Structural insights on anti-biof ilm mechanism of heated slightly acidic electrolyzed water technology against multi-resistant Staphylococcus aureus biof ilm on food contact surface
2024-01-24PinpinYnRmchnrnChellihKyoungHeeJoXiuqinChenAknkshTygiHyeonYeongJoFzleElhiNmChnWooMinSeungWookDeogHwnOh
Pinpin Yn, Rmchnrn Chellih,b,c, Kyoung Hee Jo, Xiuqin Chen, Aknksh Tygi,Hyeon Yeong Jo, Fzle Elhi, Nm Chn Woo, Min Seung Wook, Deog Hwn Oh,
a Department of Food Science and Biotechnology, College of Agriculture and Life Sciences, Kangwon National University, Chuncheon 24341, South Korea
b Kangwon Institute ofInclusive Technology (KIIT), Kangwon National University, Chuncheon 24341, South Korea
c Saveetha School of Engineering, (SIMATS) University, Sriperumbudur 600124, India
d Seoulin Bioscience Company, Seongnam-si 13488, South Korea
Keywords: Multi-resistant Staphylococcus aureus Metabolic prof ile SAEW Biof ilm Hurdle technology Electrode material
ABSTRACT Slightly acidic electrolyzed water (SAEW) has proven to be an efficient and novel sanitizer in food and agriculture field. This study assessed the efficacy of SAEW (30 mg/L) at 40 °C on the inactivation of foodborne pathogens and detachment of multi-resistant Staphylococcus aureus (MRSA) biof ilm. Furthermore,the underlying mechanism of MRSA biof ilm under heated SAEW at 40 °C treatment on metabolic prof iles was investigated. The results showed that the heated SAEW at 40 °C significantly effectively against foodborne pathogens of 1.96–7.56 (lg (CFU/g)) reduction in pork, chicken, spinach, and lettuce. The heated SAEW at 40 °C treatment signif icantly reduced MRSA biof ilm cells by 2.41 (lg (CFU/cm2)). The synergistic effect of SAEW treatment showed intense anti-biofilm activity in decreasing cell density and impairing biof ilm cell membranes. Global metabolic response of MRSA biof ilms, treated by SAEW at 40 °C, revealed the alterations of intracellular metabolites, including amino acids, organic acid, fatty acid, and lipid. Moreover,signaling pathways involved in amino acid metabolism, energy metabolism, nucleotide synthesis, carbohydrate metabolites, and lipid biosynthesis were functionally disrupted by the SAEW at 40 °C treatment. As per our knowledge, this is the f irst research to uncover the potential mechanism of heated SAEW treatment against MRSA biof ilm on food contact surface.
1. Introduction
Antimicrobial resistance has become the most critical and severe world issue in recent years, compromising food safety and public health. Multi-resistantStaphylococcus aureus(MRSA) is an opportunistic pathogen and biofilm producer and acquires various drug-resistant genes[1]. The World Health Organization has recently listed MRSA as a high-priority antibiotic-resistant pathogen[2].In addition,S. aureus, is known to form biofilms on both abiotic and biotic surfaces, which gain considerable resistance to various antimicrobial agents, severe environmental conditions, and host immunity[3]. Previous reports show that 64.8% ofS. aureusisolated strains exhibited a high biof ilm formation, and 90.3% were resistant to at least one antimicrobial[4]. More signif icantly, biof ilm growth can greatly stimulate spontaneous mutations inS. aureusand enhance the ability to acquire or propagate antimicrobial resistance determinants via horizontal gene transfer[5]. The contamination and infection caused by MRSA are challenging to treat, mainly due to antibiotic resistance and limited penetration of sanitizers to the MRSA biof ilm matrix[6].Their association with the cross-contamination of food contact surfaces and particular foods, such as ready-to-eat products, fruits and vegetables, has increased public health concerns. Therefore, it is crucial to develop innovative anti-MRSA biofilm strategies.
In food and agriculture fields, slightly acidic electrolyzed water(SAEW) has been shown to be an efficient and novel sanitizer. A solution known as SAEW is generated when a diluted electrolyte,including HCl and/or NaCl, is electrolyzed in an electrolytic cell without a diaphragm[7]. Hypochloric acid (HOCl) generated from SAEW is 80 times effective than an equivalent hypochlorite ion (ClO−) concentration for inactivating foodborne pathogens,includingSalmonella Enteritidis,Escherichia coliO157:H7,Listeria monocytogenes, andS. aureus[8]. The commercially generated SAEW,possessing the added advantage of being low-cost, easy-to-use, and eco-friendly, is becoming increasingly popular in the food industry.The researcher found that various critical reaction parameters, such as electrode material, water hardness, and electrolyte composition,significantly impacted the production of such oxidants (Cl2, ClO−,and HOCl)[9]. One of the essential critical parameters influencing the yield and species of produced oxidants is the electrode material[10-11].During the electrolysis, ruthenium (Ru), iridium (Ir), and platinum(Pt) as platinum group metals (PGMs) used catalysts on electrodes can increase chlorine production by reducing the redox potential of chlorine ions. However, developing a highly efficient SAEW generator through optimizing the type and shape of electrode material has rarely been studied.
Furthermore, to enhance the antimicrobial activity of SAEW,the researchers used the hurdle technology to combine SAEW with mild heat, organic acid, ultraviolet light, and ultrasound[12]. It was hypothesized that hurdle technology using a combination treatment of heated water with SAEW would assist in reducing microbial load than cold water alone[13]. However, it is commonly expected to produce the SAEW and then heat it to the targeted temperature for studies of the exact nature. The effect of heated SAEW on the MRSA biofilm mechanism is still not well explored.
Metabolomics can track the overall environmental stress results on the cell[14]. In recent days, cellular metabolomics may reveal how the bacteria respond to stress by analyzing the whole metabolite pool[15-16]. Mass spectrometry (MS) has emerged as a prominent tool for metabolic profiling, making the high-throughput profiles of bacteria metabolic states more possible and creating a complete understanding of the mechanisms underlying oxidative stress and ensuring bacterial response[17]. Thus, the integrated metabolomics of MRSA biofilm treated with SAEW and preheating combined treatment may offer valuable information regarding the adaptive processes.
Therefore, the objectives of this study were: 1) to optimize the SAEW generation system, including the electrode material and temperature variation and the stability and antimicrobial effect of SAEW; 2) to assess the synergistic impact of SAEW and preheating on the inactivation of biofilm cells and detachment of the MRSA biofilm; 3) to investigate the metabolic changes and distributed pathway of MRSA biofilm in response to SAEW and preheating treatment. To the best of our knowledge, this is the first study on the effectiveness and biofilm metabolism of SAEW combined with preheating as an antimicrobial agent on MRSA biofilm’s metabolic response.
2. Materials and methods
2.1 Bacterial cultures and growth conditions
The list of microbes applied in the study such asS. aureus(ATCC 13565),S. aureusCCARM 3080 (MRSA),S. enteritidis(ATCC 13076),E. coliO157:H7 (ATCC 43895),Bacillus cereus(ATCC 10987), andL. monocytogenesScott A (ATCC 43251) was obtained from Department of Food Science and Biotechnology,Kangwon National University. The 6 strains were activated from a frozen stock at −80 °C, and 100 μL of the stock was inoculated into 8 mL of fresh Brain Heart Infusion Broth (BHI; Becton Dickinson Diagnostic Systems, Sparks, MD, USA). Then, the prepared solution was transferred twice at 37 °C for 18–24 h and used for the following experiments.
2.2 Optimization of SAEW generation conditions
2.2.1 Optimization of electrode material
The influence of electrode material on free chlorine generation was studied using an innocuous electrolyte. The following mixture optimized the electrolyte: 1% HCl at 0, 1, 2, and 2 mol/L NaCl, 1.5%HCl at 0, 1, 2, and 2 mol/L NaCl, and 2.0% HCl at 0, 1, 2, and 2 mol/L NaCl. The anode, with a working geometric area of 4 170 mm2,was made of three different electrode materials, including Ru,Ir and Pt. Each electrode material has two types of shapes: plate and mesh. The titanium (Ti) was applied for the cathode. The electrolyte materials were applied in the resultant electrochemical cell, shown in Fig. S1. The constant current density ranged from 6 to 8 ampere during the electrolysis. The electrolyzed water samples were collected at timed intervals (1, 2, and 3 h), and the available chlorine concentration (ACC) and pH were analyzed,as previously described by Yan et al.[18]. Briefly, a dual-scale pH meter was used to determine the pH of all produced SAEWs(Accumet model 15; Fisher Scientific Co., Hampton, VA, USA).The ACC was measured using a digital chlorine test (20J3A;Kasahara Chemical Instruments Corp., Japan).
2.2.2 SAEW storage test
The changes in bactericidal effect and physicochemical properties of SAEW under two storage condition (close-30 and 50 mg/L and open-30 and 50 mg/L) were tested in this study. The bottle material was made of high density polyethylen with a volume of 1 L. The 4 samples were stored in a growth chamber (Biofree, Bucheon,Korea) at 25 °C and 60% humidity for 6 months. The properties of the sample (pH and ACC) were tested on the storage of 0, 3, and 6 months. Meanwhile, the bactericidal efficacy was assessed at 0,3, and 6 months after storage using MRSA andS. aureus13565,S. enteritidis,L. monocytogenesScott A,E. coliO157:H7, andB. cereusbroth culture[18]. Briefly, 1 mL of targeted bacterial suspension(108CFU/mL) was mixed with 9 mL of tested SAEW solution, shaken immediately and reacted for 1 min. Further, the residual SAEW of the sample was neutralized using a solution containing 0.5% sodium thiosulphate and 0.85% sodium chloride. The serial dilutions were performed using buffered peptone water (0.1% BPW; Difco, Sparks,MD, USA), and 100 μL of the bacterial suspensions were spread out on BHI plates and incubated at 37 °C overnight.
2.2.3 Effect of heated SAEW on sanitization efficacy
The effect of heated SAEW on sanitization efficacy was studied at 30, 40, and 50 °C. The sterilization water (SAEW) generator of this research was provided by Seoulin Bioscience (Seongnam,South Korea, ecoTree®). The pH and ACC of fresh-produced SAEW were tested immediately. The bactericidal efficacy of heated SAEW was studied usingS. aureus13565,L. monocytogenesScott A,S. enteritidis,E. coliO157:H7, andB. cereus, broth cultures, as followed by section 2.2.1. In addition, the disinfection of heated SAEW at 40 °Cin vivofollowed Yan et al.[18]. Spinach, lettuce, chicken, and pork were purchased in the local market. A cocktail ofS. enteritidis,L. monocytogenes,S. aureus, andE. coliO157:H7 at a concentration of 1 × 108CFU/mL was inoculated on the surface of each chicken,lettuce, pork, and spinach sample, respectively. Subsequently, the inoculated samples were immersed in 200 mL of each treatment solution (distilled water (DW) at room temperature, 30, 40, and 50 °C; SAEW at room temperature, 30, 40, and 50 °C) and shaken for 1 min. After treatment, the samples were immediately added to the 200 mL of neutralizing solution and reacted for 1 min to neutralize the residual SAEW. The serial dilutions were performed using BPW,and 100 μL of the bacterial suspensions were then spread out on BHI plates, and incubated at 37 °C for 12 h, following the reference of Forghani et al.[19].
2.3 Elimination of biofilm with SAEW treatment
MRSA andS. aureus13565 were cultivated from a single colony and incubated overnight. Sterile stainless steel (1.8 cm × 1.8 cm)was put into 12-well microtiter plates. Then, 4 mL of approximately 106CFU of MRSA orS. aureus13565 cells was added into each well respectively and incubated at 37 °C anaerobically for 48 h to form biofilm. After the mature biofilms had developed, the coupons were taken out of the media using terile forceps. The coupons were rinsed thrice with 0.1% sterile peptone water to remove unattached cells.Further, the mature MRSA andS. aureus13565 biofilms were put into SAEW solution (4 mL) and allowed to react for 5 min.
2.3.1 Quantification of biofilm production
After treatment, biofilm production on the coupon was quantified using the crystal violet (CV) staining assay. Briefly, each coupon biofilm was washed thrice with phosphate buffered saline (PBS) to remove loosely attached cells and was dried for 30 min. After drying,each coupon was stained with 4 mL of autoclave 0.1% (m/V) CV solution incubated for 30 min at room temperature. The coupons were washed with PBS. The dye adhering to the biofilm cell on the coupon was re-soluble in 4 mL of 95.0% ethanol for 45 min. The samples’optical density (OD) was measured at 600 nm using a microplate reader (Infinite 200, Tecan, Switzerland).
2.3.2 Cell enumeration
After treatment, the coupon was transferred into a 50 mL sterile tube containing 10 mL BPW and vortexed for 1 min to detach MRSA andS. aureus13565 from the stainless coupon. After serial dilutions(1:10) with 9 mL of BPW, the bacterial suspensions were grown using BHI media using spread plate technique. Further, the colonies were enumerated after incubating at 37 °C for 24 h and the results were expressed as lg (CFU/cm2).
2.3.3 Confocal laser scanning microscopy (CLSM)
The MRSA andS. aureus13565 biofilms were further observed by CLSM according to the method of Yan et al.[20]. Briefly, the biofilm on the coupon was treated by the protocol described in section 2.3.The dead and living biofilm cells were stained for 30 min using propidium Iodide (PI) and SYTO-9 (Invitrogen) in the dark condition,respectively. The PI and SYTO-9 were excited by 561 and 488 nm solid state laser beams, respectively. The excitation wavelengths were between 570 and 620 nm (red, representing dead bacteria), and 500 and 550 nm (green, representing active bacteria). Dead and live bacteria in the biofilms were observed using an SR-CLSM microscope(LSM880 with Airyscan, ZEISS, Oberkochen, Germany) at 60 ×magnification. ZEN 3.1 was used to analyze thex,yandz-stacks of each bacterial biofilm[21]. The structural parameters were measured by using Images J software[22].
2.4 Extraction of intracellular metabolites
The metabolic differences between the control and the treatment group of MRSA andS. aureus13565 biofilms were determined using ultra-high performance liquid chromatography with quadrupole timeof-flight mass spectrometry (UPLC-QTOF-MS/MS)[23]. The protocol of intracellular metabolites extraction was followed by Chen et al.[24].Briefly, after treatment with DW, SAEW, heated DW, and heated SAEW for 5 min, the neutralizing solution tube was added to each tube to stop SAEW decontamination activity and then centrifuged(10 000 r/min, 10 min). The collected biofilm pellets were immediately suspended in a 5 mL extraction solution of 80:20 methanol/water. To rupture the cell membrane, the mixtures were submerged in ice and sonicated for 20 cycles with 5 s pulses and 10 s stop. Further, the cell suspension was centrifuged at 6 000 ×gfor 5 min at 4 °C in a microfuge. Intracellular metabolites were obtained from the supernatant of the lysed cells. The samples were filtered by millex springe filter with 0.22 μm pore size ((Merck KGaA Darmstadt, Germany) and transferred to the chromatography vials for further analysis.
The extracts were analyzed by EXionLC UHPLC system (SCIEX ExionLC AD machine, MA, USA) and equipped with a C18100 × 3 mm Accucore column (Thermo Fisher Scientific, Waltham, MA,USA). The protocol of Tyagi et al.[25]was applied in the detection methodology with some modifications. Briefly, the mobile phase consists of 0.1% formic acid (A) and methanol (B). A quadrupole time-of-flight mass spectrometer (Q-TOF-MS) was used to perform mass spectrometry in both negative (ESI–) and positive (ESI+) ion modes (X500R QTOF). Them/zrange was from 115 to 1 000 and the scanning time was approximately 1 s.
2.5 Statistical analysis
In this study, IBM SPSS Statistics version 19 was applied for statistical analysis, including mean values of pH, ACC, and microbial populations from each treatment. Principal component analysis(PCA) was used for MRSA andS. aureus13565 biofilm screening the significant metabolites, identifying the metabolic differences, and investigating the group discriminations after SAEW, heated DW,and heated DW SAEW at 40 °C treatment[25]. The significance of the difference atP≤ 0.05 in the tukey multiple-range tests set. Metlin(https://metlin.scripps.edu/) identified the compounds. ClustVis(http://biit.cs.ut.ee/clustvis/) and Origin software 2021 were used to generate PCA graphs. The MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/) and Kyoto Encyclopedia of Genes and Genomes(KEGG) database (https://www.genome.jp/kegg/) were used to perform the pathway analysis and interpretation.
3. Results and discussion
3.1 Effect of electrode material on the production of reactive oxygen species in the electrolysis process
The preparation process, including electrode material, has been demonstrated to influence SAEW properties. Pt, Ru, and Ir have been considered to maintain stable performance during the production of SAEW[26]. The concentration of electrolytes also influence the SAEW effectiveness. Therefore, the production efficacy of SAEW using Pt,Ru, or Ir with the addition of different concentrations of HCl (1%,1.5%, and 2%) and NaCl (0, 1, 2, and 3 mol/L) was tested separately,as shown in Table 1. The result showed that the concentration of ACC increased with the electrolyte concentration. This may be caused by higher electrolyte concentrations leading to higher conductivity, thus generating more chlorine. Notably, the acceptable SAEW with higher ACC (26 mg/L) and proper pH (5.91) was generated by using a Ru plate sample with 3 mol/L NaCl and 1.5 % HCl.
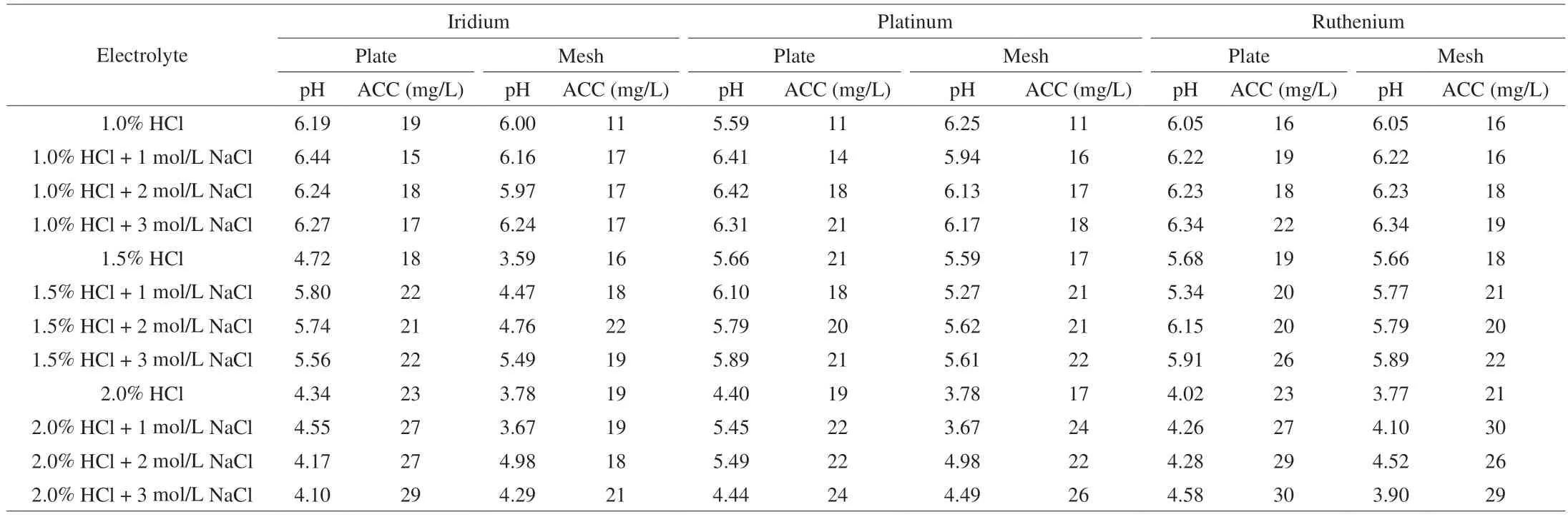
Table 1 The effect of electrolyte materials on the pH and ACC of electrolyzed water.
Previous research showed that the synthesis of active chlorine is the direct oxidation of chloride ions on the surface of the metal oxide electrode [2Cl–– 2e–= Cl2(g)], followed by the production of HOCl (Cl2+ H2O → HOCl + H++ Cl–)[18]. Therefore, the oxidation performance of the anode is essential for the generation system’s efficiency in electrolytic reactions. Ru, Ir and Pt belong to the platinum group metals. According to the structural characteristics of the three materials, there are two electrons in the outermost layer of Ir, while only one electron is in the outermost layer of Pt and Ru.The most effective electrical conductor is a metal that has univalent electrons, which can move freely and accelerate the ion exchange in the electrolysis process. Based on our data, Ru-plate exhibited highly enhanced electrocatalytic activity with a yield of 0.86 g-Cl2/Ah (Fig. S2).The data was consistent in with previous research. Lee et al.[27]also reported that Ru had enhanced electrocatalytic activity for the production of active chlorine.
The stabilities of SAEW produced by the Ru-plate generation system were further tested, shown in Tables S1 and S2. When kept in closed condition, the electrolyzed waters (30 and 50 mg/L) were more stable, with a minimal decline in ACC. The closed state effectively inhibits the inflow of external gas and reduces the volatilization rate of Cl2, reducing the degree of ACC attenuation. Following the 6-month opened and closed storage, SAEW at 30 and 50 mg/L can inactivateS. aureus,E. coli,S. enteridis,L. monocytogenes, andB. cereuscells in about 8 (lg (CFU/mL)) to undetected level within 1 min,respectively (Fig. S3). Hence, the SAEW produced by Ru-plate was selected for further investigation.
3.2 Synergist effect of mild heated SAEW on sanitization efficacy
It is commonly known that heating improves the sanitization effectiveness of SAEW[28]. However, it is customary for related studies first to generate the SAEW and then heat it to the desired temperature. It means that preservation hurdle technology contains two processes, which are not effective and convenient for application in the food industry. Therefore, the heated SAEW system for one step was developed in this study. Figs. 1 and 2 depict the characteristics and antimicrobial efficacy of mild heated SAEW produced by Ru electrode material. There is no significant difference in physical properties (pH and ACC) of SAEW among the preheating temperature variation. There was no loss of ACC when SAEW was heated to 50 °C after production.
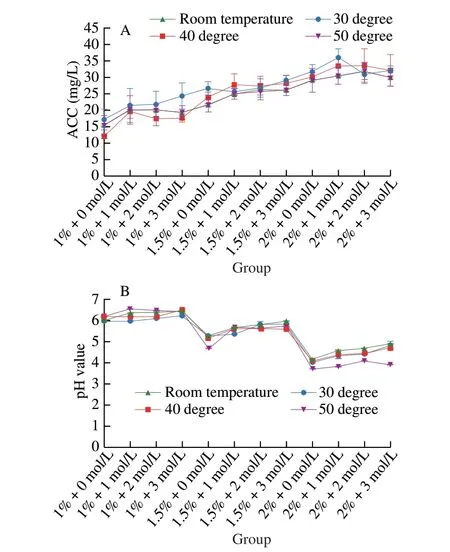
Fig. 1 Physical properties of optimized SAEW using water temperature variations during generation.
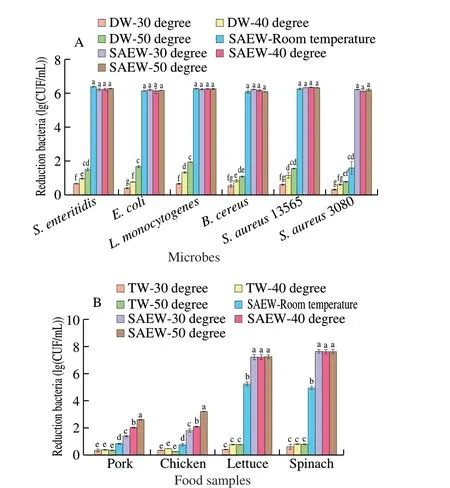
Fig. 2 (A) Effect of heated SAEW with variation temperature on the antimicrobial effect in 1 min reaction. (B) The inactivation effect of SAEW on inoculated pathogens artificially on food samples. Control, untreated samples;Pork was inoculated with S. aureus (ATCC 13565); Chicken was inoculated with S. enteritidis (ATCC 13076); Lettuce was inoculated with L. monocytogenes Scott A (ATCC 43251); and Spinach was inoculated with E. coli O157:H7 (ATCC 43895).
The survival results ofE. coli,S. enteridis,L. monocytogenes,B. cereus,S. aureus13565, and MRSA treated tap water at 30, 40 and 50 °C had no significant difference compared with the control group.In contrast, the mild heated SAEW at 30, 40 and 50 °C reducedE. coli,S.enteridis,L. monocytogenes,B. cereus,S. aureus13565 to undetected levels after 1 min treatment. The sanitization effect of mild heated SAEW at 30 °C (4.49 (lg (CFU/mL)) reduction),40 °C (5.65 (lg (CFU/mL)) reduction), and 50 °C (6.71 (lg (CFU/mL))reduction) against MRSA was greater than that SAEW at room temperature(1.54 (lg (CFU/mL)) reduction). Thus, the hurdle technology of mild heated SAEW showed a synergistic bactericidal efficacy.
Considering different cell attachment levels and organic matter on the sample, the effect of heated SAEW on pathogens inactivation artificially inoculated on food samples was also evaluated. Treatment with SAEW at various temperature resulted in of 5.14–7.16,4.88–7.56, 0.67–3.14, and 0.76–2.55 (lg (CFU/g) reduction ofL. monocytogenes,E. coli,S. enteritidis, andS. aureusseparate inoculated on lettuce, spinach, chicken, and pork reduced respectively.The disinfectant efficacy of SAEW was ranked as follows: 50 °C >40 °C > 30 °C > room temperature. These findings were consistent with the previous research[16,19,29]. For example, Forghani et al.[19]indicated that the combination of EW and heating induced more inactivation than individual treatment. Wu et al.[16]reported that AEW combined with heating reducedL. monocytogenesin salmon(2.1–2.2 (lg (CFU/g)). Reports based on Xie et al.[29], AEW at 50 °C treatment resulted in a 3.1 (lg (CFU/g)) reduction ofVibrio parahaemolyticuscells on shrimp, which had no adverse effect on the visual quality. Additionally, compared to the control group, the overall appearance of the pork and chicken after washing with SAEW at 40 °C shows no significant difference (Fig. 3 and Table S3).Overall, the most efficient intervention for sanitizing food samples should be the application of mild heated SAEW at 40 °C.

Fig. 3 The general appearance of chicken, pork, lettuce and spinach after washing by SAEW at room temperature, 30, 40 , and 50 °C compared with control group (distilled water).
3.3 Synergistic efficacy of mild heated SAEW at 40 °C against multi-resistance S. aureus biofilm
Biofilm formed by foodborne pathogenic bacteria on the food contact surfaces leads to cross-contamination during food processing, which is a severe concern in the food industry[30].S. aureusis a highly adaptable pathogen with a significant propensity to form biofilms on solid-phase surfaces, particularly on food equipment[31]. Stainless steel is widely used for food contact surface in food processing plants[32]. Therefore, the antibiofilm efficacy of SAEW, heating and mild heated SAEW at 40 °C was investigated against twoS. aureusstrains on 304 stainless steel.
According to our results in Fig. 4, the biofilm biomass indicated that mild heated SAEW at 40 °C was more effective in eradicating MRSA (41.23% reduction ratio) andS. aureus13565 biofilms(57.72% reduction ratio). Notably,S. aureus13565 biofilms were more susceptible than the MRSA biofilms under mild heated SAEW treatment. This might be becauseS. aureus13565 has a strong biofilm production phenotype, while MRSA has a strong resistance ability with weak biofilm production. At the same time, individual mild heated water treatment did not effectively inactivate MRSA andS. aureusbiofilm cells. DW at 40 °C alone for 5 min only resulted in 0.46 and 0.32 (lg (CFU/cm2)) of MRSA andS. aureusbiofilm cells, respectively. Liu et al.[33]also reported that treatment at 40 °C only for 5 min inactivated 0.16 (lg (CFU/mL)) ofE. coliO157:H7 biofilm cells. Treatment with SAEW alone for 5 min inactivated 1.55 and 1.87 (lg (CFU/cm2)) in MRSA andS. aureusbiofilm cells, respectively. However, treatment with mild heated SAEW at 40 °C achieved 2.41 and 3.49 (lg (CFU/cm2)) reduction in MRSA andS. aureusbiofilm cells. Similarly, Hussain et al.[34]indicated that combination treatment with SAEW (80 mg/L) at 60 °C for 10 min reducedB. cereusATCC 10897 biofilms at 1.28 (lg (CFU/cm2)). Wu et al.[16]reported that the combination treatment of heat and acidic electrolyzed water (ACC 100 mg/L)had better inactivation effects than a single treatment. Hence, the significant potential of heated SAEW at 40 °C is a promising strategy for reducing viable cells in the pathogenic microbial biofilm.
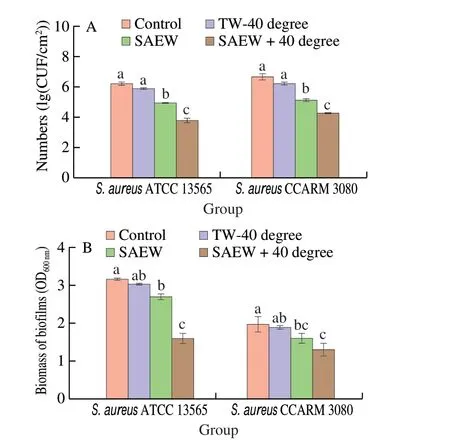
Fig. 4 Effect of heated SAEW at 40 °C treatment against the S. aureus 13565 and MRSA biofilms on the stainless steel surfaces. (A) CFU numbers;(B) Crystal violet staining. The temperature of control and SAEW alone was room temperature. The plate counting method was used for the selective agar for S. aureus (Baird-Parker agar). Values are expressed as the means ± standard deviations (n = 3). Different letters represent the significant difference (P < 0.05).
3.4 CLSM-based observation of biofilms by SAEW combined with preheating
The effects of mild heated SAEW at 40 °C on the permeability of the mature biofilm cell membrane of MRSA andS. aureus13565 were revealed by CLSM. As shown in Fig. 5, the control MRSA andS. aureusbiofilm cells emitted green fluorescence, indicating that all biofilm cells adhered to the stainless steel surface in densely populated areas without membrane damage. However, the proportion of both biofilms cells emitting red fluorescence sharply increased after the mild heated SAEW at 40 °C treatment. This finding implies that the heated SAEW at 40 °C significantly increased the number of damaged cells. Furthermore, Table 2 displays the observed key parameters of untreated and treated MRSA andS. aureusbiofilms as determined from the CLSM images. The combined treatment performed the best in terms of reducing average thickness (from 16.55 to 3.10 μm for MRSA and from 16.82 to 4.35 μm forS. aureus13565) and biomass biovolume (from 5.73 to 3.46 μm3/μm2for MRSA and from 5.19 to 2.10 μm3/μm2forS. aureus13565). These occurrences were ascribed to the destruction of biofilm’s basic structure, resulting in more micro holes when treated by mild heated SAEW at 40 °C.
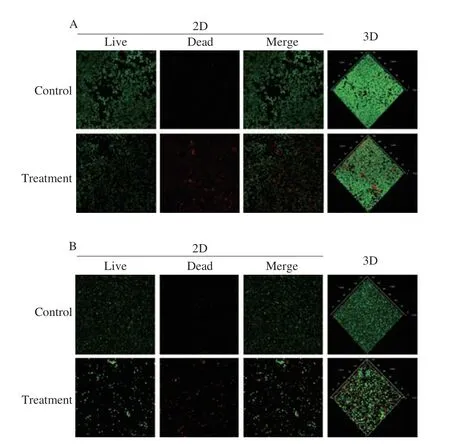
Fig. 5 Confocal laser scanning microscopy images of S. aureus biofilm treated by the combination of SAEW (30 mg/L) and heating (40 °C).(A) S. aureus 13565 strain; (B) MRSA strain.
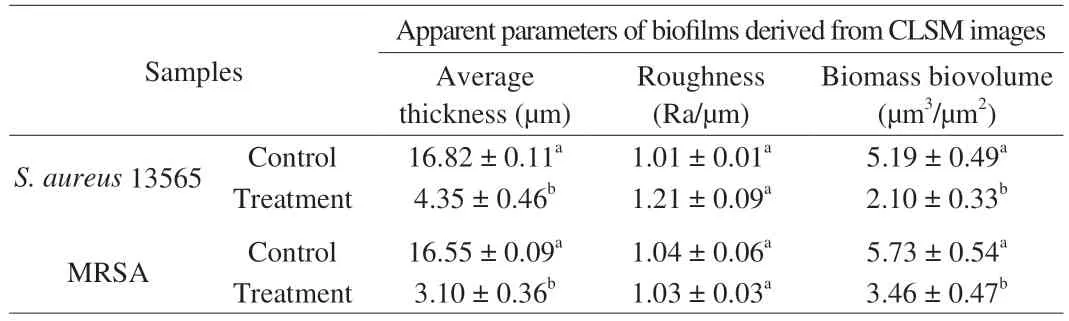
Table 2 Changes of the average thickness, roughness, and porosity of MRSA and S. aureus 13565 biofilm treated by SAEW combined preheating treatment.
A synergistic impact might be attributed to heat conduction generated by mild temperature, which promotes ClO–penetration and diffusion into the biofilm structure[35]. In addition, the high temperature could reduce the hydrophilic group composition in the biofilm, leading to the disintegration of the dense biofilm[36]. The OCl–generated by SAEW would induce the degradation of polysaccharides in the extracellular polymeric substance (EPS) and amino residues on protein chains[37-39]. Enhancement of disinfectant agents transfers to a deeper depth of the biofilm or through the cell membrane, resulting in the synergistic effect of mild heated SAEW treatment.
3.5 Metabolomics profiling and pathway analysis
Metabolite profiles were investigated to explore the potential mechanism underlying biofilm oxidative and heat response changes.The overall metabolic responses of twoS. aureusbiofilms exposed to mild heated SAEW treatment were studied using UPLC-QTOF-MS/MS.To date, this is the first study to assess metabolite profiles and pathways to look into the oxidative response of MRSA andS. aureusbiofilm.
Metabolic analysis of SAEW, heat, and heated SAEW at 40 °C treatment biofilm cells revealed that various metabolites were altered in both MRSA andS. aureus13565 strains biofilm. The contents of 25 intracellular metabolites were altered in MRSA, while the levels of 32 metabolites inS. aureus13565, including organic acid, amino acids, fatty acid, oligopeptide, carbohydrate, an analogue of the amino acid lysine, peptide and other derivatives (Figs. 6 and 7). The analysis showed that heated SAEW affects the biochemical level of MRSA andS. aureus13565 differently. Specifically, four significantly changed amino acids, includingL-phenylalanine,L-leucine, glutamic acid,andL-tryptophan, were seen to decline onS. aureus13565 biofilm,while only three amino acid was altered in MRSA. The accumulation of glutamic acid,L-leucine andL-tryptophan in MRSA was 2, 8,and 2 times less than that ofS. aureus13565 under heated SAEW at 40 °C treatment. Besides,S. aureus13565 biofilm had considerably decreased levels of acetic acid and succinic acid. Glucose and fructose had lower intensity in MRSA than thatS. aureusin 13565. Wu et al.indicated slight differences in diversity among threeListeriastrains after EW combined moderate heat treatment[16].
It was shown that PC1 and PC2 revealed 81.60% of the overall dataset (PC1 for 51.2%; PC2 for 30.4%) forS. aureus13565 biofilm.For MRSA, the first 2 principal components explained 83.8% of variances with PC1 explaining 59.6% and PC2 explaining 24.2%.Each of the 4 samples and metabolites was separated in Fig. 6B plot. Overall, it demonstrated that the model correctly predicted the data. The metabolic profile of the control biofilm was shown to be distinct from all other treatment samples forS. aureus13565, with more significant amino acid diversity towards combined treatment,followed by individual treatment.

Fig. 6 (A) Heatmap of 25 identified metabolites in the MRSA biofilm under untreated control, SAEW at room temperature, tap water at 40 °C, and combination treatment of SAEW and preheating at 40 °C. (B) PCA of the MRSA biofilm under untreated control, SAEW at room temperature, tap water at 40 °C, and combination treatment of SAEW and preheating at 40 °C was shown by comparing PC1 with PC2. (C) KEGG pathway analysis of MRSA biofilm in the control vs. combined treatment.

Fig. 7 (A) Heatmap of 32 identified metabolites in the S. aureus ATCC 13565 biofilm under untreated control, SAEW at room temperature, tap water at 40 °C,and combination treatment of SAEW and preheating at 40 °C. (B) PCA of the S. aureus ATCC 13565 biofilm under untreated control, SAEW at room temperature,tap water at 40 °C, and combination treatment of SAEW and preheating at 40 °C was shown by comparing PC1 with PC 2. (C) KEGG pathway analysis of S. aureus ATCC 13565 biofilm in the control vs. combined treatment.
Moreover, the pathway analysis was performed based on the significant screened metabolites, shown in Figs. 6C and 7C.AP-value ≤ 0.05 indicates strong enrichment in the annotation categories. Effective pathways in the MRSA andS. aureus13565 biofilm cells treated with heated SAEW at 40 °C were identified by setting the statistical threshold atP< 0.05. The metabolites were summarized using literature and the KEGG database to generate a simplified schematic metabolic map. A total of 27 and 24 pathways were predicted, of which 10 and 7 wereP< 0.05 forS. aureus13565 and MRSA, respectively.
Disturbed pathways are mainly related to energy metabolism,carbohydrate metabolites, nucleotides synthesis, amino acid metabolism, and lipid biosynthesis. Seven pathways, including Taurine and hypotaurine metabolism, Sulfur metabolism, Glyoxylate and dicarboxylate metabolism, Streptomycin biosynthesis,D-glutamine andD-glutamate metabolism, Aminoacyl-tRNA biosynthesis, and Nitrogen metabolism, were markedly altered for twoS. aureusstrains biofilm under heated SAEW at 40 °C treatment. ForS. aureus13565, three more pathways, Phenylalanine metabolism,Phenylalanine, tyrosine and tryptophan biosynthesis, and glycolysis/gluconeogenesis, were also significantly altered. The considerably changed pathways demonstrated that the heated SAEW at 40 °C treatment greatly impactedS. aureus13565 more than MRSA.
Based on comparative literature and KEGG, a hypothetical metabolic diagram was constructed to show the response ofS. aureus13565 and MRSA under SAEW, heat and heated SAEW at 40 °C treatment (Fig. 8). According to section 3.4 previously discussed, the mechanisms of individual treatment are that SAEW stress disturbed the metabolic flux of the biofilm bacteria while heat treatment activated their defense system. In the heated SAEW treatment, the anti-biofilm effect of SAEW was strengthened by heat, which might be due to the facilitation of penetration and diffusion of ClO-of SAEW into the biofilm structure.
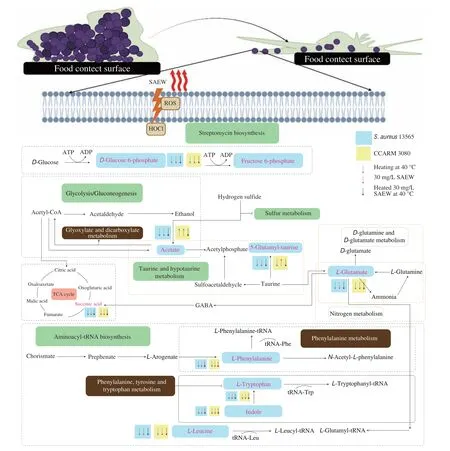
Fig. 8 Overall view of metabolic alteration by the SAEW, heating, and heated SAEW treatment in two S. aureus biofilm cells. Arrow up, the increase of metabolite compared with the control group; Arrow down, the decrease of metabolites compared with the control group. The main pathway affected markedly by the combined treatment were labeled green for two S. aureus strains biofilm. The main pathway affected markedly by the combined treatment were labeled grey for S. aureus 13565 biofilm.
Initially,S. aureus13565 and MRSA must quickly adapt to various carbon and nitrogen sources during the invasion of a host[40].Glucose is generally considered the main source of cell energy, which can be utilized in energy generation and biosynthetic pathways. As a glucose catabolism pathway, glycolysis metabolizes glucose, provide biosynthetic precursors such as fructose-6-phosphate and glucose-6-phosphate, and rapidly produces a small amount of NADH, ATP, and pyruvate can enter the Krebs cycle (TCA). The reduction of essential precursors might be due to the disturbance of the metabolic system.InS. aureus13565 and MRSA biofilm cells, TCA-related metabolites such as succinic acid decreased.
The TCA cycle provides intermediates and precursors for other metabolic pathways and is supplied by other metabolites.γ-Aminobutyric acid (GABA) can be synthesized fromL-glutamate and further regulates succinic acid involved in TCA cycle[41].Glutamine is synthesized by nitrogen metabolism, which catalyzes the ATP-dependent amidation of glutamate using ammonium as the nitrogen supply. It can be used to produce succinate for the maintenance of TCA cycle. As a result, a disruption in one metabolic pathway might impact the whole metabolic system.
In MRSA andS. aureus13565 biofilm cells, the level of glutamic acid,L-leucine andL-tryptophan was notably reduced under decreased after heat SAEW at 40 °C treatment. Amino acids were associated closely with cell fitness maintenance and stress adaptation[42]. For instance, Guo et al.[43]reported that the metabolites ofS. entericaincluding tryptophan, methionine, and valine decreased significantly after organic acid treatment (1.5% acetic acidic, 1% citric acid, and 1.5% lactic acid).L. monocytogenesadapts to the AEW stress by inhibiting most amino acids, such as leucine, phenylalanine, and glutamic acid[16].
As the primary osmoregulation solutes, amino acids can maintain cell ground substance osmolality and avoid disintegrating subcellular compositions[44].L-tryptophan, the most chemically complex, is a biosynthesis precursor for a broader range of complex microbial natural products[45]. However, amino acid metabolism is vital and complex during stress adaption. Additionally, SAEW with high oxidative may damage the biofilm and membrane and induce osmotic imbalance[46]. Previous research has also found that stress, such as ultrasound, essential oil, and acid, cause significant alterations in these pathways in bacteria[24,19,43]. Liu et al.[47]reported that lowconcentration EW caused severe disruption across a wide range of biochemical processes inListeria innocua, especially amino acid metabolism, nucleotides biosynthesis, and peptidoglycan synthesis.Overall, the imbalance may lead to the loss of cell repair function and self-destruction ofS. aureus13565 and MRSA biofilm cells.
4. Conclusions
The synergistic effectiveness of the combined SAEW and preheating treatment against the MRSA biofilm was evaluated in this study. The 5-min treatment of either SAEW (30 mg/L) or preheating(40 °C) did not have a significant bactericidal impact on MRSA biofilms. The combination of SAEW (30 mg/L) produced by Ruplate material and preheating at 40 °C synergistically reduced the number of sublethal cells and efficiently detached the MRSA biofilm.The combination treatment significantly (P< 0.05) reducedS. aureusbiofilm cells by 2.41–3.49 (lg (CFU/cm2)). When the combined SAEW and preheating were conducted for 5 min, the destruction of biofilm’s fundamental structure led to entire intact surfaces and more holes. Moreover, metabolomics analysis revealed that the contents of intracellular metabolites were altered in biofilms, including amino acids, organic acid, fatty acid, and lipid. The number of significantly changed pathways for MRSA andS. aureus13565 was 4 and 8,respectively.S. aureus13565 biofilms were more susceptible to MRSA biofilms under the combination treatment. However, a variety of gene expression, post-transcription and post-translational events are present in bacteria, which is crucial for bacterial metabolism modulation, and will be further studied.
Conflict of interest
All the authors declare no competing interest.
Acknowledgments
This work was supported by Brain Korea (BK) 21 Plus Project(4299990913942) funded by the Korean Government, Korea, the Collabo Project funded by the Ministry of SMEs and Startups(C1016120-01-02), and the National Research Foundation of Korea(NRF) (2018007551).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.26599/FSHW.2022.9250131.
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango