Preventive effect of kiwi berry (Actinidia arguta) on loperamide-induced constipation
2024-01-24JiyueZhangBinLiNingxuanGaoHaikunLiXingyueCuiHanqianJiangSiyiTangChenyuJinJinlongTian
Jiyue Zhang, Bin Li, Ningxuan Gao, Haikun Li, Xingyue Cui, Hanqian Jiang,Siyi Tang, Chenyu Jin, Jinlong Tian
College of Food Science, Shenyang Agricultural University, Shenyang 110866, China
Keywords: Kiwi berry Constipation VIP-cAMP-PKA-AQP3 Gut microbiota
ABSTRACT Constipation is a common intestinal disease. Kiwi berries can effectively prevent constipation. However,studies have yet to be done to determine how kiwi berries prevent constipation. For two weeks, mice in this study were continually orally gavaged with kiwi berry, loperamide, or a combination of the 2. This study found that the kiwi group’s feces had more water than the constipated mice. In addition, kiwi berries can speed up gastrointestinal transit (GI), shorten the time it takes to pass the f irst dark stool, and dramatically enhance body weight gain. In the interstitial cells of Cajal (ICC) cells and colon tissues, alterations in the protein expression of vasoactive intestinal peptide (VIP), cyclic adenosine monophosphate (cAMP), protein kinase A (PKA), and aquaporin-3 (AQP3) were found. At 3, 6, and 12 h of ICC cells and mouse colon, the kiwi group’s VIP, cAMP, PKA, and AQP3 protein expression levels were lower than those of the constipated mice. The kiwi berry can decrease the Firmicutes to Bacteroidetes ratio and boost the diversity and quantity of gut microbiota. By inf luencing the gut microbiota and VIP-cAMP-PKA-AQP3 signaling pathway, kiwi berries prevent constipation.
1. Introduction
Fewer than three bowel movements per week, complicated bowel movements, hard and dry stools, stomach pain, nausea, dyspepsia,and bloating are all signs of constipation[1]. With the accelerated pace of life, increasing social pressure, and bad eating practices in modern culture, around 70% of individuals are in a sub-health state, frequently experiencing digestive discomfort, with constipation being the most common type[2]. Constipation can also be brought on by medication,unclean living, abusing laxatives, hormonal issues, and illnesses in other sections of the body[3]. Constipation has a highly complex etiology that involves the patient’s bowel function being damaged.The results in varying levels of psychological stress, affecting the patient’s quality of life[4-5]. Currently, laxatives are mainly used to cure constipation, although this treatment method can have several undesirable side effects, including constricted diarrhea and drug dependence[6]. Therefore, preventing constipation at its source has become a new study focus to reduce the harm that constipation and laxative side effects cause to the human body. The most crucial approach to enhancing intestinal health is to prevent constipation with diet. Still, there are no clinical trials or scientif ic studies on functional foods, particularly berries, to do so.
First, bowel movement time and intestine advance value are essential indicators to measure the intestinal physiological activity,which are benef icial to assess the degree of constipation in mice since the incidence of constipation are associated with problems of colonic motility[7]. A shorter time to f irst bowel movement and an increased rate of advancement indicated improvement in constipation[8]. It has been suggested that persistent constipation and neurotransmitter and water channel levels are strongly correlated[9]. Vasoactive intestinal peptide (VIP), a crucial enteric neurotransmitter, can control intestinal motility and intestinal fluid metabolism[10]. VIP binds to the corresponding receptor, allowing ATP to be converted to cyclic adenosine monophosphate (cAMP), and then cAMPdependent protein kinase A (PKA) is activated, which phosphorylates intracellular aquaporin-3 (AQP3)[11]. The water transport system and the development of constipation are both closely tied to AQP3[12].Therefore, it’s crucial to identify alterations in the VIP-cAMP-PKAAQP3 signaling pathway. Reduced levels of substance P (SP), an excitatory neuropeptide that can stimulate bowel movement and is closely associated with colon sensory dysfunction[13]. Constipation may result from increased rectal compliance brought on by impaired sensory function in the colon. The smooth muscle of the colon is stimulated to contract by an intestinal hormone called motilin (MTL),which quickens the passage of intestinal contents through it[14]. To alleviate constipation, 5-hydroxytryptamine (5-HT) encourages the production of cells linked to intestinal motility[15].
Constipation causes oxidative and inflammatory reactions,changes gut microbiota composition, and makes the gut more permeable to pathogens and endotoxins[16]. Constipation causes hazardous germs to multiply, excrement to remain in the small intestine for much longer than usual, and it can injure other organs[17].Intestinal health is linked to the interstitial cells of Cajal (ICC).Constipation and bowel dysfunction will result from alterations in the content and structure of ICCs in the intestine[18].
Prebiotics has been the focus of studies on regulating intestinal health and treating constipation[19-21], and there has also been researched on berries such as mulberry[22]. The material for this paper was the kiwi berry, a highly unique berry. Its fruit has a distinct flavor, a small fruit form, green fruit skin when it is young,dark green fruit skin when it is old, a smooth, hairless surface, and green or green-yellow meat when it is ripe[23]. Kiwi berry (Actinidia argute), also known as rattan pear, rattan jujube, and macaque pear,is a perennial dioecious deciduous vine berry in the Actinidiaceae family[24-25]. The kiwi berry is whole and healthy, containing many vitamins, proteins, and fats, and its VC content is 80 to 100 times higher than that of apples and pears[26]. Kiwi berry is also a medicinal and edible homologous plant, its fruit, stem, root, and leaves all have therapeutic significance[27]. The kiwi berry’s polysaccharides and flavonoids moisten the intestines and cleanse the bowels[28].
Additionally, kiwi berries can lower blood sugar, regulate intestinal health, support fetal development, and prevent and treat disorders of the white inner[29]. Resources for wild kiwi berries can be found in China, Japan, Russia, and the Korean Peninsula. The kiwi berry macaque arrived in the United States from Japan in the late 19thcentury and was cultivated for trade in the 20thcentury[30]. Wild kiwi berry resources are particularly abundant in Liaoning, Shandong,Jilin, Heilongjiang, and other provinces in China. The Longcheng No. 2 cultivar from Dandong, Liaoning Province, was chosen in this study.
This study examined whether kiwi berries might prevent constipation by modulating the VIP-cAMP-PKA-AQP3 signaling pathway and gut microbiota composition in loperamide-induced constipated mice and the ICCs experiment. These findings demonstrate that kiwi berries can be used as a different approach to treat constipation. It is advantageous to facilitate clinical tests and result in the exploitation of kiwi berries.
2. Materials and methods
2.1 Chemicals
Loperamide hydrochloride (CAS No. 34552-83-5; 2 mg per capsule), loperamide (dissolved in sterile saline, 1 mg/mL) and activated carbon (CAS No. 7440-44-0) was procured from Sigma Aldrich (St. Louis, MO, USA). To prepare the activated carbon solution, 100 g of gum arabic and 50 g of activated carbon were added to 1 L of water. SP, MTL and 5-HT were detected by enzymelinked immunosorbent assay (ELISA) kits (Shanghai Enzyme-Linked Biotechnology Co., Ltd., Shanghai, China).
2.2 Sample preparation
Commercial maturity Longcheng No. 2 kiwi berries were collected from Dandong (124°23’ E, 40°07’ N, China). Two hundred grams kiwi berry was divided into two equal parts, which are respectively used for the Kiwi and Kiwi+Lop groups. Obtained kiwi berry extract by vacuum microwave drying and ultrafine grinding.
2.3 Animal experiment
Male BALB/c mice (7-week-old, 18–22 g) were acquired from Liaoning Changsheng Biotechnology Co., Ltd. (Liaoning,China). Before the investigation began, acclimatize mice for a week(temperature (25 ± 2) °C, humidity (50 ± 5)%). Mice were casually assigned to four groups (n= 12), and the prevention experiment was conducted by gavage for 2 weeks: control group (Con),0.25 mL sterile saline; kiwi group (Kiwi), 0.25 mL 300 mg/kg kiwi;loperamide and kiwi berry group (Kiwi + Lop), 0.125 mL 10 mg/kg Lope + 0.125 mL 300 mg/kg kiwi berry; Model group (Lop), 0.25 mL 10 mg/kg loperamide. All experimental procedures were conducted according to the guidelines provided by the Animal Care Committee.The Ethical Committee approved all experiments for the Experimental Use of Animals at Shenyang Agricultural University (LACUC Issue No. 2022030315, Appr Date: 2022.03.03).
2.4 Interstitial cell experiment
Six constipated mice from the Lop group were randomly selected for ICCs extraction. The mice were sacrificed by cervical dislocation,and the colonic tissue was removed from the cell cleansing station.The colonic tissue was rinsed with PBS before adding 2 mL of the enzymatic solution. After digestion with type I collagenase (37 °C,30 min), serum-containing complete medium was added, passed through a 200-mesh sieve, and the cell filtrate was collected for subsequent experiments. The group names of the cell experiment are consistent with the animal experiment: control group, not added (Con),30 ng/L kiwi berry (Kiwi), the mixture of kiwi berry and loperamide(Kiwi + Lop, 1 ng/L Lope + 30 ng/L kiwi berry), constipation group(Lop, 1 ng/L loperamide).
2.5 Real-time quantitative PCR and Western blot of VIPcAMP-PKA-AQP3 pathway
Measured the expression level of proteins and genes of VIP,cAMP, PKA, and AQP3 in ICC cells (3, 6 and 12 h time points) and mouse colonic tissue, respectively. The TRIpure reagent isolated total RNA from colon tissue (BioTeke, Beijing, China). Utilizing BeyoRT II M-MLV reverse transcriptase, the extracted RNA(500 ng) was converted into cDNA (Beyotime Bio, Shanghai, China).The ExicyclerTM96 fluorescence quantifier (BIONEER Bio, Daejeon,South Korea) was employed for this investigation. The relative gene expression was calculated using the 2–ΔΔCttechnique. Table 1 displays the primer sequences forVIP,cAMP,PKA,andAQP3.
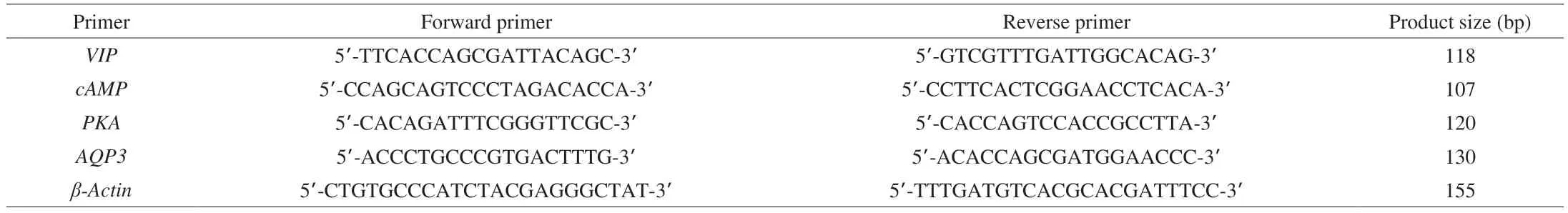
Table 1 Primer sequences used in the experiment.
Proteins were abstracted from colon tissue and interstitial cells utilizing total protein extraction kits (Wanlei bio, Shenyang, China).The thickness of protein lysates was measured by the bicinchoninic acid (BCA) protein assay kit (Wanlei bio, Shenyang, China).Protein was divided by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene difluoride (PVDF) membranes. Subsequently, blocking 1 h with 5% skim milk in Tris-buffered saline Tween-20 (TBST), the membranes were cultivated overnight at 4 °C with primary antibodies(VIP, cAMP, PKA, AQP3, andβ-actin antibodies). Next, washing membranes and conjugating with horseradish peroxidase (HRP)-labeled secondary antibodies (Cell Signaling Technology, MA, USA)were investigated. Enhanced chemiluminescence (ECL) (Wanlei Bio,Shenyang, China) was utilized to explore protein bands.
2.6 Determination of constipation-related indicators
After two weeks of experimentation, the body weight of each mouse was monitored at 9 a.m. Mice were fasted overnight, provided only with water, and fed with activated carbon powder 18 h later.Then mice were immediately transferred to clean, empty individual cages. Free food and water for mice. The interval between feeding the carbon powder and the first stool’s arrival was noted. The fecal particle count was determined for each mouse within 3 h. The water content of the fecal particles was determined after drying them at 90 °C for 3 h. The mice were starved for the entire night in order to measure their gastrointestinal transit (GI) rate. Activated carbon powder was then given to the mice. After 60 min, the mice were sacrificed by cervical dislocation.
2.7 Biochemical assays
Serum and colonic tissue levels of the neurotransmitter (SP, MTL,5-HT) were determined using ELISA kits based on the manufacturer’s instructions. Optical density (OD) values were determined at 450 nm using a microplate reader (FlexStation 3, Molecular Devices,FL, USA). Each assay was performed in triplicate.
2.8 Immunohistochemistry (IHC) analysis
IHC analysis further analyzes the protein expression of VIP,cAMP, PKA, and AQP3. The fixed tissues were repaired, rinsed with tap water for 4 h, embedded in paraffin, sliced into 5 μm glass slides and placed in a warm water dish for expansion. IHC analysis of colonic tissues was performed using HRP-labeled goat anti-rabbit IgG (Thermo Fisher, CN, USA). The staining effect was observed by microscope (BX53, OLYMPUS, Japan), and representative photomicrographs were taken (DP73, OLYMPUS, Japan).
2.9 16S rDNA sequencing and analysis
Fecal microbial composition was measured by 16S rDNA sequencing. Total genomic DNA samples were extracted using the Omega Mag-bind soil DNA kit (Omega M5635-02) (Omega Bio-Tek,Norcross, GA, USA) following the manufacturer’s instructions and stored at –20 °C until further analysis. The bacterial 16S rDNA gene V3–V4 region was amplified by PCR using forward primer 338F(5’-ACTCCTACGGGAGGCAGCA-3’) and reverse primer 806R(5’-GGACTACHVGGGTWTCTAAT-3’). The PCR components include 5 μL of buffer (5×), 2 μL of dNTP solution (2.5 mmol/L),1 μL of forward and reverse primers (10 μmol/L), 1 μL of DNA template solution, 14.75 μL of ddH2O and 0.25 μL of FastPfuDNA polymerase (5 U/μL). PCR amplification was performed as follows:initial 5 min at 98 °C, followed by 25 cycles of 30 s denaturation at 98 °C, 30 s annealing at 52 °C, 45 s extension at 72 °C, and a final extension at 72 °C for 5 min.
2.10 Statistical analysis
Results in triplicate are presented as mean ± SD. One-way ANOVA and Tukey’s multiple comparison test were used (GraphPad Prism 9.1.1). APvalue less than 0.05 indicates statistical significance.Correlation analysis was performed by Spearman’s method.
3. Results and discussion
3.1 VIP-cAMP-PKA-AQP3 signaling pathway expression of ICC experiment
The goal of this research is to determine how kiwi berries reduce constipation. To improve the health of the mouse gut, kiwi berries can increase mouse growth, alter the fecal condition, up-regulate excitatory neurotransmitters directly linked to intestinal motility,and regulate the intestinal flora. The majority of earlier studies on constipation employed animals[31]. Based on these findings, this study coupled ICC cell assays with VIP-cAMP-PKA-AQP3 pathway regulation to investigate the preventative mechanism of kiwi berries on constipation. Combining cell and animal research provides a novel perspective on preventing constipation bothin vitroandin vivo.
Kiwi berry prevented constipation by down-regulating the VIPcAMP-PKA-AQP3 pathway. At the 3, 6, and 12 h different times,the kiwi berry group’s relative mRNA levels ofVIP,cAMP,PKA,andAQP3were the lowest (Figs. 1–4). VIP, cAMP, PKA, and AQP3 protein expression in the Kiwi group was significantly lower than in the Con group at the 3, 6, and 12 h, , respectively. It was, however,noticeably higher in the Lop group. The enteric nerve’s central target cell is the ICC[32]. Taking part in gastrointestinal pacing and the spread of myoelectric activity facilitates the transfer of nerve signals and controls the motility of the gastrointestinal tract[33].
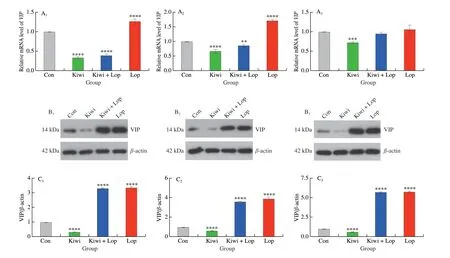
Fig. 1 Effects of kiwi berry on the VIP in interstitial cell experiments. (A1-A3) Relative expression level of VIP mRNA; (B1–B3) Representative immunohistochemical images of VIP (Original magnification ×200); (C1–C3) The protein levels of VIP performed by Western blotting at 3, 6, 12 h, respectively. Data were represented as mean ± SD (n = 3). ****P < 0.000 1, ***P < 0.001, **P < 0.01, vs. Con group.

Fig. 2 Effects of kiwi berry on the cAMP in interstitial cell experiments. (A1–A3) Relative expression level of cAMP mRNA; (B1–B3) Representative immunohistochemical images of cAMP (Original magnification ×200); (C1–C3)The protein levels of cAMP performed by Western blotting at 3, 6, 12 h,respectively. Data were represented as mean ± SD (n = 3). ****P < 0.000 1, ***P < 0.001, vs. Con group.
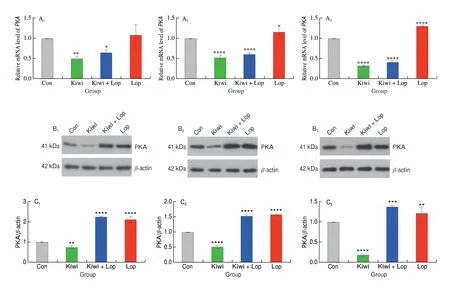
Fig. 3 Effects of kiwi berry on the PKA in interstitial cell experiments. (A1–A3) Relative expression level of PKA mRNA; (B1–B3) Representative immunohistochemical images of PKA (Original magnification ×200); (C1–C3) The protein levels of PKA performed by Western blotting at 3, 6, 12 h, respectively.Data were represented as mean ± SD (n = 3). ****P < 0.000 1, ***P < 0.001, **P < 0.01, *P < 0.05, vs. Con group.
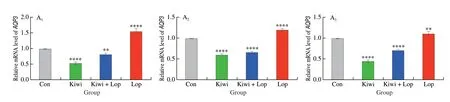
Fig. 4 Effects of kiwi berry on the AQP3 in interstitial cell experiments. (A1–A3) Relative expression level of AQP3 mRNA; (B1–B3) Representative immunohistochemical images of AQP3 (Original magnification ×200); (C1–C3) The protein levels of AQP3 performed by Western blotting at 3, 6, 12 h,respectively. Data were represented as mean ± SD (n = 3). ****P < 0.000 1, **P < 0.01, *P < 0.05, vs. Con group.
3.2 The efficacy of kiwi berries to prevent constipation symptoms
The mice in the Kiwi group were substantially heavier than the mice in the Con group. In contrast, the constipated mice weighed a lot less (Fig. 5A). The Kiwi group experienced the quickest onset of first black stools compared to the Con group, with a 47.3% decrease(Fig. 5B). Within 3 h, the Kiwi group’s fecal number increased by 30% (Con), 30% (Kiwi + Lop), and 150% (Lop), respectively(Fig. 5C). Additionally, the consumption of kiwi berries increased fecal water content which was 18.8%, 9.3%, and 110.3% greater than that of the Con, Kiwi + Lop, and Lop groups, respectively (Fig. 5D).The Kiwi group had the most significant GI rate, which was higher than that of the Con, Kiwi + Lop, and Lop groups by 25.0%, 10.5%,and 46.2% respectively (Fig. 5E).
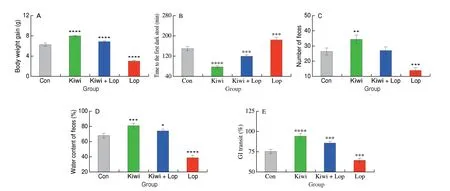
Fig. 5 Effect of kiwi berry on body weight and defecation parameters. (A) Body weight gain; (B) Time to the first dark stool; (C) Number of feces; (D) Water content of feces; (E) Gastrointestinal transit rate. Data were represented as mean ± SD (n = 3). ****P < 0.000 1, ***P < 0.001, **P < 0.01, *P < 0.05, vs. Con group.
Peristalsis, whose degree is determined by the rate of intestinal propulsion, is primarily responsible for facilitating the passage of intestinal contents. The stool will pass more efficiently with a quicker bowel rate; otherwise, constipation is more likely[34]. According to a study, kiwi berries reduce constipation by speeding up digestive propulsion. The study shows thatBifidobacteriumsupplementation dramatically reduces the frequency and consistency of defecations and relieves constipation by enhancing intestinal transit time and stool quality[35]. The time to the first melena excretion, which is the total transit times through the small and large intestines, reflects the length of time stools pass through the gut. The stool retaining too much water and remaining in the colon for an extended period is one of the leading causes of constipation. As a result, the better adjustment impact on constipation is associated with a shorter defecation duration of the first black stool[36]. In line with our findings,Bifidobacteriumreduces the time until the first stool,which relieves constipation[35].
3.3 Kiwi berry modulates neurotransmitter levels in serum and colon tissue
The neurotransmitters’ components, intimately linked to intestinal function, were examined in mouse serum and colon samples to further evaluate the kiwi berry’s ability to prevent constipation. As seen in Fig. 6, the Kiwi group had the highest amounts of neurotransmitters in both serum and colon samples, whereas the Lop group had the lowest neurotransmitter levels. Compared to the constipated mice, SP levels in serum and colon tissue of the Kiwi group increased by 76.5% and 36.9%, respectively. Compared to serum and colon samples from the Lop group, MTL and 5-HT levels in the Kiwi group rose by 90.6%and 52.0%, 61.8% and 55.9%, respectively.
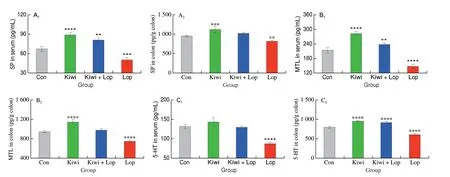
Fig. 6 Kiwi berry enhanced the excitability neurotransmitter levels in serum and colon tissue. The levels of (A1, A2) SP, (B1, B2) MTL and (C1, C2) 5-HT in serum and colon tissue. Data were represented as mean ± SD (n = 3). ****P < 0.000 1, ***P < 0.001, **P < 0.01, vs. Con group.
Multiple neurotransmitters are involved in gastrointestinal motility’s complex process and reaction[37]. Confirmed the presence of excitatory neurotransmitter receptors in the colon, which receive neurotransmitters and facilitate intestinal motility[38-40]. Increased excitatory neurotransmitter levels will encourage bowel movement and alleviate constipation. In loperamide-induced constipated rats, taurine-xylose supplementation dramatically raised SP and MTL serum levels[41-42]. In loperamide-induced rats,Anemarrhena asphodeloidesBge. can effectively increase the MTL and SP levels,alleviating constipation[38]. When administered different konjac mannan oligosaccharides, diphenoxylate-induced constipated mice had higher MTL and SP levels in their serum[43]. Previous research solely examined the variations in serum or colon sampled excitatory neurotransmitters. This study thoroughly examined mouse serum and colonic samples for neurotransmitter alterations.
3.4 VIP-cAMP-PKA-AQP3 signaling pathway expression of animal experiment
Real-time quantitative PCR and Western blot approaches are crucial for identifying molecular pathways. The expression of several essential regulators, including VIP, cAMP, PKA, and AQP3, connected to colonic water uptake and the gastrointestinal nervous system, were examined using RT-qPCR and Western blot to determine whether kiwi berries had a protective effect against loperamide-induced intestinal tissue damage. Notably, the Kiwi group had relatively lower values of the VIP-cAMP-PKA-AQP3 signaling pathway than the constipated mice (Fig. 7).
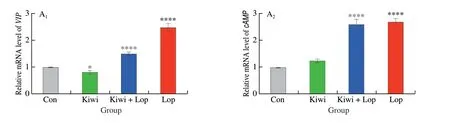
Fig. 7 Effects of kiwi berry on the VIP-cAMP-PKA-AQP3 signaling pathway in animal experiments. (A1–A4) Relative mRNA levels of VIP, cAMP, PKA and AQP3; (B1–B4) Representative immunohistochemical images of VIP, cAMP, PKA and AQP3 (Original magnification ×200); (C1–C4) The protein levels of VIP,cAMP, PKA and AQP3 performed by Western blotting at 3, 6, 12 h, respectively. Data were represented as mean ± SD (n = 3).****P < 0.000 1, ***P < 0.001, *P < 0.05, vs. Con group.
The three primary pathogenic causes of constipation are gut microbiota imbalance, intestinal motility dysfunction, and cognitive stress[44]. The brain-gut-microbiome axis (BGMA) system has emerged as an essential study topic in the pathophysiology of constipation in recent years[45-46]. The VIP-cAMP-PKA-AQP3 signaling route is one of the most significant signaling networks[11].The AQP3 has a unique water transport function that is directly linked to the secretion, absorption, and equilibrium of water in cells[12]. By controlling the amount of AQP3 in colonic epithelial cells, VIP can influence water metabolism in the colon, affecting the colonic VIPcAMP-PKA-AQP3 signaling pathway[12,32,47]. It is consistent with the findings of this study that high specific volume polysaccharid lowered the levels of serum VIP and downregulated the expression of c AMP,PKA, and AQP3 protein in the gut[48].
3.5 Characterization of intestinal tissue parameters
IHC was used to investigate the changes in VIP, cAMP, PKA, and AQP3 in the colon. Compared to mice with constipation, VIP, cAMP,PKA, and AQP3 were all significantly lower in the Kiwi group by 73.9%, 27.0%, 60.0%, and 60.0%, respectively (Fig. 8). According to the results of immunohistochemistry, kiwi berry inhibited the VIP-cAMP-PKA-AQP3 signaling pathway, which in turn prevented constipation from occurring.
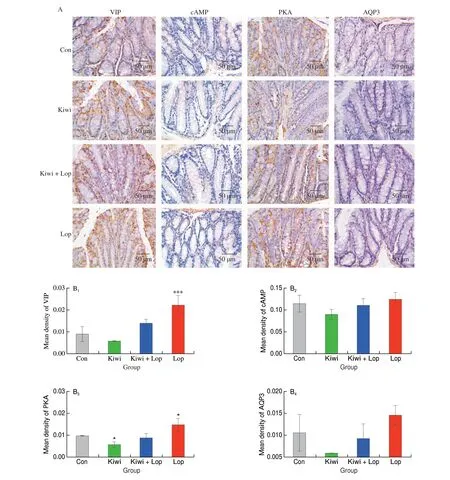
Fig. 8 Effects of kiwi berry on the colon structure in mice. (A) Immunohistochemistry analysis of VIP, cAMP, PKA and AQP3 (400 ×); (B1–B4) Mean density of VIP , cAMP, PKA and AQP3, respectively. Data were represented as mean ± SD (n = 3). ***P < 0.001, *P < 0.05, vs. Con group.
The analysis findings of the expression of essential components in colon tissue are given more understandably thanks to immunohistochemical detection. Identifying inflammatory variables was the main focus of the IHC analysis of earlier studies on constipation. The IHC staining ofBifidobacterium pseudocatenulatumameliorates dextran sulfate sodium-induced colitis revealed that there was a significant decrease in the expression of inflammatory markers like p-p65, TLR4,p65, p-IκB and IκB[49]. In mice with constipation treated with or without chitosan oligosaccharides, histochemical staining was used to identify the expression of Claudin-1, ENaC-γ and CD45. The findings showed that T lymphocytes in the intestinal mucosa and submucosa were significantly increased in mice with constipation. Following the administration of chitosan oligosaccharides, the immunological activation is caused by loperamide[19]. Additionally, IHC research has been done on neuronal aspects of intestinal contraction and gastrointestinal transit. By increasing the expression of the proteins c-kit and stem cell factors in intestinal tissue, konjac oligosaccharides can improve gastrointestinal motility and cure constipation[20].
3.6 Kiwi berry alters the composition of gut microbiota
Feces from 4 experimental groups of mice were subjected to 16S rDNA sequencing to investigate how kiwi berries reduce constipation in mice by altering gut bacterial ecosystems. Shannon, Simpson, and Chao1 indices are instances ofα-diversity. The Chao1 index displays the diversity of gut microbiota. The differences in gut microbiota between samples are reflected by the Simpson and Shannon indices.The kiwi fruit group’s Chao1, Simpson, and Shannon indices were all higher than those of the Lop group, kiwi berries may be able to boost the species variety and abundance of the fecal microbiota(Fig. 9A). Firmicutesand Bacteroidetes were the two most prevalent taxa just at the phylum level, trailed by Proteobacteria and Actinobacteria (Fig. 9B). Treatment with kiwi berry extract decreased Firmicutes abundance but increasedBacteroidetes abundance. Subsequently, interaction principal component analysis(PCA) was used to assess variations in the complexity of fecal microbial communities and structural alterations in various samples.The PCA-based findings demonstrated a substantial difference between the gut microbiota of the Kiwi group and the Lop group(Fig. 9C). Through heatmap analysis, the regulation effect of kiwi berry on fecal flora was discussed at the genus level. As a result, Fig. 9D displays the abundance of 20 significantly different genera.Prevotella,Helicobacter,Ruminococcus,andCoprococcuswere more prevalent in the Kiwi group than in the Lop group.
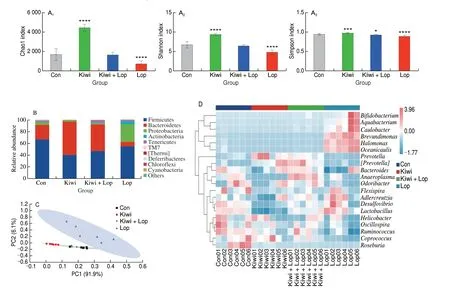
Fig. 9 Effects of kiwi berry on the fecal microbiota. (A1–A3) Chao1, Shannon and Simpson indexes; (B) Relative abundance of gut microbiota at the phylum level; (C) PCA; (D) Heatmap of the distribution of dominant groups at the genus level. Data were represented as mean ± SD (n = 3). ****P < 0.000 1, ***P < 0.001,*P < 0.05, vs. Con group.
Through the neuronal signaling pathway, gut bacteria can affect the central nervous system functions[50]. The zymolytic effects of the gut microbiota support the diversity of various neurotransmitters and control the host’s release of signaling molecules[51]. By releasing neurotransmitters, the hypothalamus stimulates primary sensory neurons within the gastrointestinal wall and results in aberrant gut flora[52]. Neurotransmitters promote gut bacteria by causing neurons to communicate once again[43]. Research into constipation mechanisms must include a thorough examination of the gut microbiota.
Chao1 and Shannon indexes of the polysaccharide extract group were greater than those of the Lop group, according to the outcomes of microbial research on improving functional constipation byChrysanthemum morifoliumpolysaccharide[53]. In mice given antibiotic treatment, ginsenoside Rk3 reduces the dysregulation of theα-diversity of the gut microbiota, which is also in line with our findings[54]. In agreement with our study, oat phenolic compounds significantly increased the abundance of Bacteroidetes and reduced the diversity of Firmicutes, improving the metabolic syndrome by modulating metabolites and gut microbiota composition[55-56]. By managing intestinal flora, foods likeC. morifoliumare becoming more well-acknowledged as efficient and secure laxatives[53].
3.7 Correlation between constipation-related indicators and relative abundances of gut bacteria
Spearman correlation coefficients were computed to investigate the relationship between indicators linked to constipation. As shown in Fig. 10, body weight and defecation parameters (fecal number,fecal water content, and GI rate) were positively correlated with Bacteroidetes. Excitatory neurotransmitter (SP, MTL, and 5-HT)levels in colon and serum samples were also positively correlated with Bacteroidetes. Therefore, Bacteroidetes promotes weight gain in mice, enhances fecal status, and increases excitatory neurotransmitters closely associated with the gut. Actinobacteriaand the expression of the VIP-cAMP-PKA-AQP3 signaling pathway were positively associated.
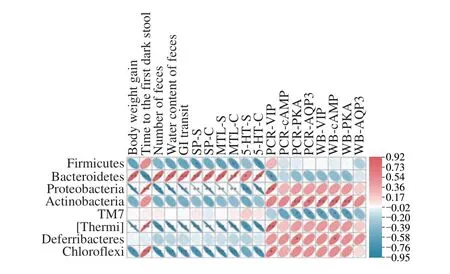
Fig. 10 Correlation between constipation-related indicators in animal experiment. SP-S, SP level in the serum; SP-C, SP level in the colon. The red and blue colors stand for the positive and negative correlation between two indicators, *P < 0.05, **P < 0.01.
To better understand the relationship between the variety and abundance of gut bacteria and the incidence of constipation, Spearman correlation analysis was also used in earlier studies on constipation.The correlation analysis of lactulose regulating gut microbiota and intestinal metabolites in treating constipated mice revealed that the majority of intestinal bacteria (gut integrity and motility,inflammatory cells factors, intestinal metabolites) that changed at the genus level were associated with constipation are closely related to the physicochemical characteristics[43]. Intestinal bacteria related to intestinal metabolite synthesis were impacted in constipated mice,according to a Spearman correlation analysis between 27 bacterial genera altered by chitosan oligosaccharides and biomarkers associated with constipation. These findings imply that the remodeling of gut microbiota structure in constipated mice may be connected to the regulating action of chitosan oligosaccharides on the production of intestinal metabolites[19].Studies have shown that the neurotransmitter levels in mouse colon tissue after honey treatment were associated with microorganisms.Loperamide-induced constipation in mice can be alleviated by regulating neurotransmitters and gut microbiota through honey supplementation,which is consistent with our findings[57].
Earlier research on constipation dealt with its regulation after it had already occurred when colon damage could still exist. Therefore,taking proactive actions to stop intestine damage before it is affected is crucial for intestinal health.Dendrobium candidumWall. ex Lindl., resveratrol, and a high-fiber diet have all been suggested for preventing constipation, although the underlying mechanisms and effects are unclear[31,58-59]. Because kiwi berry has no adverse effects and is biologically active in people, research into its ability to prevent constipation is vital in light of the studies mentioned above. This study created a special “ICC cell-animal model-microbe” system to study the constipation prevention mechanism of kiwi berry, supporting the use of kiwi berry as an alternative treatment for constipation, and they could also help in clinical trials and commercialization of functional berries.
4. Conclusion
The study provides proof of the beneficial preventative effect on mice with constipation, especially enhancing mice’s fecal health and encouraging their intestinal peristalsis. Kiwi berries can regulate the VIP-cAMP-PKA-AQP3 signal pathway, change the composition of gut bacteria and improve intestinal health. These results suggest that kiwi berries could be used as a treatment candidate to treat constipation.
Conflict of interest
The authors declare there is no conflict of interest.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (U21A20273); China Agriculture Research System of MOF and MARA(CARS-29); the First Batch of Liaoning “Unveiling Leader” Scientific and Technological Projects(2021JH1/10400036).
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango