020-IAR), and the Earmarked Fund for CARS (CARS-44).
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango
- Learning about good nutrition with the 5-color front-of-package label“Nutri-Score”: an experimental study