Punicalagin prevents obesity-related cardiac dysfunction through promoting DNA demethylation in mice
2024-01-24ShngjiPiRunLiuQingqingPngJingXinZhongshiQiJihngFngXuYngZiruiYoXioqinLiuXinfngJingLiChnDuoLi
Shngji Pi, Run Liu, Qingqing M, Png Jing, Xin H, Zhongshi Qi, Jihng Fng,Xu Yng, Zirui Yo, Xioqin Liu, Xinfng Jing, Li Chn,, Duo Li,
a Institute of Nutrition & Health, Qingdao University, Qingdao 266071, China
b School of Public Health, Qingdao University, Qingdao 266071, China
c Central Laboratory, Guizhou Aerospace Hospital, Zunyi 563000, China
d Health Centers of Licha Town, Qingdao 266071, China
e College of Computer Science and Technology, Qingdao University, Qingdao 266071, China
Keywords: DNA demethylation Mitochondrial function Obesity-related cardiac dysfunction Punicalagin Ten-eleven translocation family enzymes
ABSTRACT The aim of this study was to investigate whether punicalagin (PU) could prevent obesity-related cardiac dysfunction by promoting DNA demethylation, and to explore its possible mechanism. C57BL/6J mice were fed with standard diet, high-fat diet (HFD), HFD supplemented with resveratrol, low-dose PU (LPU) and high-dose PU (HPU) for 8 weeks. Compared with HFD group, body weight was signif icantly lower in PU treatment groups, number of cardiomyocytes and the protein level of myosin heavy chain 7B were signif icantly higher in PU treatment groups. Levels of 5-hydroxymethylcytosine and 5-formylcytosine were signif icantly lower in HFD group than in other groups. Compared with the HFD group, the protein level of ten-eleven translocation enzyme (TET) 2 was significantly higher in PU treatment groups, p-AMP-activated protein kinase (AMPK) was signif icantly higher in LPU group. Levels of total antioxidant capacity and the protein levels of complexes II/III/V, oxoglutarate dehydrogenase, succinate dehydrogenase B and fumarate hydrolase were signif icantly lower in HFD group than PU treatment group. The ratio of (succinic acid + fumaric acid)/α-ketoglutarate was signif icantly higher in HFD group than other groups. In conclusion, PU up-regulated TETs enzyme activities and TET2 protein stability through alleviating mitochondrial dysfunction and activating AMPK, so as to promote DNA demethylation, thus preventing obesity-related cardiac dysfunction.
1. Introduction
The prevalence of obesity is increasing and has become the most serious public health problem all over the world[1]. Obesity contributes to cardiac complications, such as cardiac dysfunction and heart failure, which can be prevented by taking appropriate measures[2].Currently, the principal therapy for preventing or alleviating obesityrelated cardiac dysfunction is pharmacotherapy. However, these drugs have side effects on the body, such as developing gastrointestinal discomfort, eating disorders and others[3-4]. It has prompted to search for a preventive measure to alleviate obesity-related cardiac dysfunction.
The dysregulation of DNA epigenetic modification plays an important role in obesity-related cardiac dysfunction[5]. In mammals, DNA methyltransferases (DNMTs) modify cytosine to 5-methylcytosine (5mC). The ten-eleven translocation enzymes(TETs) convert 5mC into 5-hydroxymethylcytosine (5hmC),5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) in turn,resulting in DNA demethylation[6]. Previous studies found that the increasing level of DNA methylation was positively correlated with the incidence rate of obesity and cardiac dysfunction, and the mortality increases with the occurrence of hypermethylation in patients with cardiac injury[7-8]. In addition, the level of DNA 5mC was significantly up-regulated in patients with cardiac dysfunction[9]. However, the level of DNA 5hmC in obese mice with cardiac dysfunction was significantly lower than that in normal mice. When TETs-mediated DNA demethylation was inhibited,it was not conducive to heart development[5]. The mechanism of DNA demethylation dynamically changed or reversed abnormal methylation, for example, hypermethylation of specific sites occurred in the fibrotic heart, whereas myocardial fibrosis improved with the increase of demethylation[10-11]. In conclusion, the inhibition of DNA demethylation was one of the important reasons for cardiac dysfunction.
TETs family proteins are the key enzymes of DNA demethylation.Fumaric acid and succinic acid, as intermediate metabolites of the tricarboxylic acid (TCA) cycle, competitively inhibitα-ketoglutarate(α-KG), thus altering the activities of TETs family enzymes[12].Moreover, AMP-activated protein kinase (AMPK) maintains TET2 stability by phosphorylation, which in turn regulates TET2 protein level[13]. However, p-AMPK was inhibited and mitochondrial dysfunction occurred in the hearts of obese mice[14-15]. These hinted that regulating TETs by activating AMPK and improving mitochondrial function, thus promoting DNA demethylation may be an effective measure to prevent obesity-related cardiac dysfunction.
Punicalagin (PU), a kind of polyphenol in pomegranate, has been proven to prevent cardiac injury with obesity[15]. Previous studies found that PU not only protected against myocardial ischemiareperfusion-induced oxidative stress and myocardial injury through activating the AMPK signaling pathway, but also prevented cardiac metabolic disorders and injury by improving mitochondrial function[14,16]. Based on previous research, we speculated that PU acted on TETs by activating AMPK and improving mitochondrial function, thus preventing obesity-related cardiac dysfunction by promoting DNA demethylation. The purpose of this study was to investigate whether PU could prevent obesity-related cardiac dysfunction by promoting DNA demethylation, and to explore its possible mechanism.
2. Materials and methods
2.1 Materials
The standard diet (10% calories from fat, D12450J) and the high-fat diet (60% calories from fat, D12492) were purchased from FBSH Biotechnology Co., Ltd. (Shanghai, China). Resveratrol (RES,purity≥ 98%) and punicalagin (PU, purity≥ 40%) were purchased from Xi’an Victory Biochemical Technology Co., Ltd. (Xi’an, China).Detection kits for total antioxidant capacity (T-AOC), catalase (CAT),superoxide dismutase (SOD) and glutathione (GSH) were from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).Antibodies against 5mC, 5hmC, 5fC, TET1, and TET3 were purchased from Active Motif (U.S.A.). Other primary antibodies used in this study, such as TET2 and glyceraldehyde-3-phosphate dehydrogenase(GAPDH), were purchased from Cell Signaling Technology (Danvers,MA, U.S.A.). The real-time polymerase chain reaction (RT-PCR) kit and primers were purchased from Tsingke Biotechnology Co., Ltd.(Beijing, China). Ultrapure water was prepared with a Mill-Q water purification system (Millpore, U.S.A.). All other chemicals used were purchased from market resources.
2.2 Animals and experiments design
Fifty C57BL/6J mice (18–22 g weight, male, and approximately 50 days of age) were purchased by Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All animals were housed in a temperature (22–28 °C) and humidity (50%–60%)controlled room and maintained on a 12 h light/dark cycle (08:00–20:00 light/20:00–08:00 dark) with food and water provided during the experiments. After one week of acclimatization, the mice were randomly distributed into 5 groups (n= 10 in each group) and fed for 8 weeks as follows: (1) Mice fed a standard diet and given oral gavage of saline solution daily (CON); (2) Mice fed a highfat diet and given oral gavage of saline solution daily (HFD);(3) Mice fed a high-fat diet and administered a daily oral gavage of 100 mg/(kg·day) resveratrol solution (RES); (4) Mice fed a high-fat diet and administered a daily oral gavage of 50 mg/(kg·day) PU solution (LPU); (5) Mice fed a high-fat diet and administered a daily oral gavage of 100 mg/(kg·day) PU solution (HPU). Body weight,food intake, and water consumption were recorded weekly. After the intervention, the mice were fasted overnight and sacrificed. The mice were weighed, and hearts and serum were collected. The hearts were rapidly frozen in -80 °C liquid nitrogen or soaked in TRIzol,or directly stored in 4% paraformaldehyde for histological analysis.All efforts were made to minimize animal suffering. All animal experiment procedures were adhered to the Qingdao University Guide for the Care and Use of Laboratory Animals and approved by Qingdao University Laboratory Animal Welfare Ethics Committee(No. 20201105C575020210110033).
2.3 Dosage regimen
During the experiment, PU was dissolved in ultra-pure water and given a daily oral gavage (50 and 100 mg/(kg·day)) for 8 weeks. Previous studies evaluated the toxicity of PU in doses and showed that a 6% PU content in the rat diet (4 800 mg/(kg·day))during 37 days did not cause tissue changes and most of the serum biochemical and hematological parameters were essentially normal[17].Based on the dose conversion method of the Food and Drug Administration, the human equivalent doses of PU were calculated as 244 and 488 mg/day for adults weighting 60 kg, which could be achievable by intake of commercial pomegranate juice or other available supplements[18].
2.4 Blood samples and biochemical measurements
After sacrificing mice, blood was gathered from the retroorbital sinus into 1.5 mL sterile enzyme-free centrifuge tubes. The supernatant serum was separated by centrifugation (3 000 r/min for 10 min at 4 °C) for analysis. The serum concentrations of total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol(HDL-C) and the activities of creatine kinase (CK) and aspartate amino-transferase (AST) were measured by an automatic biochemical analyzer (Sysmex XN-10).
2.5 Histological analysis of mice hearts
Histopathological examination of cardiac tissues was performed using hematoxylin and eosin (H&E) staining. The hearts of mice stored in 4% paraformaldehyde were embedded in paraffin, cut into 4-5 μm sections, and stained with H&E. Examination of these sections was carried out under a light microscope. The number of cardiomyocytes was calculated in different areas under the perspective of 90 × H&E staining vision.
2.6 Western blot analysis
Western blot was performed as previously described[19]. The heart samples (30 mg) were lysed with Western and IP lysis buffer.The lysates were homogenized and centrifuged at 13 000 ×gfor 15 min at 4 °C. The supernatants were collected, and the protein concentrations were determined using a BCA protein assay kit(ThermoFisher, Rockford, U.S.A.). Equal aliquots of protein were separated by SDS-PAGE gel, transferred to pure nitrocellulose membranes of PerkinElmer Life Sciences (Boston, Massachusetts,U.S.A.), and blocked with 5% skim milk in tris-buffered saline and Tween 20 (TBST) buffer for 1 h. Then, the membranes were incubated with primary antibodies at 4 °C overnight. The membranes were incubated with secondary antibodies at room temperature for 2 h. Chemiluminescent detection was performed using an ECL Western blotting detection kit (ThermoFisher, Rockford, U.S.A.). The results were analyzed by Image J software (Version 1.8.0) to obtain the optical density ration of the target proteins relative to GAPDH.
2.7 Dot-blot analysis
Dot-blot was prepared following our previously described method[20]. Genomic DNA was extracted from hearts (20 mg)according to the TIANamp Genomic DNA kit instructions and DNA concentrations were determined using a NanoDrop (Thermo Scientific). The genomic DNA was sampled onto nylon membranes and baked at 80 °C for 2 h, then blocked with 5% skim milk in TBST for 1 h, followed by incubation with the antibody overnight at 4 °C.Secondary antibodies were incubated after three washes with TBST.Detections were performed using an ECL western blotting detection kit (ThermoFisher, Rockford, U.S.A.). The results were analyzed by Image J software (Version 1.8.0).
2.8 Total RNA isolation and real-time PCR
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad,CA, U.S.A.) following the manufacturer’s protocol. The cDNAs were generated from total RNA using an RT-PCR kit according to the manufacturer’s protocol, followed by semiquantitative real-time PCR using specific primers. Data were normalized to the mRNA levels ofGAPDH, which was used as a housekeeping gene, and the data were analyzed by the 2−ΔΔCtmethod. Primers used in this experiment include: TET1 (F: 5’-GAAGGAAGTGACGTGAAAAC-3’;R: 5’-AAAAATCCATGCAACAGGTG-3’); TET2 (F:5’-GCCATTCTCAGGAGTCACTGC-3’; R: 5’-ACTTCTCGATTGTC TTCTCTATTGAGG-3’); TET3 (F: 5’-CCCTCGGCGGGGATAAT-3’;R: 5’-TGCTTAGCTGCCTTGAATCT-3’); GAPDH(F: 5’-ACCACAGTCCATGCCATCAC-3’; R:5’-CACCACCCTGTTGCTGTAGCC-3’).
2.9 Detection of biochemical kits
The heart tissues were weighed, then added normal saline according to 1:9. The samples were lapped to prepare 10% cardiac homogenates, measured the protein concentration. Tested the levels of T-AOC, SOD, CAT and GSH according to the instructions of the kits.
2.10 Genomic DNA extraction and detection of mitochondrial DNA (mtDNA) copy number
Real-time PCR was used to quantify mtDNA copy number after DNA isolation. Data were analyzed by 2−ΔΔCtmethod. Primers used in this experiment include:D-loop (F: 5’-AATCTACCATCCTCCGTG-3’;R: 5’-GACTAATGATTCTTCACCGT-3’); 18S (F:5’-CATTCGAACGTCTGCCCTATC; R: CCTGCTGCCTTCCTTGGA-3’).
2.11 Quantification of TCA cycle metabolites by gas chromatography-mass spectrometry (GC-MS)
According to our previous experimental method, the intermediate metabolites of the TCA cycle were detected by GC-MS[21]. The weighted heart tissues (30 mg) were ground with liquid nitrogen and added with an extraction buffer. The samples were stored at-80 °C overnight, and the supernatant was freeze-dried in a vacuum after centrifugation. The sample was dissolved by adding 50 μL of methoxyamine (10 mg/mL in pyridine) and incubated at 37 °C for 1 h. The derivative reaction occurred after the addition of 50 μL BSTFA (1% TMCS, Sigma) at 60 °C for 1 h. Samples were analyzed by the Agilent 7890A Meteorological Chromatography-5975C Mass Spectrometer. Under the same chromatographic conditions, retention time was used to identify the metabolites. After denoise processing,mass spectrometric data were analyzed and quantified by AMDIS deconvolution according to the Fiehn and NIST compound databases.Metabolite contents were normalized with an internal standard(myristic acid d27), and a two-dimensional matrix of metabolite content and sample information was finally constructed.
2.12 Statistical analysis
All the statistical analysis was performed by the Statistical Package Social Sciences 19.0 system (SPSS Inc., U.S.A.), and the data were expressed as means values ± standard error of the mean (SEM).The statistical significance of body weight was evaluated with two-way analysis of variance (two-way ANOVA). Other indicators were analyzed by one-way ANOVA. The level of significance was set atP< 0.05 in all comparisons.
3. Results
3.1 The effects of PU on parameters in HFD-induced obese mice
The body weight of mice in the HFD group increased weekly,and showed a significant difference (P< 0.05) from the mice of the CON and LPU groups after one week of intervention. However, the difference in body weight between the HPU and HFD groups was not significant until the second week of intervention (Fig. 1A). There was no significant difference between groups on food intake (Fig. S1).Moreover, the heart weight and heart weight/body weight in the HFD group were significantly higher (P< 0.05) than the other groups(Figs. 1B and 1C).

Fig. 1 Effects of PU on the parameters of obese mice induced by high-fat diet. (A) Body weight weekly; (B) Heart weight; (C) Heart weight/body weight. Serum concentrations of (D) TC, (E) TG, (F) HDL-C and (G) LDL-C; (H) Serum AST activity; (I) Serum CK activity. The values were presented as the means ± SEM(n = 6); In Fig. 1A, *P < 0.05, **P < 0.01 means CON group vs. HFD group, #P < 0.05, ##P < 0.01 means LPU group vs. HFD group and &P < 0.05, &&P < 0.01 means HPU group vs. HFD group. In Figs. 1B-I, *P < 0.05, **P < 0.01 means HFD group vs. other groups.
The HFD-induced obesity model was characterized by hyperlipidaemia[22]. The serum concentrations of TC and LDL-C were significantly higher (P< 0.05) in the HFD group than in the CON,RES and LPU groups (Figs. 1D and G). Compared with the HFD group, serum concentration of TG was significantly lower (P< 0.05)in the RES, LPU and HPU groups (Fig. 1E). The serum concentrations of serum HDL-C and the activities of AST and CK were significantly higher (P< 0.05) in the HFD group than in the other groups(Figs. 1F, H and I).
3.2 The preventive effects of PU on cardiac dysfunction in HFD-induced obese mice
The cardiac morphology and 1.5× H&E staining showed that the hearts of mice in the HFD group were obviously larger than mice of other groups. Under observation of 90× H&E staining, the cardiomyocytes of mice in the CON group were consistent in size and arranged in an orderly manner, whereas the cardiomyocytes of HFD-induced obese mice were arranged in a disordered manner and appeared with obvious gaps. The performance of cardiomyocytes in the RES, LPU and HPU groups were consistent with that of the CON group (Fig. 2A). In addition, the number of cardiomyocytes was significantly lower (P< 0.05) in the HFD group than in the other groups (Fig. 2B).
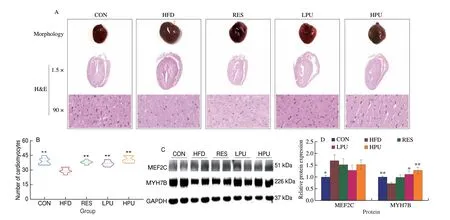
Fig. 2 PU ameliorated cardiac dysfunction in obese mice. (A) The heart morphology in mice, and the H&E staining of heart were photographed at 1.5×magnification and 90× magnification; (B) Number of cardiomyocytes in different regions; (C) Western blot images of MEF2C and MYHTB protein levels and(D) statistical analysis. The values were presented as the means ± SEM (n ≥ 4); *P < 0.05, **P < 0.01 means HFD group vs. other groups.
The expression of myocyte enhancer factor-2 (MEF2) C and myosin heavy chain (MYH) 7B were closely related to cardiac function[23]. The results of Western blot showed that the protein expression level of MEF2C was significantly lower (P< 0.05) in the CON group than in the HFD group. Compared with the HFD group,the protein expression level of MYH7B was significantly higher(P< 0.05) in the CON, LPU and HPU groups (Figs. 2C and D).
3.3 PU promoted the DNA demethylation in obese mice hearts
To probe into the effects of PU on DNA demethylation in obese mice hearts, the global levels of DNA 5mC, 5hmC and 5fC in mice hearts were measured by dot-blot. There was no statistical difference in DNA 5mC level among all groups (Fig. 3A). The levels of DNA 5hmC and 5fC were significantly lower (P< 0.05) in the HFD group than in the other groups (Figs. 3B-D). The results indicated that PU had the potential to promote DNA demethylation.
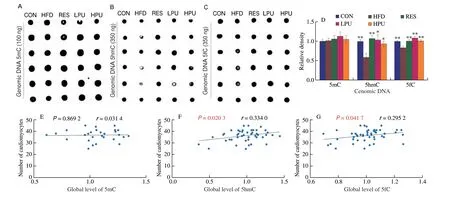
Fig. 3 Effects of PU on the global levels of DNA 5mC, 5hmC and 5fC in obese mice hearts and correlation analysis. The dot-blot image of (A) DNA 5mC,(B) DNA 5hmC and (C) DNA 5fC; (D) statistical analysis of dot-blot; correlation analysis between the number of cardiomyocytes with (E) DNA 5mC, (F) DNA 5hmC and (G) DNA 5fC. The values were presented as the means ± SEM (n ≥ 4); *P < 0.05, **P < 0.01 means HFD group vs. other groups.
Correlation analysis showed that there was no relationship between the global level of 5mC and the number of cardiomyocytes,but the levels of 5hmC and 5fC were significantly positive associated with the number of cardiomyocytes (Figs. 3E-G).
3.4 PU up-regulated the expression of TETs and activated AMPK
DNMTs and TETs play important roles in DNA demethylation[24].We detected the protein expression levels of DNMTs, and found that the protein expression level of DNMT1 was significantly lower(P< 0.05) in the HFD group than in the CON group. No statistical difference was observed in protein expression levels of DNMT3A and DNMT3B between all groups (Figs. 4A and B). The protein expression level of TET2 was significantly lower (P< 0.05) in the HFD group than in the other groups. In addition, the protein expression levels of TET1 and TET3 were significantly higher(P< 0.05) in the CON and HPU groups when compared with the HFD group, but no significant difference between the HFD and RES group (Figs. 4C and D). However, it was found that the mRNA levels of TET1, TET2 and TET3 did not statistically change among the five groups (Fig. 4E).

Fig. 4 Effects of PU on the DNMTs, TETs enzyme family and AMPK signal pathway in obese mice hearts. (A) Western blot images of DNMT enzyme family protein levels and (B) statistical analysis; (C) Western blot images of TETs enzyme family protein levels and (D) statistical analysis; (E) the levels of mRNA for TETs; (F) Western blot images of AMPK signal pathway and (G) statistical analysis. The values were presented as the means ± SEM (n ≥ 4); *P < 0.05, **P < 0.01 means HFD group vs. other groups.
Due to the fact that p-AMPK affects the protein stability of TET2[13], we examined the protein expression levels of the AMPK-ACC signaling pathway. We found that p-AMPK/AMPK was significantly lower (P< 0.05) in the HFD group than in the CON,RES and LPU groups. There was no significant difference in p-AMPK/AMPK between the HFD and HPU groups. Moreover, there was no statistical change in p-ACC/ACC between all groups (Figs. 4F and G).
3.5 PU improved mitochondrial function in obese mice hearts
The activities of TETs were influenced by mitochondrial function[12], so we detected the mitochondrial function in mice hearts.The levels of T-AOC, SOD, CAT and GSH were significantly lower(P< 0.05) in the HFD group than those in the other groups (Figs. 5A-D).In addition, the mtDNA copy number was significantly higher(P< 0.05) in both LPU and HPU groups than in the HFD group (Fig. 5E).By measuring the protein expression levels of mitochondrial respiratory chain complex enzymes, we found that the protein expression levels of complexes III and V were significantly lower(P< 0.05) in the HFD group than in the other groups. And the protein expression level of complex II was significantly higher(P< 0.05) in the CON, LPU and HPU groups when compared with the HFD group. Moreover, the protein expression levels of complex I were significantly lower (P< 0.05) in the HFD group than in the HPU group. There was no statistically difference when compared the protein expression levels of complex IV among the five groups(Figs. 5F and G).

Fig. 5 PU improved mitochondrial function in the hearts of obese mice induced-by high-fat diet. The determination of (A) T-AOC, (B) SOD, (C) CAT and(D) GSH in mice heart tissues; (E) the results of mitochondrial DNA copy number; (F) Western blot images of Complex protein levels and (G) statistical analysis.The values were presented as the means ± SEM (n ≥ 4); *P < 0.05, **P < 0.01 means HFD group vs. other groups.
3.6 PU maintained the homeostasis of TCA cycle in obese mice hearts
The homeostasis of TCA was destroyed by mitochondrial dysfunction[25]. We detected the protein expression levels of TCA cycle-related enzymes in heart tissue, and found that the protein expression levels of aconitase 2 (ACO2), oxoglutarate dehydrogenase(OGDH), succinate dehydrogenase (SDH) B and malate dehydrogenase 2 (MDH2) were significantly lower (P< 0.05) in the HFD group than in the other groups. The protein expression levels of isocitrate dehydrogenase (IDH) 2 and fumarate hydrolase (FH) were significantly lower (P< 0.05) in the HFD group than in the CON,LPU and HPU groups. And the protein expression levels of IDH1 and SDHA were significantly lower (P< 0.05) in the HFD group than in the CON and LPU groups. In addition, when compared with the HFD group, the protein expression level of citrate synthase (CS)was significantly higher (P< 0.05) in the RES group and the protein expression of succinyl-CoA synthetase (SCS) was significantly higher(P< 0.05) in HPU group (Figs. 6A-C). The results of GC-MS in mice hearts showed that the content ofcis-aconite acid andα-KG did not change statistically among the groups (Figs. 6D and F). However, the content of malic acid was significantly lower (P< 0.05) in the HFD group than in the CON group (Fig. 6E), and the contents of fumaric acid and succinic acid were significantly higher (P< 0.05) in the HFD group (P< 0.05) than in the CON, LPU and HPU groups (Figs. 6G and H). Moreover, the ratio of (fumaric acid + succinic acid)/α-KG was significantly higher (P< 0.05) in the HFD group than in the other groups (Fig. 6I).
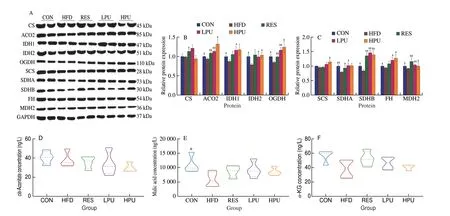
Fig. 6 Effects of PU on the TCA cycle homeostasis in obese mice hearts. (A) Western blot images of TCA cycle related enzymes proteins levels and (B,C)statistical analysis; The content of (D) cis-aconite acid, (E) malic acid, (F) α-KG, (G) fumaric acid, and (H) succinic acid. (I) The ratio of (fumaric acid +succinic acid)/α-KG. The values were presented as the means ± SEM (n ≥ 4); *P < 0.05, **P < 0.01 means HFD group vs. other groups.
4. Discussion
Previous studies found that the obese mice induced-by HFD had increased body weight and occurred hyperlipidemia, and the concentrations of serum TC, TG, HDL-C and LDL-C were significantly higher than the normal mice[26]. Consistent with previous studies[27], we found that PU decreased body weight and improved blood lipid levels(Fig. 1), thus preventing obesity in mice fed with HFD.
PU showed beneficial effects on hearts. Previous studies demonstrated that PU alleviated cardiac dysfunction through relieving myocardial infarction and inhibiting cardiomyocyte apoptosis[14,28].In our study, the morphological and the H&E results of mice hearts showed that PU restored the cardiac hypertrophy which occurred in obese mice to normal state (Fig. 2A). When myocardial hypertrophy occurs, the size of cardiomyocytes was increased and the number was decreased within the same size field of vision[29]. Similarly, we found that the HFD group had fewer cardiomyocytes than the other groups(Fig. 2B). MEF2C, one of the MEF2 family proteins, was highly expressed in hearts and involved in various processes of cardiac development[30-31]. The expression of MEF2C was up-regulated when myocardial hypertrophy occurred[32]. We found that the protein expression level of MEF2C was significantly higher (P< 0.05) in the HFD group than in the CON group, and PU had the potential to lower its level (Figs. 2C and D). Previous found that rats which knocked out MYH7B presented with hypertrophic cardiomyopathy, cardiomyocyte disorders and cardiac fibrosis[33]. However, polyphenols such as apple polyphenol promoted the formation of muscle fibers including the heart muscle by up-regulating the expression of MYH7B[34]. We also found PU up-regulated MYH7B protein expression level in obese mice hearts (Fig. 2C and D). In conclusion, our results showed that PU alleviated HFD-induced cardiac dysfunction in mice. Moreover,the gene of MYH7 was mainly regulated by 5hmC during heart development[35]. And MEF2C was involved in the establishment of epigenetic signatures, in particular the deposition of 5hmC on enhancers[36]. Therefore, we hypothesized that PU showed beneficial effects in obese mice hearts through acting on DNA epigenetic modifications.
DNA epigenetic modifications are closely related to cardiac function[35]. DNA hypermethylation appeared in patients with cardiac dysfunction, congenital heart disease in children and their mothers,which was associated with an increasing risk of cardiovascular death in the population[37-38]. When DNA demethylation was inhibited, the level of 5hmC was decreased, which in turn inhibited the expression of pivotal structural genes in cardiac such as MYH7, resulting in cardiac dysfunction[36,39]. In this study, the results of dot-blot pointed out that the level of 5mC was not statistically change among groups,while PU significantly increased the levels of DNA 5hmC and 5fC in the hearts of obese mice (Figs. 3A-D), so we speculated that PU had the ability to promote DNA demethylation. The 5mC is produced under the catalysis of DNMTs, and the change of DNMTs is the major reason for the different methylation levels between healthy hearts and hearts with cardiac dysfunction[40]. In this study, there was no significant difference in the level of 5mC and the protein expression levels of DNMT3A and DNMT3B among groups. Although we found that the protein expression level of DNMT1 was significantly lower(P< 0.05) in the HFD group than in the CON group (Figs. 4A and B),its change did not affect the level of 5mC. Previously, it was found that DNMTs that played a major role in human and mice hearts were mainly DNMT3A and DNMT3B, rather than DNMT1[40-41].
TETs are key enzymes in the demethylation process, so we detected the protein expression levels of TETs and found that the level of TET2 was significantly lower (P< 0.05) in the HFD group than in the PU treatment groups, and the protein expression levels of TET1 and TET3 were significantly higher (P< 0.05) in the HPU group than in the HFD group (Figs. 4C and D). Compared with the RES group, PU intervention groups significantly up-regulated the protein expression levels of TETs in the mice hearts which fed with HFD. However, although the treatment of low-dose PU did not significantly up-regulate the protein expression levels of TET1 and TET3, it still up-regulated the levels of DNA 5hmC and 5fC(Figs. 3B-D), indicating that TET2 played a key role in the process of cardiac demethylation. Previous found that TET2 is the most important enzyme which regulates DNA demethylation in the hearts,the level of 5hmC in the hearts of mice with TET2 knockout was significantly lower than mice with other TETs knockout[42]. Many regulatory factors regulate the levels of TETs in the process of transcription and translation[43]. We found that the mRNA levels of TETs did not statistically change among all groups (Fig. 4E).Therefore, we speculated that the change in TETs was caused by posttranslational modification. It is well known that phosphorylation is the most extensive covalent modification in protein post-translational modification and AMPK maintained the stability of TET2 by phosphorylation of TET2 serine 99[13,44]. It was confirmed that PU alleviated myocardial hypertrophy and cardiac dysfunction in HFDinduced obese mice by increasing the ratio of p-AMPK/AMPK[15,45],which was consistent with our result. Therefore, we revealed that PU up-regulated the protein stability of TET2 by activating AMPK, thus promoting DNA demethylation in obese mice hearts.
The activities of TETs were regulated by mitochondrial function[20]. Previous studies pointed out that PU improved mitochondrial dysfunction. For example, it inhibited oxidative stress,increased the levels of T-AOC and activated antioxidant defense system[14]. Moreover, PU increased the copy number of mtDNA and the protein expression levels of complexes in HFD-induced obese mice[46-47]. In this study, we detected the levels of T-AOC, SOD,GSH, CAT, the mtDNA copy number and the protein expression of complexes in mice hearts, the results were similar with previous studies, so we confirmed that PU improved mitochondrial dysfunction in the hearts of obese mice (Fig. 5). The TCA cycle not only plays an important role in maintaining the mitochondrial function, its intermediates such as succinic acid are also circulating biomarkers of obesity-related cardiac dysfunction[48-50]. In addition, fumaric acid and succinic acid, as intermediates of TCA cycle, are competitively inhibitedα-KG, thus decreasing the enzyme activities of TETs[12,51]. It was found that PU improved the homeostasis of TCA cycle through acting on the intermediates of TCA cycle in mouse cerebral cortex[52].Through detecting the protein expression levels of TCA cycle-related enzymes and the content of TCA cycle intermediates, we found that PU improved the homeostasis of TCA cycle in the hearts of obese mice (Figs. 6A-H). The ratio of (fumaric acid + succinic acid)/α-KG represents the enzyme activities of TETs, which was decreased with the increasing of the ratio. We found that the ratio was significantly lower (P< 0.05) in the LPU and HPU groups than in the HFD group (Fig. 6I), which indicated that PU alleviated mitochondrial dysfunction through improving the homeostasis of TCA, thus up-regulating TETs activities, so as to promote DNA demethylation.
5. Conclusion
Collectively, our study found that PU up-regulated TETs enzyme activities and TET2 protein stability through alleviating mitochondrial dysfunction and activating AMPK, thus promoting DNA demethylation, which may be one of the mechanisms of PU preventing obesity-related cardiac dysfunction. These findings provide a new perspective for PU to prevent cardiac dysfunction in obese mice.
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020QH294 and ZR2021QH342).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.26599/FSHW.2022.9250123.
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango