Parboiled rice supplementation alleviates high-fat diet-induced hyperlipidemia by regulating genes and gut microbiota in mice
2024-01-24XiuxiuWuTinyiGuoBioLiShuiHnZuominHuYiLuoDndnQinYpingZhouFeijunLuoQinluLin
Xiuxiu Wu, Tinyi Guo, Bio Li, Shui Hn, Zuomin Hu, Yi Luo,Dndn Qin, Yping Zhou, Feijun Luo,, Qinlu Lin,
a National Research Center of Rice Deep Process and Byproducts, Hunan Provincial Key Laboratory of Grain-oil Deep Process and Quality Control,Hunan Provincial Key Laboratory of Forestry Edible Resources Safety and Processing, Central South University of Forestry and Technology, Changsha 410004, China
b College of Life Science and Technology, Central South University of Forestry and Technology, Changsha 410004, China
c Department of Clinical Medicine, Medical College of Xiangya, Central South University, Changsha 410008, China
Keywords: Parboiled rice Blood lipids Transcriptome Gut microbiota PPAR Signaling pathway
ABSTRACT Dietary parboiled rice (PR) has a low risk of disease, but little is known about the contribution of PR to the prevention of hyperlipidemia. The potential role and underlying mechanisms of PR in hyperlipidemia were evaluated in this study. Male C57BL/6J mice were fed with a normal diet, high-fat diet (HFD) containing ref ined rice (HFDRR) or PR (HFDPR). It was found that PR intervention improved lipid accumulation in mice.Transcriptomic data analysis revealed that 27 genes were up-regulated (mostly involved in lipid breakdown)and 86 genes were down-regulated (mostly involved in inflammatory responses) in the HFDPR group compared to the HFDRR group. And 15 differentially expressed genes (DEGs) were validated by quantitative real-time PCR (RT-qPCR), while protein interaction network showed that protein tyrosine phosphatase receptor type C (PTPRC) has a central role. The gut microbiota of mice was also altered after different dietary treatments, with higher ratio of Firmicutes and Bacteroidetes, increased abundances of Ruminococcaceae,Lachnospiraceae, Christensenellaceae, Porphyromonadaceae, Rikenellaceae and Prevotellaceae, and decreased abundances of Lactobacillaceae, Peptostreptococcaceae, Erysipelotrichaceae and Actinobacteria in the HFDRR group. In addition, it was observed that PPAR signaling pathway may act as a bridge between DEGs and differential gut microbiota. These results suggested that PR can prevent hyperlipidemia by modulating liver genes and gut microbiota.
1. Introduction
Hyperlipidemia is a common metabolic disease, which is related to the increased risk of cardiovascular diseases (CVDs) such as atherosclerosis, coronary heart disease and stroke[1], and has become a widespread social health problem. Hyperlipidemia is characterized by abnormal elevation of blood lipids, and improving dyslipidemia can effectively prevent the occurrence of CVDs, which include drug control and lifestyle interventions such as exercise and diet. However,lipid-lowering drugs are usually accompanied by side effects.Therefore, it is considered desirable to maintain normal blood lipids by using alternative diet methods.
Rice (Oryza sativaL.) is one of the important staple foods for human beings, but it is often consumed in the form of refined rice(RR) in daily life[2]. RR is ref ined white rice produced by f ine milling.During milling, rice bran, aleurone layer and rice germ are removed.The nutrients such as protein, fat, vitamins and minerals in RR are lost, and the nutritional value is greatly reduced and long-term consumption of RR will threaten human health. A kind of pure natural and nutritious rice, parboiled rice (PR), is made by cleaning, soaking,cooking, drying and other hydrothermal treatment of paddy, then removing the husk of the paddy and milling the rice according to the conventional method[3]. Compared with RR, PR retains the nutrients in the outer layer and bran during processing and has elevated resistant starch content, and it is more conducive to human health. In addition, PR has the advantages of increased nutrient content, reduced broken rice yield, slower digestion, and better storage properties, and it is gaining increasing attention among researchers and consumers[4].Studies on the physiological functions of PR already reported its various effects, such as neuroprotection[5], prevention of beriberi[6],antioxidation[7], anti-inflammation[8], anti-diabetes[9], and renal protection[10]. However, the lipid-lowering effect of PR has not been reported.
Chronic diseases such as CVDs are the result of a complex interaction of genetic and environmental factors, of which diet plays an important role. The effects of diet on chronic disease may be masked by the heterogeneity of the effects associated with genetic variation among individuals, and consideration of diet-gene interactions may help us understand the pathogenesis of CVDs[11]. For example,previous studies reported that heptamethoxyflavone could reverse the mRNA levels of lipid catabolism genes (Cpt1b,Cyp8b1,Crat,Slc27a5,Ucp3,Cd36andFabp7), lipid biosynthesis genes (FasnandSrebp1c) and inflammation-related genes (Ccl2,Ccl4,Cxcl10,IL-1β,Tlr2,Fos,RelbandNfkbia) in rats fed with high-fat diet (HFD)[12].Rice bran polysaccharide treatment of mice fed with HFD can affect liver lipid accumulation by regulating lipid metabolism-related genes (Fasn,Acc,Cd36,Srebp1c,Sirt,Pparα,PparγandPparδ) and inflammation-related genes (IL-6,iNOSandTNFα)[13]. Understanding diet-gene interactions can provide theoretical support for the development of dietary interventions, which can help to eliminate the influence of genetic factors on disease risk and to develop personalized dietary recommendations.
In recent years, more and more studies have confirmed that intestinal microbes are related to many chronic diseases and metabolic dysfunctions, and they play an important role in regulating human health. Markers of microbial stability, such as richness and diversity, are often used as indicators of gut health. The overgrowth of intestinal bacteria, the change of intestinal microbiota, and the translocation of bacteria and their products are the common pathways for the occurrences and developments of alcoholic liver disease,non-alcoholic fatty liver disease and liver cirrhosis[14]. Although the composition of gut microbes varies from person to person, it can be changed through internal and external stimuli, which may reshape the structure and biological output of gut microbes. Dietary alterations can regulate intestinal microbes and improve human health, and previous studies have long recognized that diet can change the microbial ecology[15]. In recent decades, it has been discovered that changes in the structure of microorganisms caused by diet have an important impact on human physiology and disease processes. For example,it is reported that the health benefits of a vegan diet for metabolic syndrome, cardiovascular disease, and rheumatoid arthritis are related to gut microbiota[16]. Intake of whole-grain rice can regulate intestinal microbes and reduce the risk of disease[17]. Therefore, the interaction between diet and intestinal microbes is closely related to the body’s metabolism, but there is no relevant research on PR in this area.
Here, the diets of C57BL/6J mice were prepared by mixing RR or PR powder with basic laboratory feed. The effects of HFD and normal diet on mice were compared, and the effects of RR and PR on HFD were also evaluated in mice. We assessed the effect of PR on pathological indicators, biochemical indicators, and lipid deposition in liver and epididymal adipose tissue in mice. In addition, in this study,changes in liver genes and intestinal microbial composition were analyzed, and transcriptome and microbiome data were integrated and systemically analyzed. The purpose of the research is to explore possible molecular mechanisms of lipid-lowering effect of PR.
2. Materials and methods
2.1 Determination of nutrients
The nitrogen content was evaluated using the microKjeldahl method and crude protein was determined as N × 6.25. Lipids were determined gravimetrically after Soxhlet extraction with anhydrous ether. Carbohydrates were calculated as 100 − Moisture content− Ash content − Protein content − Fat content − Dietary fiber content. Dietary fiber, ash, thiamine, riboflavin and Fe contents were determined according to GB 5009.88-2014, GB 5009.4-2016,GB 5009.84-2016, GB 5009.85-2016 and GB 5009.90-2016 (China Standard Press, China).
2.2 Formula of feed
The nutritional compositions of the basic laboratory diet (Con,acquired from SLAC Company, Changsha, Hunan, China) and other diets were as Table S1. The feed consists of 69% carbohydrate, 20%protein and 10% fat. HFD containing refined rice (HFDRR) contained approximately 53.5% carbohydrate, 20.5% protein and 25% fat.In the HFDPR protection group, it provides approximately 53.2%carbohydrate, 20.8% protein, and 25% fat[13,18]. Both RR and PR were originated from the same species (Huanghuazhan, HHZ) and obtained from Hunan Jinjian Rice Industry Co., Ltd. (China).
2.3 Animal trial
The study was carried out in mice to assess the lipid-lowering effects of PR. The study was approved by the medical school of Hunan Normal University following the Guide for the Care and Use of Laboratory Animals (SYXK-Xiang 2019-0006). After 7 days of acclimation, 30 C57BL/6J male mice (8 weeks old, 22−24 g,purchased from SLAC Co., Ltd., Changsha, Hunan, China) were randomly divided into 3 groups (n= 10) according to the following:Con group, HFDRR group, and HFDP group. Each group of mice maintained a 12-h light/dark cycle for 8 weeks, with different diets and free access to drinking water, as well as a room temperature of(22 ± 2) °C and humidity of (50 ± 5)% and suitable housing. The bioinformatics analysis was conducted using 6 mice from each group.
2.4 Determination of physiological index and total food intake
During the feeding experiment, the body weight and food consumption of mice were determined every week. Following the sacrifice of the mice at 8 weeks, the liver, spleen, and epididymal fat were harvested and weighed immediately, placed in liquid nitrogen to ensure quick freezing, and then stored at −80 °C.
2.5 Biochemical analysis
After fasting overnight (12−14 h), mice were sacrificed. Following CO2anesthesia, all blood samples were collected and centrifuged at 4 °C, 1 400 ×g, 10 min, serum samples were collected and stored at −80 °C until biochemical analysis. Assay kits (Lei Du Life Technology, Shenzhen, China) were used to determine the concentrations of serum total cholesterol (TC), total triglyceride(TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C).
2.6 Fasting blood glucose and oral glucose tolerance test
In each group (n= 10), samples of blood from the tail vein after an overnight fast were collected every week to measure fasting blood glucose levels, and at the end of the study, oral glucose tolerance tests were performed. An oral glucose dose of 2 g/kg was administered to the mice, and tail vein blood was collected at 0, 15, 60, 90, and 120 min. The blood glucose was measured using an Accu-Chek Active blood glucose meter (Roche, Mannheim, Germany).
2.7 Liver and epididymal fat histopathology
After washing with 1 × PBS, the liver and epididymal adipose tissue were fixed in formaldehyde fixative for 48 h at room temperature. Following dehydration, xylene was used to permeabilize the specimens and paraffin was used for embedding. Slicing the tissue into 6−8 μm slices after cooling, and then dried at 45 °C at a constant temperature. Afterwards, the wax was removed, and the specimens were soaked in ethanol solution for 5 min, dyed with hematoxylin for 5 min, rinsed with distilled water for 1 h, and then soaked in ethanol for a further 10 min. Following this, the specimens were counterstained with eosin staining solution for 3 min, and then dehydrated with 100% ethanol. It was finally sealed with neutral resin and examined under a microscope.
2.8 RNA-seq
Total RNA was extracted from the tissue using TRIzol®Reagent(Invitrogen, USA) and genomic DNA was removed using DNase I(TaKaRa, Japan). Then RNA quality was determined by 2100 Bioanalyser (Agilent, USA) and quantified using the ND-2000(NanoDrop Technologies, USA). RNA-seq transcriptome librariy was prepared following TruSeqTMRNA sample preparation Kit from Illumina (San Diego, CA, USA) using 1 μg of total RNA.Sequencing was performed on Illumina Hiseq xten/Nova seq 6000 platform at Majorbio Corporation (Shanghai, China). Con, HFDRR and HFDPR groups were compared and genes with Con/HFDRR≥ 2 or ≤ 0.5, HFDPR/HFDRR ≥ 1.5 or ≤ 0.75,P≤ 0.05 were considered differentially expressed genes (DEGs). In addition, protein interactions were mapped using String website and imported into Cytoscape software for beautification. Functional enrichment analysis of DEGs was performed using Gene Ontology (GO) database and Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.bioinformatics.com.cn/?keywords=pathway).
2.9 Quantitative real-time PCR (RT-qPCR)
RT-qPCR assay was carried out utilizing the SYBR®Select Master Mix kit (Applied Biosystems, USA). Based on the manufacturer’s protocol (Applied Biosystems, USA), the relative expression levels of target genes andβ-actinwere assessed. An initial denaturation at 95 °C for 3 min was followed by 45 cycles of 95 °C for 15 s and 56 °C for 1 min in each RT-qPCR reaction. Dissociation curve analysis in conjunction with 1.2% agarose gel electrophoresis were utilized to verify the specificity of PCR reactions. In order to quantify the relative expression of mRNA, the ratio of the target gene toβ-actinwas calculated using the PCR system (Applied Biosystems,USA). The primer sequences were listed in Table S2.
2.10 16S rRNA sequencing
According to the manufacturer’s instructions, total bacterial DNA from samples of colon contents (n= 6 per group) was extracted and purified. The V3-V4 hypervariable region was determined by PCR amplification, using forward primer 338F(5’-ACTCCTACGGGAGGCAGCAG-3’) and reverse primer 806R(5’-GGACTACHVGGGTWTCTAAT-3’). The PCR products were identified by 2% agarose gel, extracted and further purified using Axy Prep DNA Gel Extraction Kit (Axygen, USA). QuantiFluor™-ST (Promega, USA) was used to quantify the purified amplicons for sequencing on the Illumina MiSeq platform according to standard protocols (Majorbio Corporation, Shanghai, China).
2.11 Western blotting analysis
The proteins were boiled for 10 min under 95 °C and then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis for separation (Bio-Rad, USA) and further transferred to the PVDF membrane. Next, the PVDF membranes were blocked with 5% BSA in 1× TBST for 1 h at room temperature followed by incubating with corresponding primary antibodies overnight at 4 °C.β-Actin (Cat. #: KM9001) was purchased from SUNGENE BIOTECH(China). Peroxisome proliferator-activated receptor gama (PPARγ,Cat. #: 16643-1-AP) was purchased from Proteintech (USA). Fatty acid translocase CD36 (CD36, Cat. #: 14347S) was brought from Cell Signaling (USA). Silent mating type information regulation 2 homolog-1 (SIRT1, Cat. #: sc-15404) was purchased from Santa Cruz Biotechnology (USA). Protein tyrosine phosphatase receptor type C (PTPRC, also called CD45, Lot. #: GR3381590-5) was brought from Abcam (China). Then the membranes were washed with TBST three times, each time 10 min and incubated with appropriate secondary-antibody (horseradish peroxidase conjugated Goat Anti-Rabbit IgG (H + L), Cat. #: SA009, AURAGENE, China; horseradish peroxidase conjugated Goat Anti-Mouse IgG (H + L), Cat. #: SA001,AURAGENE, China) for 1 h at room temperature. After three times of final wash, the membranes were incubated with ECL Plus™ (Pierce,Rockford, USA) and observed in the imaging system (ChemiDoc™XRS+, Bio-Rad, USA).
2.12 Statistical analysis
Data were expressed as means ± standard deviation (SD).Differences among groups were analyzed by one-way analysis of variance and subsequent Tukey post-hoc test using R 4.0 software.ForP-values below 0.05, a statistically significant difference was set.
3. Results
3.1 Nutrients composition analysis of RR and PR
Although RR and PR are derived from the same rice species,there are significant differences in their nutritional components due to different processing techniques. The contents of protein, fat, dietary fiber, vitamins and minerals in PR were significantly higher than those in RR. It suggests that PR is more nutritive than RR, as shown in Table 1.

Table 1 Nutrients composition analysis of RR and PR.

Table 2 Genes up-regulated by PR supplementation from RNA-seq analysis.

Table 3 Genes down-regulated by PR supplementation from RNA-seq analysis.
3.2 Effects of PR supplementation on the phenotype alterations
At the end of the feeding phase of the experiment, mice in the HFDRR group had the highest body weight, and weight of liver,epididymal fat and spleen (Figs. 1A, G-I), which was significantly different from Con and HFDPR groups (P< 0.05). Supplementation with PR reduced the values of these indicators to varying degrees,suggesting that PR could inhibit lipid over-accumulation and partially restore the phenotype. It can also be seen from the photographs of body organs that compared with the Con group, liver and spleen were enlarged and lipid accumulation in epididymal fat was excessive in HFDRR group, while the appearance of liver, spleen and epididymal fat was improved in HFDPR group (Figs. 1D-F). The total food intake (Fig. 1B) showed no significant difference among the 3 groups(P> 0.05). In addition, we explored the effect of PR on blood glucose(Fig. 1C). The results showed that the fasting blood glucose of the Con and HFDPR groups were lower than that of the HFDRR group.After 15 min of feeding, the blood glucose levels of the 3 groups increased rapidly, and continued to decline in the subsequent time.Compared with the Con and HFDPR groups, the blood glucose of the HFDRR group was still at a high level.

Fig. 1 Effect of PR supplementation on the phenotype alterations. (A) Body weight, (B) total food intake, (C) blood glucose, (D) liver performance,(E) epididymal fat performance, (F) spleen performance, (G) liver weight, (H) epididymal fat weight, (I) spleen weight. Organs of two mice in each group were randomly selected to observe their appearance. Data are expressed as mean ± SD, n = 6. Con vs HFDRR or HFDRR vs HFDPR, *P < 0.05, **P < 0.01 according to Tukey’s post hoc test. Same with Figs. 2, 5, 6, 9.
3.3 Effects of PR on lipid accumulation in mice
The blood samples of mice were collected to analyze the serum lipid profiles, which include TC, TG, LDL-C and HDL-C(Figs. 2A-D). Among the 3 groups, the HFDRR group had the highest content of TC, TG and LDL-C, and the lowest content of HDL-C.Compared with the HFDRR group, the HFDPR group significantly reduced the content of TC, TG and LDL-C, and increased the content of HDL-C (P< 0.05). It shows that adding PR to feed mice can significantly improve the cholesterol distribution in the serum and ameliorate HFD-induced hyperlipemia.
Histological analysis of liver (Fig. 2E) and epididymal fat(Fig. 2F) was performed using hematoxylin and eosin (H&E)histological staining. Compared with Con group, the hepatocytes in HFDRR group were more swollen, significantly increased in volume and had more fat vacuoles. In HFDRR group, hepatocyte boundary was unclear and hepatocyte steatosis was severe. The fat vacuoles in HFDPR group were smaller and the cell boundary was clearer than that in HFDRR group, indicating that PR could reduce the fat accumulation in hepatocytes. The size of epididymal adipocyte was the largest in the HFDRR group. In contrast, it was significantly reduced in the HFDPR group, which alleviated lipid accumulation in epididymal adipose tissue.
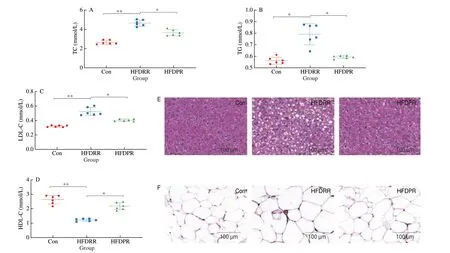
Fig. 2 Effect of PR on lipid accumulation in mice. (A) TC, (B) TG, (C) LDL-C, (D) HDL-C, (E) histology of liver, and (F) epididymal adipose tissue.
3.4 Effects of PR on hepatic gene expression profiles in mice
To elucidate the underlying mechanism by which PR prevents hyperlipidemia, the gene expression profile of mouse liver tissue was analyzed by RNA-seq. It can be seen from Table S3 that the GC content of the three groups was between 49.39% and 51.1%, while the Q30 was higher than 94.21%, indicating that the sequencing quality was good. 44.44−69.35 million clean reads of high-quality was obtained from mice in the Con, HFDRR and HFDPR groups, with over 86.03% of the clean reads uniquely mapped toMus musculusin each sample. Our data demonstrate that all libraries are of good quality and suitable for subsequent analysis.
Compared with Con group, the genes with |log2FC| > 1 in the HFDRR group were screened. Meanwhile, the differences between the HFDRR group and the HFDPR group were analyzed, and the DEGs were further screened. These DEGs were used to perform hierarchical clustering analysis (Fig. 3A), and 27 up-regulated genes and 86 down-regulated genes were identified as being regulated by PR treatment (Fig. 3B). The names and fold changes of DEGs were listed in Tables 2 and 3.

Fig. 3 (A) Cluster heatmap analysis of DEGs. 113 genes were screened by RNA-seq analysis, all of which were significantly different among the Con,HFDRR and HFDPR groups. Different colors represent different logarithmic intensities of gene expression levels, and red and green represent high and low expression of genes, respectively. The cluster dendrogram on the left shows the correlation of genes. (B) Number of DEGs. HFDRR/Con ≤ 0.5,HFDPR/HFDRR ≥ 1.5, and HFDRR/Con ≥ 2, HFDPR/HFDRR ≤ 0.75.
3.5 PR regulated the expressions of lipid metabolism and inflammation-related genes
To investigate the role of the above DEGs in hyperlipidemia,GO and KEGG analyses were used to elucidate the functions and important pathways of DEGs (Fig. 4). GO analysis showed that the processes enriched by DEGs with significantly increased expression in HFDPR mice were mainly associated with lipid metabolism, such as fatty acid metabolic process, cholesterol metabolic process, sterol metabolic process, steroid metabolic process (Fig. 4A). Furthermore,KEGG pathway analysis exhibited that up-regulated DEGs were mainly enriched in PPAR signaling pathway, retinol metabolism,fatty acid degradation, and primary bile acid biosynthesis (Fig. 4C).Most of the genes with increased expressions are involved in lipid metabolism.

Fig. 4 Analysis of functional enrichment and KEGG pathways enrichment of DEGs with increased or decreased expression in mice fed with HFD supplemented with PR. (A) Functional enrichment of DEGs with increased expression affected by PR supplementation. (B) Functional enrichment of DEGs with decreased expression affected by PR supplementation.(C) The significantly enriched pathways of up-regulated DEGs affected by supplemental PR. (D) The significantly enriched pathways of down-regulated DEGs affected by supplemental PR.
GO analysis of genes with significantly decreased expression revealed that they were primarily associated with inflammation,such as response to interferon-γ, leukocyte cell-cell adhesion,cellular response to interferon-γ and regulation of leukocyte cell-cell adhesion (Fig. 4B). Furthermore, KEGG analysis showed that the enriched pathways of DEGs with decreased expression were mainly phagosome, intestinal immune network for IgA production, asthma,viral myocarditis, type I diabetes mellitus, rheumatoid arthritis,and type II diabetes mellitus (Fig. 4D). Most genes with decreased expression are involved in inflammatory responses.
3.6 PR up-regulated gene expressions of lipid metabolism and down-regulated gene expressions of inflammatory response in hepatic tissues
Given the role of PR in improving lipid accumulation and steatosis from the above experimental results, DEGs participated in lipid metabolism and inflammation were further analyzed in order to identify any genes that might be related to the lipid-lowering function of PR. The heatmap of the genes related to lipid metabolism process was shown in Fig. 5A, it can be seen that the expression of these genes such asFads2,Cpt1a,Cyp4a14,Fads1,Elovl5,Acacb,were increased, whileCav1was decreased in the HFDPR group.Moreover, genes related to metabolic process of cholesterol, sterol and steroid, includingTm7sf2,Cyp51,Nsdhl,Pmvk, were increased,whereasAbcg1was decreased in the HFDPR group.Egr1was also up-regulated in the regulation of steroid metabolic process. In addition,down-regulated genesOsbpl8,Abcg1andCav1were associated with lipid storage. Interestingly, up-regulatedAcacbwas also involvedin lipid storage. Over 40 genes linked to inflammation, and these genes,exceptNab2, were significantly down-regulated in the HFDPR mice compared to the HFDRR mice (Fig. 5B). These genes contained(a) chemokines:Cxcl9,Cxcl10; (b) cytokine-regulated genes:H2-Ab1,H2-Eb1,H2-Aa; (c) guanylate binding proteins:GBP2,GBP4,GBP5,GBP7; (d) integrins:Itgb7,Itga4; (e) programmed death ligand:Cd274; (f) inflammatory factor:Aif1; (g) chemokine-like receptor:Cmklr1; (h) pro-inflammatory gene:Cd44; (i) macrophage migration inhibitory factor receptor:Cd74; (j) macrophage clearance receptor:Msr1; (k) heat shock protein:Hsp90aa1.
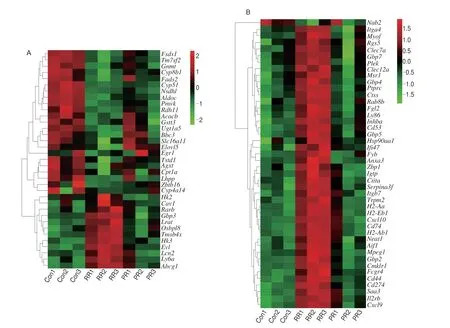
Fig. 5 Effects of PR supplementation on DEGs associated with lipid metabolism and inflammation in mouse liver. (A) Heatmap for genes participated in lipid metabolism. (B) Heatmap for genes participated in inflammation. Colors represent the logarithmic intensity of gene expression levels, with red representing high expression and green representing low expression, and the cluster dendrogram on the left shows the correlation of genes. (C) Relative mRNA levels of genes involved in lipid metabolism. (D) Relative mRNA levels of genes involved in inflammation. (E) Diagram of protein interaction network. The node size and edge thickness are determined according to the degree and the color is determined according to the combined score. The larger the value of degree and combined score,the larger the node, the thicker the edge, and the redder the color. (F) Protein expression levels of PTPRC. Values are expressed as mean ± SD (n = 3). β-Actin was used for internal control.
Among the DEGs related to lipid metabolism and inflammation,15 genes were selected and assayed by RT-qPCR (Figs. 5C and D).It was found that the expression trends of these genes were consistent with the results of RNA-seq analysis of HFDRRvsHFDPR.HFDRR feeding significantly down-regulated the expression of lipid catabolism-related genes (Fads2,Fads1,Elovl5,Gnmt,Cyp51,Cyp8b1andCpt1a) and up-regulated the expression of lipid biosynthesis-related genes (Abcg1andCav1) compared with the Con group (P< 0.05), while supplemental PR effectively reversed most of the changes. In addition, the mRNA levels of 6 inflammation-related genes in the HFDRR group were significantly higher than those in the Con group. PR supplementation significantly down-regulated inflammatory factorAif1, chemokineCxcl10, chemokine-like receptorCmklr1and pro-inflammatory geneCd44in mouse liver tissues(P< 0.05). Furthermore, the expression levels ofRgs5andSaa3in the HFDPR group were also significantly lower than those in the HFDRR group (P< 0.05). Changes in the expression of these genes in the HFDPR group of mice may help reduce steatosis and maintain normal physiological functions of lipids.
The protein interactions of lipid metabolism and inflammationrelated genes were further analyzed in String website and Cytoscape software. It can be seen that PTPRC occupied the most important position in the interaction network (Fig. 5E), and PTPRC is usually associated with inflammation, diabetes, atherosclerosis, etc. Western blotting analysis confirmed that PR supplementation could inhibit the protein expression level of PTPRC. Secondly, proteins such as CXCL10, CXCL9, CD274, CD74, H2-AB1, CTSS and FCGR4 were also key targets. Our data suggest that PTPRC may have an important function in the lipid-lowering efficacy of PR treatment.
3.7 PR supplementation altered gut microbiota
To evaluate the effect of PR supplementation on mouse gut microbiota, we performed 16S rRNA gene-based taxonomic profiling.Our results showed that less gut microbiota diversity was exhibited in mice of HFDRR group than that of Con and HFDPR group (Figs. 6A and B), as indicated by the Shannon and Simpson indexes. Compared with the HFDRR group, the Shannon and Simpson indexes of the HFDPR group were found to be revised (P> 0.05 in Shannon index andP< 0.05 in Simpson index), which indicates that PR feeding can partially restore the host gut microbiota diversity. The principal coordinate analysis (PCoA) showed that 3 treatment groups had differential clustering (Fig. 6C). According to the results, when a 90%ellipse is provided, HFDRR mice have differential clusters compared with Con mice. After PR treatment, the HFDPR group was closer to the Con group on theX-axis, indicating that PR treatment can improve the mouse gut microbiota.

Fig. 6 Effects of PR supplementation on gut microbiota. (A) Shannon index, (B) Simpson index, (C) principal co-ordinates analysis (PCoA). (D) Percentage of community abundance at the phylum, (E) family, and (F) genus levels, (G) LEfSe multi-level species hierarchical tree diagram, using different colors to represent certain enriched taxa, (H) only taxa meeting an LDA significance threshold > 4 were listed in the LEfSe Bar.
The structural changes of microbiota in the colon contents of 3 treatment groups were analyzed by 16S rRNA sequencing. After 8 weeks of dietary interventions, the differences in the relative abundance of specific microbes among groups were also tested in the present study. The taxonomic characteristics of the taxa showed that the relative abundance of the taxonomic sequences of the phylum was mainly composed of Firmicutes and Bacteroidetes (Fig. 6D).It was worth noting that the relative abundance of Proteobacteria in the HFDPR group was higher than that in the Con group (10.29%vs3.60%) and the HFDRR group (10.29%vs3.09%), while the Con group and the HFDRR group were closer (3.60%vs3.09%).In addition, the Actinobacteria in the HFDRR group had the highest relative abundance (4.40%), and the HFDRR group (1.36%)and the Con group (0.48%) were closer. At the family level(Fig. 6E), the abundance of Erysipelotrichaceae (31.33%vs4.62%)and Lactobacillaceae (17.99%vs8.24%) in the gut microbiota of the HFDRR group was higher than that of the Con group, Bacteroidales_S24-7_group (30.33%vs55.58%), Lachnospiraceae (5.62%vs9.63%)and Ruminococcaceae (2.43%vs4.28%) were lower than the Con group. Compared with the HFDRR group, the HFDPR group reduced the abundance of Erysipelotrichaceae (8.36%) and Lactobacillaceae(16.26%), and increased the abundance of Bacteroidales_S24-7_group(36.49%), Lachnospiraceae (17.15%) and Ruminococcaceae (4.80%).Among them, Lachnospiraceae and Ruminococcaceae are the two most abundant families in phylum Firmicutes. They can degrade a variety of recalcitrant polysaccharides and have beneficial effects on the intestinal barrier function. However, Desulfovibrionaceae (7.52%)was more enriched in the HFDPR group than in the Con (1.69%)and HFDRR groups (2.01%). At the genus level (Fig. 6F), PR supplementation has higher abundance of norank_f_Bacteroidales_S24-7_group (36.49%vs30.33%), Lachnospiraceae_NK4A136_group (5.27%vs1.98%),Desulfovibrio(7.52%vs2.01%), norank_f_Lachnospiraceae(5.82%vs1.37%) than RR supplementation and lower abundance ofLactobacillus(16.26%vs17.99%) andFaecalibaculum(8.15%vs30.78%).
The significantly differential species among the 3 groups of C57BL/6J mice were determined by LEfSe using non-parametric factorial Kruskal-Wallis sum-rank test and linear discriminant analysis (LDA) to estimate the effect size of each species abundance(Figs. 6G and H). The Con group was characterized by significantly different communities (P< 0.05) belonging to Verrucomicrobiaceae,Christensenellaceae and Mollicutes. However, the species enriched by HFDPR intervention belonged to Aerococcaceae. In contrast, the key phylotypes of HFDRR was identified as a significantly enriched species belonging to Peptostreptococcaceae and Actinobacteria.
3.8 Correlation analysis between gut microbiota and lipidrelated indexes
Unadjusted and adjustedP-values (Benjamini-Hochberg method)(Fig. 7) were used for Spearman correlation analysis. Our correlation results indicated that 45 gut microbes were related to hyperlipidemia.The increase in the abundance of intestinal microorganisms Lactobacillaceae and Erysipelotrichaceae was related to the increase in liver tissue weight. Conversely, the increase in the abundance of certain bacteria, Bacteroidales_S24-7_group, Prevotellaceae,Bacteroidaceae, unclassified_o_Bacteroidales, Porphyromonadaceae,Christensenellaceae and Corynebacteriaceae, was associated with a decrease in liver tissue weight. In addition, unclassified_o_Bacteroidales and Christensenellaceae were negatively correlated with LDL-C content in mouse serum.

Fig. 7 Correlation between the bacterial groups and indexes. (A) Heatmap with un-adjusted P value. (B) Heatmap with adjusted P value. * and ** indicate the significance of the associations (P < 0.05 and P < 0.01, respectively). Same with Figs. 8, 10.
3.9 PR supplementation altered the gut function
The gut functional changes caused by different diets were predicted by Tax4Fun and exhibited in Fig. 8, based on the KEGG.Compared with the HFDRR group, we found that the HFDPR group significantly enriched starch and sucrose metabolism (P= 0.005 07),D-alanine metabolism (P= 0.005 075), citrate cycle (TCA cycle)(P= 0.005 075), nicotinate and nicotinamide metabolism (P= 0.045 33),and PPAR signaling pathway (P= 0.045 33). These results indicate that PR is involved in the regulation of energy metabolism, amino acid metabolism and PPAR metabolic pathway.

Fig. 8 Tax4Fun-predicted microbial community functional changes in Con,HFDRR and HFDPR groups. Based on the KEGG database.
3.10 PPAR signaling pathway may be an important bridge between liver genes and gut microbiota
Combined analysis of liver genes and gut microbiota revealed that both alterations involved PPAR signaling. To demonstrate whether PPAR signaling was regulated, the mRNA and protein levels of several genes associated with PPAR in liver tissues were examined (Fig. 9). Obviously, HFD increasedPparγandCd36, and decreasedSirt1mRNA levels (P< 0.01). Western blotting analysis further confirmed that PR could inhibit the expression levels of PPARγ and CD36, and promote expression levels of SIRT1. It indicates that PPAR signaling pathway is involved in PR-mediated lipid-lowering effect.

Fig. 9 Relative expression levels of mRNA and protein of genes related to PPAR signaling pathway. (A) Relative mRNA expression levels of Pparγ,Cd36 and Sirt1. (B) Relative protein expression levels of PPARγ, CD36 and SIRT1. Values are expressed as mean ± SD (n = 3). β-Actin was used for internal control.
3.11 Integrative analysis of gut microbiota and DEGs
To understand the relationship between the transcriptome and the microbiome with the impact of PR, the correlations between the gut microbiota and lipid metabolism-and inflammatory responserelated DEGs identified by RNA-seq analysis were explored by Spearman’s correlation analysis (Fig. 10). The results of family-level analysis showed that the abundance changes of Bifidobacteriaceae,Corynebacteriaceae, Erysipelotrichaceae and Coriobacteriaceae were positively correlated with lipid storage-related genes (such asCav1,Abcg1), and negatively correlated with lipid decompositionrelated genes (such asFads2,Cyp8b1). These four bacteria and Lactobacillaceae were positively associated with most of the inflammation-related genes. In addition, increased abundances of Christensenellaceae, Bacteroidales_S24-7_group, Bacteroidaceae,Porphyromonadaceae, unclassified_o_Bacteroidales, Prevotellaceae,Rikenellaceae, Ruminococcaceae and Helicobacteraceae were associated with decreased levels of some lipid synthesis-related DEGs and increased levels of some lipid decomposition-related DEGs.Increases in these bacteria were also associated with decreased levels of most inflammation-related DEGs.
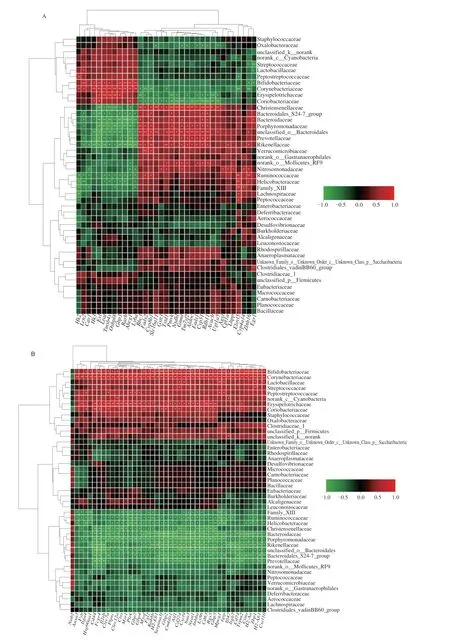
Fig. 10 Integrative analysis of the transcriptome and the microbiome data. (A) Correlation analysis of gut microbiota and lipid-related genes. (B) Correlation analysis of gut microbiota and inflammation-related genes.
4. Discussion
Lipids are one of the important nutrients for the human body.They provide the energy and essential fatty acids needed by the human body and play an important role in maintaining the stability of the intracellular environment. In the present study, our data indicate that PR supplementation in diet upregulates the mRNA levels ofFads1,Fads2,Elovl5andCyp51in the liver, which are involved in fatty acid metabolism. High expressions ofFads1,Fads2andElovl5may increase the conversion of potentially harmful fatty acids such as palmitate, stearate or oleate to unsaturated long-chain fatty acids and lead to the degradation of these lipid species[19].Carnitine palmitoyltransferase,Cpt1a, can promoteβ-oxidation of fatty acids[20], and induction of its expression improves obesity,hyperlipidemia[21-22]and fatty liver[23]. In addition,Cpt1ais involved in activation of brown adipose tissue, which promote thermogenesis and energy expenditure[24]. Conversely,Abcg1, regulated byPparsis involved in early cholesterol reverse transport[25]. Compared withn-6 polyunsaturated fatty acid, the intake of saturated fatty acids could modulate gene expression of cholesterol inflow and outflow mediators in peripheral blood mononuclear cells, such as up-regulated expression ofAbca1/Abcg1and down-regulated expression ofLDLR,which may explain the phenomenon of elevated cholesterol caused by excessive intake of saturated fatty acids[26].Cav1has been reported to be increased expression in adipose tissue in obese subjects,attenuating leptin-dependent increases in adiponectin, potentially providing a target for ameliorating obesity[27]. These findings are similar to our observations. Therefore, the regulation of fatty acid metabolism process may be the reason for the lower levels of serum TC, TG, and LDL-C in the HFDPR group.
Metabolic inflammation plays an important role in the occurrence and development of many diseases. PR supplementation can inhibit the expressions of inflammatory factors in liver tissues.Transcriptome analysis reveals that PR supplementation also reduces the expression of other inflammation-related factors. For example,Aif1, an inflammatory factor mainly produced by macrophages in human white adipose tissue, is a putative obesity gene and its expression is increased in obese women[28].Aif1may be an important predictor of activated macrophages and increase expression in inflammatory response[29]. Our transcript profile also shows that proinflammatory chemokineCxcl10expression is decreased. Downregulated expression ofCxcl10could reduce liver inflammation,injury and fibrosis[30]. In HFD-fed obese KKAy mice,Cxcl10expression was elevated in the intraperitoneal adipose tissues[31].Elevated expression ofCd44could exacerbate the inflammatory response, and consumption of fish oil can reduceCd44and other proinflammatory gene expressions induced by high-fat, high-cholesterol diet[32]. These results are consistent with our results, suggesting that PR may attenuate lipid accumulation by inhibiting the expressions of inflammatory factors, thereby preventing hepatic steatosis in HFD mice. Obesity can cause chronic inflammation. Can inflammation also promote obesity? Studies have shown that hypothalamic inflammation leads to increased food intake and nutrient storage in mice[33]. Whether PR supplementation in diet can inhibit hypothalamic inflammation? It needs further study.
Protein interaction networks were used to analyze key proteins.In the present study, PTPRC may play an important role in the lipidlowering effect of PR supplementation. PTPRC is a member of inflammatory markers and plays an important role in inflammation,diabetes, atherosclerosis and other diseases. In addition, PTPRC also plays an important role in lipid metabolism processes. PTPRC is also associated with intestinal bacteria. Dimitriu et al.[34]found that PTPRC regulated immune responses by selecting for the presence of specific taxa. CD274 plays an important role in obesity. Eljaafari et al.[35]found that PD-L1 was overexpressed in the white adipose tissue of diet-induced obese mice. CD74 is a ligand for macrophage migration inhibitor. Li et al.[36]showed that CD74 in apoptotic macrophages was associated with inflammation and thrombosis during human atherosclerotic plaque progression, lipid metabolism and clinical manifestations of atherosclerosis. Cathepsin S (CTSS) was reported to be overexpressed in broiler ovaries leading to excessive lipid deposition, oxidative stress and potential damage to ovulation[37].These proteins may have important effects on the homeostasis of lipid metabolism, and their interference and signaling pathways can be studied in the future. In addition, whether these key proteins are related to the gut microbes deserves further investigation.
The proportion of Firmicutes and Bacteroides in obese people is relatively higher than that in healthy people. In this study, at the family and genus level, Bacteroidales/norank_f_Bacteroidales_S24-7_group and butyric acid-producing Lachnospiraceae/Lachnospiraceae_NK4A136_group/norank_f_Lachnospiraceae have higher abundance in Con and HFDPR groups. Butyric acid is a shortchain fatty acid that plays an important role in preventing or treating obesity. Erysipelotrichaceae, Lactobacillaceae/LactobacillusandFaecalibaculum, which are related to weight gain and obesity, are dominant in HFDRR. Correlation analysis of intestinal microbes and lipid metabolism-related parameters can identify functional gut microbes. In this study, Bacteroidales_S24-7_group, Bacteroidaceae and unclassified_o_Bacteroidales, which showed significant correlation, all belong to the phylum Bacteroidetes and are beneficial bacteria. The relative abundance of Peptostreptococcaceae tended to decrease after the mice received PR treatment, consistent with previous studies suggesting that reducing the relative abundance of Peptostreptococcaceae was beneficial in reducing obesity and lipid disorders[38]. Liu et al.[39]studied the improvement effect ofLactobacillus plantarumY44 on inflammation and lipid metabolism in HFD obese mice, and found thatLactobacillus plantarumY44 increased the level of Rikenellaceae in obese mice. Our results show that Rikenellaceae is negatively correlated with LDL-C, and also suggest that Rikenellaceae can help improve lipid metabolism.Porphyromonadaceae, which produces butyric acid by fermenting carbohydrates, is beneficial to promote intestinal health and enhance the host immune response[40]. Here, Porphyromonadaceae reduced liver weight and serum LDL-C levels in mice, probably due to the effect of the small molecule metabolite butyric acid.Christensenellaceae is a potentially beneficial gut microbe. Li et al.[41]reported that Christensenellaceae was negatively correlated with pathological features of metabolic syndrome, such as obesity,hypertriglyceridemia, and body mass index. They found that people with high abundance of Christensenellaceae had lower levels of lipid biosynthesis and energy metabolism pathways, which may explain the negative relationship between body weight and Christensenellaceae.In view of the important role of gut microbes in the body, it is necessary to study the precise regulation of gut bacteria by means of fecal microbiota transplantation or gene editing.
Combined analysis of pathway enrichment results for liver genes and gut microbes will help to unravel the molecular mechanisms underlying the lipid-lowering efficacy of PR. Our data imply that the PPAR signaling pathway may be a key pathway. The PPAR signaling pathway is closely related to the glucose regulation and lipid profile[42]. A study on atorvastatin ester shows that it affects liver lipid metabolism and cholesterol, and the metabolism may be mediated by PPAR pathway in the hyperlipidemia model rats[43]. PPARs family,three subtypes of PPARα, PPARγ and PPARδ, belong to the ligandactivated nuclear receptor superfamily of transcription factors,and have been reported to regulate various functions such as lipid metabolism[13]. PR treatment leads to differences in the expression ofPparγ, and the expression ofCd36andSirt1that belong to the same family related to PPARs has also changed, which is consistent with the conclusions of most previous studies on lipid metabolism mechanisms.
At present, the public acceptance of PR is not high, the main reason is the low viscosity and bad taste of PR. Meanwhile, the price of PR is higher compared to RR. There are still many problems to be solved in the study of the lipid-lowering mechanisms of PR. What are the targets of active components and metabolites of PR after entering cells? What is the upstream pathway of PPAR activated by PR? The downstream target genes regulated by PPAR also need further systematic study. In addition, PR may activate other pathways.How does PPAR pathway coordinate other pathways to ameliorate hyperlipidemia in the model? What transcription factors can be activated by these signaling pathways? How do the transcription factors affect the expressions of lipid metabolism-related genes? All of these issues need to be resolved.
5. Conclusion
In this study, we determine that PR supplementation in diet can decrease the body weight, reduce the blood glucose and ameliorate the blood lipid profiles in the HFD-induced obese mice. PR supplementation can regulate the expressions of lipid metabolismrelated genes in the liver tissue of mice and promote fat catabolism.At the same time, PR can also inhibit the expressions of inflammatory factors and inhibit HFD-induced chronic inflammation of liver tissue. PR supplementation can change the gut microbiota of obese mice, which means PR can change metabolites of gut microbiota and may be participated in the lipid-lowering effect. This study also preliminarily explored the molecular mechanism of lipid-lowering effect of PR, and found that some signal pathways such as PPAR pathway may play an important role in it, but further research is needed. In-depth study of the anti-hyperlipidemia mechanism of PR will promote further development and utilization of PR and benefit our health.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The project was financially supported by Key Project of State Key R & D Program, China (2022YFF1100200), the Program for Science & Technology Innovation Platform of Hunan Province(2019TP102), Natural Science Foundation of Hunan Province(2021JJ31075, 2019JJ50984), Natural Science Foundation of Changsha City (kq2014275), Scientific Innovation Fund for Postgraduates of Central South University of Forestry and Technology(CX20200699, CX202102067) and Postgraduate Scientific Research Innovation Project of Hunan Province (CX20201018, CX20210899,CX20220701 and CX20220720).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/ 10.26599/FSHW.2022.9250120.
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango