Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango
2024-01-24HongleiGuoYanjunCong
Honglei Guo, Yanjun Cong
Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Food and Health, Beijing Technology and Business University, Beijing 100048, China
Keywords: Mango Allergen Epitope Immunocross-reactivity Prospects
ABSTRACT Mango (Mangifera indica L.) is a tropical fruit that is widely consumed as both fresh fruits and processed products around the world. The high incidence of mango allergy, on the other hand, has sparked widespread concern. Therefore, a summary and analysis of the current status and issues in mango allergen research can guide in-depth study on the mechanism of mango allergy and reveal effective desensitization methods. We described the incidence of fruit allergy, as well as the mechanism and clinical symptoms of mango allergy, in this review. We also looked into the structural properties of mango allergens, the effect of processing methods on mango allergens, prediction methods for mango allergen epitopes, and the current state of research on mango cross-reactive allergens and preventive measures. Finally, the research directions and ideas for the future are proposed and discussed.
1. Introduction
Mango (Mangifera indicaL.) is a member of the Anacardiaceae family. It grows in tropical and subtropical regions and is known as the “king of tropical fruits” because of its tender flesh, rich f lavor, and high nutritional value; it is consumed globally. Despite its growing popularity, mango has been identif ied as a signif icant allergen in several studies[1-6]. Although mango-like fruits do not fall into the World Food and Agriculture Organization’s 8 categories of allergenic foods, which primarily indicate highprotein foods such as milk, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, and soybeans, some studies have shown a high incidence of mango allergy[7].
2. Overview of fruit allergy
Food allergy is an immune response that repeatedly occurs after being exposed to a specific food. It could be immunoglobulin E(IgE) mediated (type I hypersensitivity reactions), cell-mediated, or a combination of the two[8]. The risk of allergic reactions associated with fruit consumption rises as living standards rise and fruit consumption rises. Although fruit allergies are not life-threatening,they are more common than other allergies, affecting 4.3% of the population[9]. The types of fruits that cause food allergies vary by country. According to data from European countries, the prevalence of fruit allergy in children is three times that of adults, accounting for more than 5% of the total child population[10]. Vegetables, fruits, and buckwheat cause 75% of food allergies in Japan[11]. Fruit allergens that cause allergic reactions in China are widely distributed and commonly found in Rosaceae family fruits such as apples and peaches, as well as tropical fruits such as pineapples, mangoes, and lychees[12].Mango is a popular tropical fruit that can cause allergic reactions in some people; however, no global statistical data on mango allergy exists. Mango allergies account for 0.3%, 6%, and 16% of all food allergy patients in Switzerland, France, and Thailand, respectively[13].Furthermore, the Chinese population with mango allergy is growing.For example, among 1 901 patients with allergic diseases in Kunming(China), the positive rate of mango allergen skin tests was 42.3%[14].The positive rate of mango allergen was 17.1% in 215 210 specific IgE tests performed by Wang and others[15]in the Department of Allergic Reactions of Peking Union Medical College Hospital from 2008 to 2010. Jia et al.[16]examined 4 622 children from the Shanxi(China) region who visited the pediatric department of Xijing Hospital for allergic diseases between March 2015 and February 2019 and discovered that the prevalence of mango allergy was 3.6%. Therefore,the prevalence of mango allergy varies significantly across countries,possibly due to differences in the types of mangos consumed, climate,dietary habits, geographic region, and genetic factors[17].
3. Mango allergy mechanism and clinical symptoms
Mango allergic reactions are typically classified as type I hypersensitivity or type IV delayed allergic reactions. Type I hypersensitivity mediated by IgE is a common immune reaction. The main symptoms of hypersensitivity induced by mango in humans include erythema, rubella masses, angioedema, asthmatic dyspnea,and gastroenteritis[18-22]. Type I hypersensitivity generally occurs within a few minutes to several minutes after non-first exposure to this type of antigen. Anaphylaxis caused by mango consumption has also been reported in some studies[23-24], but it is uncommon. Type IV delayed allergic reactions may manifest symptoms 8–72 h after non-first allergen exposure. Contact dermatitis, periorbital edema,an eczema-like rash around the lips, and blisters are symptoms of delayed allergic reactions caused by mangoes[25-29].
Fig. 1 depicts the mechanism underlying the allergic reaction to mango, which is primarily mediated by IgE. Allergen proteins are internalized and processed by antigen-presenting cells before being passed on to CD4+T cells via the T helper 2 cell (Th2) pathway.Following Th2 cell activation, the secretion of interleukin (IL)-4 and IL-12 induces specific B-cells to produce IgE antibodies that bind specifically to Fc receptors (FcεRI) on the surface of mast cells or basophils. Therefore, substances such as histamine are produced,which act on cellular tissues to sensitize the organism[30]. If there is no prolonged exposure to the same or similar allergen, this state will disappear on its own. When the same or similar allergens re-enter the body, it enters an activation state. These allergens bind to two or more adjacent IgE antibodies on the surface of mast cells. Therefore,mast cell degranulation is activated, and many cytokines are released,including both primary inflammatory mediators (histamine, proteases,and acid hydrolases) and secondary inflammatory mediators(leukotrienes, prostaglandins, and platelet-activating factors)[31]. In the effector phase, cytokines cause vasodilation and the recruitment of eosinophils and leukocytes, resulting in inflammation. The delayed allergic response is cell-mediated, with Th1-type CD-4 cells serving as the primary mediators.
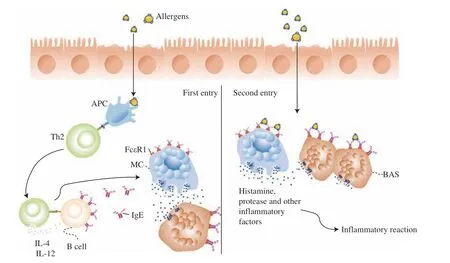
Fig. 1 IgE-mediated mechanism of mango protein allergy. This is a schematic diagram of IgE mediated mango protein allergy mechanism.
4. Current advances of mango allergens
According to earlier studies and the Allergome database (https://allergome.org), the main mango allergens are glyceraldehyde-3-phosphate dehydrogenase, 30 kDa protein, profilin, Man I chitinase,β-1,3-glucanase, lipoxygenase, and Bet v 1-like homolog (Man i 14 kDa).According to the naming criteria of the World Health Organization/International Union of Immunological Societies (WHO/IUIS)Sub-Committee on Allergen Nomenclature, glyceraldehyde-3-phosphate dehydrogenase, 30 kDa protein, profilin, Man I chitinase,and Bet v 1-like homologous protein were named as Man i 1, Man i 2,Man i 3, Man I chitinase, and Man i 14 kDa[32]. Man i 1 and Man i 2 were identified as major allergens by immunoblotting using sera from mango sensitized patients and mouse monoclonal or polyclonal antibodies[32-33]. Their details are given in Table 1, and their protein structures are schematically shown in Fig. 2.
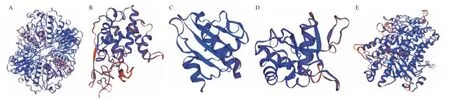
Fig. 2 3D structure of mango allergens. These are mango allergens of Man i 1 (A), Man I chitinase (B), and Man i 3 (C), β-1,3-glucanase (D), lipoxygenase (E),sourcing from uniport protein data bank.
Tsai et al.[34]used a recombinant plasmid to express a recombinant Man i 1 protein inEscherichia coliBL21 (DE3). Cao[33]used sera from mango allergic patients and mice sera to identify mango proteins and discovered a 30 kDa chitinase known as Man i 2. They expressed it inE. coliBL21 (DE3) and purified the recombinant protein. Xie[35]performed a mass spectrometry analysis of mango proteins in 2020 and discovered that the allergen Man i 2 belonged to the chitinase protein family. Plant-protective enzymes that defend against fungi and pestsin vivo, Class I chitinase allergens, can be detected in humans using a serum assay, particularly in asthmatic populations[36].
Profilin allergen is a pan-allergen with a structure similar to Artemisia defensin (Art v 1)[37]. It is a highly structurally conserved protein found in all eukaryotic cells and involved in cell motility processes. Using sera from mango allergic patients, Zhang et al.[38]performed a Western blot assay and identified a reactive band at 13 kDa as an profilin. They cloned and expressed the profilin protein from the mango fruit and obtained 14.97 and 13.99 kDa profilin proteins, Man i 3.01 and Man i 3.02[39].
Paschke et al.[40]identified Bet v 1-like homologous proteins for the first time by using enzyme allergosorbent test (EAST) inhibition and immunoblot inhibition on sera from nine patients.
The pathogenesis-related (PR-2) enzymeβ-1,3-glucanase has a protective response to pathogenic attacks. PR-2 enzymes in plants have antifungal activity, making them an important tool in crop resistance strategies against fungal pathogens[41]. Lipoxygenases are involved in many physiological activities in plants, including growth and development, pest resistance, senescence, and other responses[41].
5. Effect of processing methods on the allergenicity of mangoes
Mango is consumed throughout the year, besides fresh food,it can also be processed into milkshakes, jam, purees, dehydrated,frozen fruit juice as well as pickled mango, and is well liked by wide consumers[42]. The effect of different processing methods on mango antigenicity has been investigated as the consumer market for processed mango products has grown. Dube et al.[43]investigated the allergenicity of mangoes using various heating methods and enzymatic treatments. They discovered the mango allergens, especially Man i 1, remain stable in the processed products irrespective of the technological treatments, which include heating,mechanical or enzymatic tissue disintegration. Furthermore, no significant reduction in allergenicity was found in pure fructose and mango nectar products[44]. Most mango proteins lose thermal stability as their molecular weight increases, and the allergenicity of different mango potential allergens shows different and complex trends as heating time increases. Surprisingly, a new allergen of 34 kDa in mangoes has been discovered to be heat stable[45]. Generally, mango is processed in a thermal manner, but this often affects the quality attributes of the final product[46]. Due to the lack of an any efficient processing technology for controlling mango allergy, thus there have been a few studies needed to be done on the allergenicity of mango allergen under different processing methods, which are one of the key research directions for the future.
6. Current advances on the epitopes of mango allergens
A sequence structure specific to an antigen recognized by an antibody or antigen receptor is referred to as an epitope[47]. T-cell antigen epitopes and B-cell antigen epitopes are the two types. The Immune Epitope Database (IEDB) (https://www.IEDB.org) is a useful epitope repository that categorizes experimentally mapped B-cell and T-cell epitopes[48]. Peptide epitopes can be linear peptides or threedimensional structures made up of discontinuous peptides or amino acids, which are known as conformational epitopes. B-cell linear epitopes are typically 8−15 amino acids (aa) long[49]. Although the linear epitopes with five amino acids have been reported, the linear epitopes with highaffinity binding to IgE generally requires at least 8 aa[50].
Various bioinformatics methods for predicting allergen epitopes have been developed using computer technology and epitope databases. These prediction tools are simple, quick, and inexpensive,and they can serve as a foundation for serological methods to identify antigenic epitopes and save time. Linear epitope prediction is at the base of the physico-chemical properties of allergen protein, including hydrophilicity, secondary structure, accessibility, flexibility, and the conformational epitope prediction involves based on protein structure information and combined with phage display technology[51-53]. All of the predictive methods have certain limitations when they are used, so when performing epitope prediction, they are usually combined with multiple methods for comprehensive analysis. Several approaches had been used to predict the allergenic epitopes (Table 2). Disco tope,Bepibred, Ellipro, MHCpred, SYFPEITHI, NetMHC II, and NetMHC II pan are the most commonly used. Finally, 3D models based on predicted epitopes can be created using PyMoL or SWISS-MODEL to visualize the epitope.
There are several methods for identifying and validating epitopes,including the enzyme-linked immunosorbent assay[58-59], spot blotting[60],immunoblotting[61], peptide microarray[61], and hydrogen/deuterium exchange[62]. The enzyme-linked immunosorbent assay[63-65],spot blotting[66-67], protein microarray[68], hydrogen/deuterium exchange[69], X-ray (diffraction) crystallography[70-72], and nuclear magnetic resonance[73]can all be used to identify B-cell conformational epitopes. T-cell proliferation (3H-labeled thymidine incorporation[74-75], carboxyfluorescein succinimidyl ester dilution assay[76], intracellular cytokine staining[76]), and tetramer-guided epitope localization[77]are the methods used to identify T-cell epitopes.
Mango epitope research is in its early stages, with a focus on bioinformatics prediction and less on experimental validation. The majority of mango epitope studies have focused on B-cell epitopes,and studies on mango T-cell epitopes are unknown.
Tsai et al.[34]used multiple sequence alignment to analyze the homology of the Indian mango, wheat, and peanut and annotated the similar sequences of glyceraldehyde-3-phosphate dehydrogenase in mango, wheat, and peanut as aa 154–164, aa 215–222, and aa 318–326. These sequences were not confirmed as B-cell antigenic epitopes of mango glyceraldehyde-3-phosphate dehydrogenase by serological methods or animal experiments.
There have been few studies on the epitopes of mango profilin protein. Yan et al.[78]used the SMART software to analyze the structural domain of mango inhibitor protein and compared the amino acid sequences of mango with those of inhibitor proteins in grape,peach, citrus, and litchi and discovered a high degree of homology,but no experimental validation was performed.
Yan et al.[78]used the SMART software to analyze the structural domains of chitinase and proposed that these two structural domains could be chitinase epitopes; however, they did not validate their hypothesis experimentally. Cao et al.[33]predicted the B-cell linear epitopes of mango chitinase using Bepibred and ABCpred, predicted 6 IgE linear epitopes, and performed identification tests using speckleblotting with mango-sensitized mouse sera, and discovered that all 6 sera reacted with 8−28 aa (titin-binding domain sequence), which was identified as the major linear IgE-binding epitope. Zhang et al.[79]discovered high homology by comparing the protein sequences of mango chitinase with those of citrus, longan, poplar, and walnut chitinases. The possible regions were 38−46, 64, 67, 75−76, 89,97−101, 115, 122, 124−126, 128−129, 133−138, 144−150, 152, 176,178−181, and 222−225.
Aside from the studies on the epitopes of mango allergens Man i 1,Man i 2 and Man i 3 mentioned above, no studies on the B-cell and T-cell epitopes of other mango allergens, such as lipoxygenase andβ-1,3-glucanase, have been reported. Our group predicted the B-cell antigenic epitopes of mango lipoxygenase andβ-1,3-glucanase in the preliminary stage (to be published) using the Bepipred online tool,and the results are shown in Table 3. At a later stage, the findings will be validated using serological methods or animal experiments.
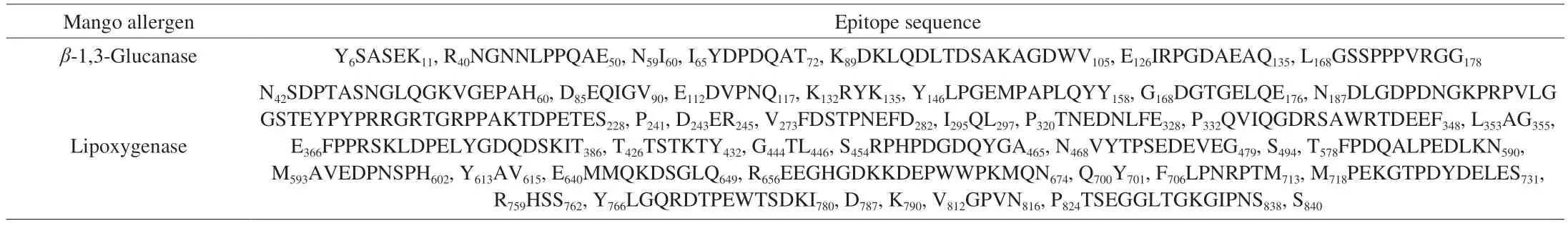
Table 3 Prediction results of mango antigenic epitopes.
Understanding the pathogenesis of mango allergy and developing accurate detection, diagnosis, and treatment methods require determining the epitope characteristics of mango allergens. B-cell conformational epitopes are important in mango allergy, but they have received far less attention than linear epitopes and, as a result, require more research.
7. Current advances on mango cross-reactive allergens
Some patients are allergic to specific foods, and when they consume other homologous or non-homologous foods, they may experience an allergic reaction, a condition known as cross-reactive allergy[80]. Cross-reactions to various allergens are possible in the same body. The OASIS study explained the mechanism of an allergic reaction; an allergic reaction may occur because two antigens have similar structural antigenic epitopes, causing IgE antibodies to bind to both and thus cause a cross-reaction[81]. The precise mechanism of immunopathology that leads to cross-allergy between different foods is unknown. Cross-allergy between allergens has received a lot of attention recently, so mechanistic studies on cross-allergic reactions have become essential.
FASTA search (using the entire sequence as a query) is the traditional method for mango cross-reactive allergen prediction,in which each query protein is resolved into a sliding window of 80 aa, and the protein amino acids to be tested are compared with the known allergen sequence. A match with > 35% sequence similarity is identified as potentially having a cross-reaction. The 1:1 FASTA with anE-value of 1.0 × 10−9as a criterion outperforms the traditional FASTA search. The 1:1 FASTA method eliminates three technical challenges: 1) the problem of differentE-values due to a lack of short sequences with high consistency for known allergens; 2) high consistency between short amino acid sequences, and 3) the problem of searching for > 35% sequence similarity greater than 80 aa. A thresholdE-value of < 1.0 × 10−9indicates the possibility of crossreactivity[82-83]. The lower theE-value, the more likely it is that the comparison of two allergen proteins will reflect true structural similarity[84].
The recognition of IgE antibodies is a common method for confirming the accuracy of immunologic cross-reactivity prediction results. Polyclonal IgE antibodies produce a high number of false positives, making accurate allergen cross-reactivity verification difficult. Some IgE is triggered by allergen-specific epitopes, while others are triggered by epitopes that can cause cross-reactivity;the proportion of this cross-reactive IgE varies depending on the patient[85]. According to some studies, immunological cross-reactivity is specific and occurs only when serum IgE antibodies from allergic patients bind to allergen-conserved epitopes. Because conformational epitopes are easily degraded during digestion in the gastrointestinal tract, linear IgE-binding epitopes are more relevant in the case of food cross-allergy[86]. Cross-reactivity strength is related to IgE antibody affinity; however, not all IgE cross-reactivity translates into clinical cross-reactivity. Therefore,in vivoexperimental validation is critical.
Identification of mango cross-reactive allergens can provide a theoretical foundation for the rational diet of mango allergy patients.This could help to avoid cross-allergy while also providing ideas and data for developing accuratein vitroor clinical methods for detecting mango allergens. Wuthrich et al.[87]were the first to propose that celery-artemisia allergy was linked to mango allergy in 1984.Because of the frequency of such cases of food cross-reactivity, it was hypothesized that there might be cross-reactivity between mango and other foods and inhalant allergens[87]. Paschke et al.[40]discovered the cross-reactivity of mango allergens with Artemisia pollen, birch pollen, celery, and carrot crude extracts. The immunoblot inhibition assay revealed that the proteins, which are about 14 kDa in mango,are cross-reactive with Bet v 1, which is found in birch pollen.Furthermore, mango’s cross-reactivity with Artemisia pollen, birch pollen, celery, and carrot were primarily associated with proteins of approximately 40, 43, and 67 kDa. Oka et al.[26]used the patch test to discover mango’s cross-reactivity with urushiol. The amino acid sequence of mango profilin protein has high homology (> 70%) with other profilin proteins from pollen, fruits, and vegetables, according to Song et al.[39]. Furthermore, the immunoblot and ELISA inhibition assays confirmed that the profilin protein was one of the causes of mango cross-reactivity with other foods or pollen (e.g., birch pollen).The mango profilin proteins were found to be highly similar to those found in other plants, including the cherry allergen Pru av 4, the pear allergen Pyr c 4, the peach allergen Pru p 4, the apple allergen Mal d 4,the peanut allergen Ara h 5, the carrot allergen Dau c 4, and the birch allergen Bet v 2. By comparing the amino acid sequences of inhibitory proteins in fruits, Yan et al.[78]discovered a high homology between mango and grape, peach, citrus, and lychee. However,no experimental validation was carried out. Mango allergens may react with latex allergens, resulting in latex fruit syndrome[88-89].By using the radioallergosorbent test (RAST) inhibition test,Funes et al.[90]discovered cross-reactivity between three lacrimal foods, namely pistachio, cashew, and mango. Other “hot” fruits, such as citrus and lychee, contain the mango allergen chitinase[91]. Through the ImmunoCap ISAC test, Ukleja-Sokolowska et al.[37]discovered significantly elevated levels of specific IgE in cattail and Artemisia asiatica in patients allergic to mangoes. Through the ImmunoCap IgE inhibition test with mango extracts, they discovered that mango and Art v 1 of Artemisia asiatica could cause cross-allergic reactions.Elvin et al.[41]used a combinatorial peptide ligand library (CPLL)approach combined with immunoproteomics to enrich low abundant proteins in mango extracts in 2018. Using sera from mango-allergic patients and immunoblotting experiments, they identified 12 protein fractions of mango allergens. They discovered that mango may cross-react with three banana fractions, Mus a 1, Mus a 2, and Mus a 5.In the same year, Bastiaan et al.[92]conducted immunoblotting experiments with cashew allergy serum and discovered significant cross-reactivity between mango and cashew, which could be attributed to chitinase andβ-1,3-glucanase.
In our early stage, the potential immunologic cross-reactivity of mango allergens with 8 major allergen groups and some fruits and vegetables was predicted using the BLAST online tool, and the results are shown in Table 4. Mango Man i 1 was found to be highly homologous with wheat; mango Man I chitinase was found to be homologous with wheat and banana; mango Man i 3 was found to be homologous with wheat, peanut, almond, shrimp, hazelnut, pistachio,peach, lychee, banana, apple, pear, celery, and carrot; and mangoβ-1,3-glucanase was found to be homologous with banana Mus a 5(to be published). In the future, the predicted results will be validated using animal antibodies and sera from mango-allergic patients.

Table 4 Potential mango cross-reactive allergens.
8. Prevention of mango cross-allergy
First and foremost, it is critical to limit or avoid exposure to mango and its cross-allergens. Of type I allergic reactions, a distinction must be made between the sensitized dose that causes sensitization and the allergen dose that triggers allergy symptoms. Even trace allergens can cause sensitization and severe allergic symptoms in allergic patients[93]. Second, the allergens and cross-allergens in mango fruit should be identified. Understanding individuals’ clinical crossreactivity symptoms to the relevant allergens are the key to better prevention, management, and development of novel therapeutic techniques. Clinical cross-reactivity occurs most commonly in mango allergy when patients consume proteins that are similar in sequence to mango allergens or contain antibody-binding epitopes that cause severe allergic symptoms. Extensive research on the epitopes of IgE antibody-bound cross-allergens can provide ideas and a foundation for clinical treatment, preventing and treating cross-allergy[94]. Third,food companies should strictly adhere to food labeling regulations and label allergen information on food packaging accurately. Furthermore,it is critical to avoid cross-allergen contact or contamination during the food manufacturing process, as well as to ensure the accuracy and precision of quantitative allergen detection methods in processed foods[95]. Fourth, reliable analytical methods are required to detect and identify allergenic ingredients in food products. In recent years,the new detection methods of food allergens have emerged, including enhanced traditional detection techniques (ic-ELISA) and emerging detection techniques with the ability high-throughput detection or screening potential food allergen, such as flexible Multi-Analyte Profiling (xMAP), biosensors, biochips, etc.[96-97]. Tsai et al.[98]developed a rapid lateral flow assay (LFA) that uses immunomagnetic nanoparticles (IMNPs) to detect mango major allergen (Man i 1)in food products. A loop-mediated isothermal amplification(LAMP) assay was developed for detection of mango in food[99].Finally, some studies have shown that early food allergen intake intervention in infants and young children is an effective preventive measure; however, the specific mechanism of its preventive effect is unknown, but it may be related to the induction of allergen-specific immunoglobulin G (IgG) 4 production and the promotion of immune tolerance[100]. Several studies have shown that introducing allergenic foods into an infant’s diet as early as 6 months of age can reduce the occurrence of food allergy by inducing oral tolerance[101].
9. Conclusions
Despite its high nutritional value and rich flavor, mango has been identified as a significant allergen in several studies. Mango allergy are typically classified as type I hypersensitivity or type IV delayed allergic reactions. Mango allergens are glyceraldehyde-3-phosphate dehydrogenase (Man i 1), 30 kDa protein (Man i 2), profilin (Man i 3),Man I chitinase,β-1,3-glucanase, lipoxygenase, and Bet v 1-like homolog (Man i 14 kDa), and some new allergens are yet to be discovered and identified. There is no change in the antigenicity of other mango allergens with the exception of Man i 1 allergen using various heating methods and enzymatic treatments, which is one of the key research directions for the future. Mango epitope research is in its early stages, with a focus on bioinformatics prediction and less on experimental validation. The majority of mango epitope studies have focused on B-cell epitopes, and studies on mango T-cell epitopes are unknown. Some studies preliminarily indicated that the crossreactivity of mango allergens with Artemisia pollen, birch pollen,celery, carrot crude extracts grape, peach, banana, citrus, lychee, latex allergens, pistachio, cashew, cattail and Artemisia asiatica, and further studies need to be carried out.
10. Suggestions and prospects
(1) Improving protein structure information and mango allergen epitope mapping. The structural characteristics and properties of the mango allergens Bet v 1-like homologous protein, lipoxygenase, andβ-1,3-glucanase are unknown. It is critical to establish an efficient and high-purity separation and purification system for mango allergens and other potential allergens. This study on mango allergen B-cell and T-cell epitopes relied heavily on bioinformatics prediction which needs to be validated further through serology,in vitroimmune cell experiments, and animal experiments. Standardization of methods for identifying allergens and their epitopes is necessary to improve the accuracy of results.
(2) In-depth research on mango cross-allergens and cross-reactive epitopes. The correlation of mango allergens with other allergens at the molecular level has been a hot topic of research. The research on mango cross-allergens and their epitopes is still in its early stages.Mango cross-allergen identification and targeted analysis of crossreactive epitopes are critical for clinical diagnosis and treatment of mango allergy. Creating a database of mango cross-allergens and their epitopes can provide a theoretical foundation for mango allergy patients’ rational diets in order to avoid cross-allergies and protect people.
(3) Develop precise methods for detecting mango allergens and epitopes and diagnosing mango allergy. It is a need for establishing a stable and precise animal evaluation model and cell evaluation model in order to determine the threshold values of the characteristic effect values of mango potential allergens and epitopes. Multiphoton microscopy must be used to rapidly observe tissue and cellular dynamic changes in model mice after allergen ingestion, and a visual evaluation model must be built. To create a three-dimensional cell model of the intestinal mucosa, intestinal epithelial cells, immune cells related to allergic processes, and effector cells could be cultured.To establish a high-throughput cell sensing model, a microfluidic intestinal cell chip could be developed. To develop an isolated culture differential evaluation model, indirect isolated cell co-culture and multi-cell three-dimensional hierarchical culture could be utilized.So far there are no accurate and sensitivein vitrodiagnostic kits for mango allergy. So clinical diagnosis is primarily dependent onin vivodiagnostic methods. Analyzing the structural information of allergens,preparing an epitope antibody library or a serum library based on mango specific allergens, developing an accurate and sensitive detection method for the clinical diagnosis and treatment of mango allergy, and developingin vitrokits all require investigating the mechanism of mango allergy.
Declaration of conflict interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by National Science and Technology Major Project of China (2019YFC1605002) and National Natural Science Foundation of China (31872886).
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Learning about good nutrition with the 5-color front-of-package label“Nutri-Score”: an experimental study