Mannogalactoglucan from mushrooms protects pancreatic islets via restoring UPR and promotes insulin secretion in T1DM mice
2024-01-24TingLiuSiChenYunheQuLujuanZhengXiaoxuanYangShuhanMenYuanningWangHanruiMaYifaZhouYuyingFan
Ting Liu, Si Chen, Yunhe Qu, Lujuan Zheng, Xiaoxuan Yang, Shuhan Men,Yuanning Wang, Hanrui Ma, Yifa Zhou, Yuying Fan
Engineering Research Center of Glycoconjugates, Ministry of Education, Jilin Provincial Key Laboratory of Chemistry and Biology of Changbai Mountain Natural Drugs,School of Life Sciences, Northeast Normal University, Changchun 130024, China
Keywords: Mannogalactoglucan Mushroom Pancreatic islets Insulin secretion Insulin synthesis Unfolded protein response (UPR)Type 1 diabetes mellitus (T1DM)
ABSTRACT Type 1 diabetes mellitus (T1DM) lacks insulin secretion due to autoimmune deficiency of pancreatic β-cells. Protecting pancreatic islets and enhancing insulin secretion has been therapeutic approaches.Mannogalactoglucan is the main type of polysaccharide from natural mushroom, which has potential medicinal prospects. Nevertheless, the antidiabetic property of mannogalactoglucan in T1DM has not been fully elucidated. In this study, we obtained the neutral fraction of alkali-soluble Armillaria mellea polysaccharide (AAMP-N) with the structure of mannogalactoglucan from the fruiting body of A. mellea and investigated the potential therapeutic value of AAMP-N in T1DM. We demonstrated that AAMP-N lowered blood glucose and improved diabetes symptoms in T1DM mice. AAMP-N activated unfolded protein response (UPR) signaling pathway to maintain ER protein folding homeostasis and promote insulin secretion in vivo. Besides that, AAMP-N promoted insulin synthesis via upregulating the expression of transcription factors, increased Ca2+ signals to stimulate intracellular insulin secretory vesicle transport via activating calcium/calmodulin-dependent kinase II (CamkII) and cAMP/PKA signals, and enhanced insulin secretory vesicle fusion with the plasma membrane via vesicle-associated membrane protein 2 (VAMP2). Collectively,these studies demonstrated that the therapeutic potential of AAMP-N on pancreatic islets function, indicating that mannogalactoglucan could be natural nutraceutical used for the treatment of T1DM.
1. Introduction
Type 1 diabetes mellitus (T1DM) is a serious chronic disease characterized by abnormal glucose metabolism due to a lack of endogenous insulin secretion from the islet β-cells (usually due to loss of β-cells)[1]. Despite intensive research, lifelong management is required due to increased incidence, no immediate prospect of a cure and development of diabetes vascular complications in T1DM[2]. A central component of therapies for T1DM should be the restoration of the damaged pancreatic β-cells[3]. Pancreatic β-cells secrete appropriate insulin to maintain blood glucose homeostasis.As circulating glucose levels rise, the insulin transcription factors induce rapid elevation of preproinsulin mRNA, and mRNA binds to the ribosome to initiate translation of preproinsulin at the endoplasmic reticulum (ER)[4]. Subsequently, the preproinsulin is formed into insulin and C-peptide by the prohormone convertases[5]. Once glucose enters the cell via glucose transporter 2 (GLUT2), and could be oxidatively metabolized to produce ATP, which causes closure of the ATP-sensitive K+channels, membrane depolarization, and initiation of voltage-gated Ca2+channels, and rapid inf lux of Ca2+into the cell[6]. The increases in intracellular Ca2+concentration promote insulin secretory granule traffic to the plasma membrane, and release insulin via solubleN-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes[7]. Restoration of insulin secretion in β-cells partially keeps glucose homeostasis and prevents the development of T1DM, suggesting that promoting the synthesis,transport and release of insulin is a potential pathway for protection of β-cell function and treatment of T1DM[8-11].
As traditional Chinese medicine and edible fungus, mushrooms can prevent or improve many diseases and provide benefits to human health. Mushrooms contain a large number of bioactive compounds and are used in the field of traditional Chinese medicine to treat T1DM[12]. Polysaccharides is the most studied and used medicinally active compound in mushrooms[13]. Mushroom polysaccharide has various physiological activities, such as hypoglycemic[14-15],hypolipidemic[16-17]and immune[18-19]. Our previous study showed that mannogalactoglucan was the main class of polysaccharides present in mushrooms, after purifying different mushroom polysaccharides and comparing their structural characteristics[20]. However, the influence of mannogalactoglucan in T1DM is not fully elucidated. Meanwhile, less is known about the mechanism of mannogalactoglucan on protecting the islet functions and promoting insulin secretion.
Herein, we isolated and purified the neutral fraction of alkalisolubleArmillaria melleapolysaccharide (AAMP-N) with the structure of mannogalactoglucan from the fruiting body ofA. mellea.Then we further investigated whether AAMP-N with the structure of mannogalactoglucan could ameliorate islet function in streptozotocin(STZ)-induced mice and explored the underlying mechanisms.
2. Materials and methods
2.1 Preparation and fractionation of A. mellea polysaccharide
The neutral polysaccharide (AAMP-N) was prepared fromA. melleafruiting body as previously described[20]. In brief, the fruiting bodies ofA. melleawere selected from Changbai Mountain in Jilin Province, China, and extracted with hot water after defatting with 95% ethanol. Then the resulting residues were extracted with 0.5 mol/L NaOH with trace amounts of NaBH4. After neutralization,concentration and precipitation with ethanol, alkali-extracted polysaccharide (AAMP) was obtained. AAMP was separated by a DEAE-cellulose (Cl−) column (7.5 cm × 30 cm, particle size > 30 μm), eluting with distilled water to give the neutral polysaccharide (AAMP-N).
2.2 Monosaccharide composition
Monosaccharide composition was determined from methods previously described[20]. 1 mL anhydrous methanol containing 2 mol/L HCl was prepared and reacted with 2 mg AAMP-N in a hydrolytic tube at 80 °C for 16 h. Then the sample was hydrolyzed with 1 mL 2 mol/L trifluoro acetic acid (TFA) in a sealed hydrolytic tube at 120 °C for 1 h. The derivatives were analyzed by HPLC after derivatization with 1-phenyl-3-methyl-5-pyrazo-lone (PMP).
2.3 Fourier transform infrared spectroscopy analysis (FT-IR)
AAMP-N was ground with KBr powder and then pressed into 1 mm pellets for FT-IR measurements using a Spectrum Two spectrometer (PerkinElmer, Waltham, MA, USA) in a range of 4 000−400 cm−1.
2.4 UV-visible spectrum analysis
The UV-visible spectrum of the AAMP-N was performed on a UV-2700 UV-visible spectrophotometer (Shimadzu, Kyoto, Japan) at the wavelength of 200−800 nm range.
2.5 Molecular weight analysis
The molecular weight distribution of AAMP-N was determined by using gel-permeation chromatography on a Shimadzu HPLC system(Shimadzu, Tokyo, Japan) coupled with a TSK-gel G-3000PWXL column (7.8 mm × 300 mm, 6 μm, TOSOH, Tokyo, Japan).
2.6 Experimental mice
Male C57BL/6J mice (7 weeks, (20.0 ± 0.8) g) were purchased from Charles River Laboratory Animal Technology Co. (Beijing,China). All experiments were approved by the Animal Care and Use Committee of Northeast Normal University (SYXK 2018-0015). The animals were housed in a room maintained at (23 ± 2) °C with relative air humidity of 45%−55% on a 12-h light/12-h dark cycle. Mice were provided a standard laboratory chow and water for 1 week prior to experimentation.
2.7 Induction of T1DM
T1DM was induced in mice by a single intraperitoneal injection of STZ (Beyotime, China) at a medium-to-high dose of 150 mg/kg BW as described previously[21]. Briefly, mice were fasted overnight and then injected intraperitoneally with a dose of STZ (150 mg/kg in 0.1 mol/L citric acid buffer, pH 4.5), while the control mice received an injection with an equal amount of citric acid-sodium citrate buffer solution. Random blood glucose was monitored within 7 days after STZ injection from tail vein blood using the One Touch Ultra Easy Glucometer (Johnson, USA). All mice exhibiting random blood glucose ≥ 16.7 mmol/L accompanied by excessive drinking, eating,urination and weight loss were considered to be T1DM[22].
2.8 Animal experimental design
These mice were then randomly divided into 4 groups(n= 8−10), including C57BL/6J group (normal control), STZ group (STZ control), STZ + acarbose group (STZ mice treated with acarbose at oral dose of 50 mg/(kg·day) as a positive control),STZ + AAMP-N group (STZ mice treated with AAMP-N at oral dose of 50 mg/(kg·day)). The oral effect of AAMP-N was investigated in T1DM model mice for 4 weeks. Fasting blood glucose (FBG)and body weight were measured each week in the experiment. The consumption of food and water as well as the production of urine were evaluated on the last day of the experiment in mice. At the end of study, the mice were anesthetized and euthanized. Their blood and organ samples were collected for the determination of biochemical parameters.
2.9 Measurement of biochemical indicators in mice
The insulin levels in serum were assayed with a Mouse Insulin ELISA Kit (Innovation Beyond Limits, Germany). The ketone body levels in serum and urine were assayed with a Mouse Ketone Body ELISA Kit (Shzkbio, China).
2.10 Immunohistochemistry
The tissues were taken out, dehydrated and embedded in optimal cutting temperature (OCT) Compound (SAKURA, 4583)and frozen in −80 °C. The tissues were sliced into 8 μm sections using freezing microtome (LEICA, Germany). Sections were fixed with 4% paraformaldehyde for 20 min, and washed with PBS for 5 min. Permeabilization for 20 min, followed washed with PBS.Sections were blocked and then incubated in the first antibodies at 4 °C overnight. After twice washes with phosphate buffered saline with 0.05% Tween-20 (PBST) for 5 min and washes with PBS for 5 min, dark incubated slides with a fluorescein-conjugated second antibody for 2 h.Slides were incubated with Hoechst 33342 (Sigma, USA) for 10 min after repeated washes. After washes, the slides were covered with fluorescence decay resistant medium (BOSTER, China). The first antibodies were anti-insulin (Cell Signaling Technology, USA) and anti-glucagon antibodies (BOSTER, China). The secondary antibodies were FITC-conjugated goat anti-rabbit immunoglobulin G (IgG) and Cy3-conjugated goat anti-rabbit IgG (ABclonal, China). The sections were observed by fluorescence microscopy (Olympus, Japan).
2.11 Proteomics
Proteins were quantified as previously described[23]. Briefly, 100 μg proteins were transferred to ultrafiltration tubes after islet lysis in RIPA buffer (Sigma, USA) and washed with 8 mol/L urea (Sigma,UAS) for two times. The proteins were reduced with 10 mmol/L dithiothreitol (DTT) (Sigma, USA) at 37 °C for 1 h and alkylated by 20 mmol/L iodoacetamide (Sigma, USA) at room temperature for 1 h,then washed with 50 mmol/L NH4HCO3(Merk, USA) for two times.Proteins were digested with trypsin (Promega, USA) and the resulting peptides were purified with methanol and acetonitrile (Thermo Fisher,USA) in C18solid phase extraction column. Peptides were redissolved in 0.1% formic acid buffer after concentration. Finally, peptides were separated using an EASY-nLC 1200 liquid chromatographic system prior to detection on a Q-Exactive mass spectrometer equipped with a nanoelectrospray ion source (Thermo Fisher Scientific, USA). Peptides were identified with the software Proteome Discoverer (Thermo Fisher Scientific, USA). Proteomics analysis was analyzed using ‘Wu Kong’platform (https://www.omicsolution.com/wkomics/main/).
2.12 Western blots
The proteins in tissues or cells were prepared in RIPA Lysis Buffer (Solarbio, China). Western blots were performed as described in the previous publication[24]using the following primary antibodies: anti-insulin (Cell Signaling Technology, USA),anti-PKA (Cell Signaling Technology, USA), anti-p-PKA (Cell Signaling Technology, USA), anti-calcium/calmodulin-dependent kinase II (CamkII) (Affinity, China), anti-p-CamkII (Affinity,China), anti-vesicle-associated membrane protein 2 (VAMP2)(OriGene, USA), anti-activating transcription factor 6 (ATF6) (ZEN BIO, China), anti-p-inositol requiring enzyme 1 alpha (IRE1α)(ZEN BIO, China), anti-IRE1α (ABclonal, China), anti-X-box binding protein 1 (XBP1) (ZEN BIO, China), anti-p-PKR-like ER kinase (PERK) (ZEN BIO, China), anti-PERK (ABclonal, China),anti-PARP (Cell Signaling Technology, USA), anti-p-JNK (Cell Signaling Technology, USA), anti-JNK (Cell Signaling Technology,USA), anti-Caspase-3 (Cell Signaling Technology, USA),β-tubulin(Cell Signaling Technology, USA), anti-GAPDH (Servicebio, China),or anti-actin (Abcam, UK) antibodies. Densitometry of the band density was determined using ImageJ software.
2.13 Cell culture
The murine insulin-secreting cell line Min6 was purchased from American Type Culture Collection. As previously described[25], the Min6 cells were cultured in Dulbecco’s modified Eagle medium(DMEM, Gibco, USA), supplemented with 15% fetal bovine serum (FBS, CellMax, China), 100 U/mL penicillin and 0.1mg/mL streptomycin (BOSTER, China), 2 mmol/L glutamax and 1 mmol/L sodium pyruvate (Gibco, USA), and 70 μmol/Lβ-mercaptoethanol(Genview, China).
2.14 Islet isolation
Islet isolation was performed as previously described[26]. In brief,the mice were euthanized by cervical dislocation and the abdominal cavity opened to isolate the islets. Islets were washed in phenol red-free HBSS buffer and digested at 37 °C for 10 min with 3 mL collagenase (Roche Diagnostics GmbH, Germany) dissolved in HBSS buffer before centrifugation. Islets cells were hand-picked and cultured in DMEM.
2.15 Measurement of insulin, C-peptide, cAMP by ELISA
Insulin levels were measured using a Mouse Insulin ELISA Kit (Innovation Beyond Limits, Germany). The Min6 cells were seeded at a density of 5 × 104cells/well for 24 h in a 48-well plate.We established three groups, including negative control group (Ctrl group), positive control group (KCl group), and experimental group(AAMP-N group). The cells were pre-incubated in Krebs-Ringer Buffer (KRB) at 37 °C for 2 h. The culture medium was changed to KRB with 5.5 mmol/L glucose (Ctrl group), KRB with 5.5 mmol/L glucose and 30 mmol/L KCl (KCl group), and KRB with 5.5 mmol/L glucose and 0.1 mg/mL AAMP-N (AAMP-N group) for 1 h.The supernatant was collected for the analysis of insulin levels measurements according to the manufacturer’s protocol. When the samples were processed as described above, C-peptide levels were measured using a Mouse C-peptide ELISA Kit (JingMei,China) and cAMP levels were measured using a Mouse cAMP ELISA Kit (Innovation Beyond Limits, Germany) according to the manufacturer’s protocol. The levels of three indexes were normalized by total protein content as determined by BCA assay.
2.16 RNA preparation and quantitative real-time PCR
Total RNA was extracted from Min6 cells using TRNzol Universal (TIANGEN, China) according to the manufacturer’s instructions. cDNA was generated from 1 μg RNA using 5× FastKing-RT SuperMix (TIANGEN, China). Quantitative PCR was performed using 2× Real Star Green Fast Mixture (Genestar, China) and a LightCycler 480 real-time PCR system (Roche Applied Science,USA). The primers used for qPCR are listed in Table 1.
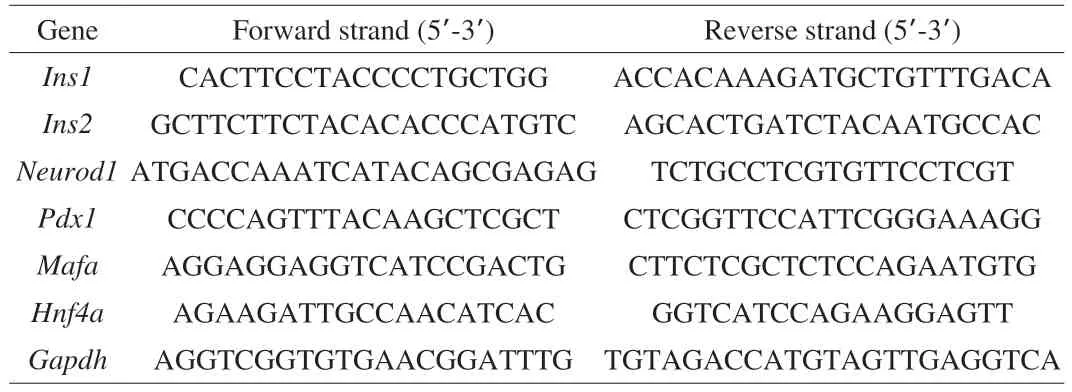
Table 1 The primers used in our study.
2.17 Measurement of intracellular Ca2+ flow
Intracellular Ca2+flow was measured as previously described[11].The Min6 cells were seeded at a density of 4 × 105cells/well for 24 h in a 6-well plate. The cells were pre-incubated in KRB at 37 °C for 1 h.The culture medium was removed and replaced with KRB containing 3 μmol/L Fluo-3AM (Abcam, UK). After incubation for 1 h, cells were washed for 3 times. Cells were collected with trypsin (Gibco,USA), resuspended by KRB after centrifugation, and diluted at a density of 5 × 104cells per 0.45 mL in a 48-well plate. Then, the plate was transferred to NovoCyte Flow Cytometer (ACEA Biosciences,USA). Fluorescence reading was taken for 70 s to establish the baseline, and then 50 μL of KCl (300 mmol/L) or 25 μL of AAMP-N(2 mg/mL) were added to corresponding wells. After that, the fluorescence readings were taken for 230 s. Background fluorescence was automatically subtracted from all Fluo-3AM fluorescence measurements.
2.18 Measurement of intracellular Ca2+ concentration
Intracellular Ca2+concentration was measured as previously described[11]. After cells were processed and washed for three times as described in section 2.13, the culture medium was changed to KRB with 5.5 mmol/L glucose (Ctrl group), KRB with 5.5 mmol/L glucose and 30 mmol/L KCl (KCl group), and KRB with 5.5 mmol/L glucose and 0.1 mg/mL AAMP-N (AAMP-N group) for 1 h. Cells were collected with trypsin (Gibco, USA), resuspended by KRB after centrifugation, and diluted at a density of 5 × 104cells per 0.5 mL in a 48-well plate. Then, the plate was transferred to NovoCyte Flow Cytometer (ACEA Biosciences, USA) to measure the fluorescence intensity.
2.19 siRNA for protein expression silencing in cells
Small interfering RNAs (siRNAs) were designed and synthesized by Sangon Biotech (China). The target sequence of VAMP2 contained forward strand (5’-GCACCUCCUCCAAACCUUATT-3’)and reverse strand (5’-AARGCACCTCCTCCAAACCTTA-3’).A non-targeting siRNA contained forward strand(5’-UUCUCCGAACGUGUCACGUTT-3’) and reverse strand(5’-ACGUGACACGUUCGGAGAATT-3’). For transient transfection,the Min6 cells were seeded at a density of 5 × 104cells/well for 24 h in a 48-well plate and transfected with Lipofectamine 3000 reagent (Invitrogen, USA) according to the manufacturer’s instructions.
2.20 Statistical analysis
The results were expressed as the means ± SEM. Statistical analysis of the data was performed using one-way ANOVA (GraphPad Prism 6.0, GraphPad Software Inc.). Differences were considered significant atP< 0.05.
3. Results
3.1 Extraction and analysis of AAMP-N
According to the extraction and fractionation scheme in Fig. 1A,we obtained neutral polysaccharide AAMP-N from the fruiting body ofA. mellea. The yield of AAMP-N was 35% relative to total polysaccharides. Monosaccharide composition analysis showed that AAMP-N was composed of glucose (Glc, 49.8%), and galactose(Gal, 25.2%), mannose (Man, 18.2%), suggesting that it might be mannogalactoglucan. The FT-IR spectrum of AAMP-N exhibited typical carbohydrate patterns (Fig. 1B). The intense and broad peak around 3 342.8 cm−1indicated the stretching vibration of O-H.The band near 2 913.7 cm−1was attributed to the C-H stretching vibrations[27]. The intense absorption band near 1 673.6 cm−1was associated with C=O. The peak at 1 398.6 cm−1was assigned to the C-H variable angle vibration. The band near 1 067.6 cm−1indicated the presence of pyranose ring[28]. The UV-visible spectrum of AAMP-N had no significant absorption peaks between 260 and 280 nm, indicating that there were no protein and nucleic acid of the purified polysaccharide (Fig. 1C). As shown in Fig. 1D, AAMP-N was composed of two fractions with molecular weight of 178 and 22.2 kDa, respectively, and the latter was the major fraction.

Fig. 1 Extraction and purification of AAMP-N. (A) Extraction and fractionation scheme of AAMP-N. (B) The FT-IR spectra of AAMP-N. (C) The UV-vis spectrum of AAMP-N. (D) The molecular weight distribution of AAMP-N.
3.2 AAMP-N ameliorated the diabetic symptoms of T1DM mice
To investigate the role of AAMP-N in T1DM, the experimental T1DM animal model in C57BL/6J mice was established by intraperitoneal injection with STZ (150 mg/kg) as shown in Fig. 2A.We investigated the oral effects of AAMP-N at a dose of 50 mg/(kg·day) for 4 weeks by recording FBG and body weight weekly, while using acarbose as a positive control[29]. The fasting glucose levels were significantly decreased by the treatment of AAMP-N compared to the STZ group (Fig. 2B), indicating that AAMP-N had an anti-diabetes potential in T1DM mice.

Fig. 2 AAMP-N ameliorated the development and severity of T1DM mice. (A) The detailed experimental scheme (n = 8−10). (B) Fasting blood glucose.(C) Body weight. (D) Food intake. (E) Water intake. (F) Urine volume. (G) Ketone body levels in serum and urine. All data was expressed as mean ± SEM. #P < 0.05,##P < 0.01, ###P < 0.001 vs. C57BL/6J mice; *P < 0.05, **P < 0.01, ***P < 0.001 vs. STZ mice. Same with Fig. 3.
Diabetic symptoms of T1DM included weight loss, polyphagia,polydipsia and polyuria. The STZ group exhibited typical symptoms of T1DM compared to C57BL/6J group, whereas the treatment of AAMP-N significantly increased the body weight of the T1DM mice (Fig. 2C) and decreased food intake (Fig. 2D), water intake(Fig. 2E) and urinary volume (Fig. 2F). Ketone body levels were another hallmark of T1DM[30]. The treatment of AAMP-N significantly decreased ketone body levels in serum and urine(Fig. 2G) compared to STZ group. Those results suggested that AAMP-N showed a significant therapeutic effect on diabetic symptoms of T1DM mice.
3.3 AAMP-N protected islet function via restoring unfolded protein response (UPR) signaling in T1DM mice
Autoimmune destruction of islet β-cell led to inadequate insulin production in T1DM. To furtherly investigate the therapeutic effects of AAMP-N, insulin- and glucagon-positive areas in the islets were measured by immunofluorescent staining with anti-glucagon and antiinsulin antibodies (Fig. 3A). C57BL/6J group showed apparently healthy islets, which β-cells were located centrally and α-cells were distributed along the periphery of the islets. However, islet β-cell area in STZ group was significantly decreased, and the α-cell area was increased. After AAMP-N treatment for 4 weeks, the islets returned to normal size, β-cell area was markedly increased and α-cell area was reduced (Fig. 3B). The insulin levels were significantly increased in the AAMP-N group compared to the STZ group (Fig. 3C). In addition, we conducted whole-islet proteomics to assess the variability of diabetes-related metabolic phenotypes after AAMP-N treatment.Venn diagram analysis was conducted to detect exclusive and shared proteins across all samples. We found that there were 495 overlapping proteins and more than 70 exclusive proteins between diabetic mice treated with AAMP-N and acarbose (Fig. 3D). AAMP-N showed the hypoglycemic effect as well as acarbose. Then we explored the Gene Ontology (GO), and the biological processes analysis showed that these proteins were significantly enriched for ER stress and protein folding. The molecular function analysis showed that these proteins were significantly enriched for unfolded protein binding(Fig. 3E). As shown in Fig. 3F, the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis and heatmap indicated that the majority of protein alterations occurred in the metabolic pathway, pancreatic secretion, and protein processing in the ER. Islet β-cells contain a highly developed ER responsible for the production and release of insulin in response to elevated blood glucose. The dysfunction of ER led to β-cells death and T1DM, which could be restored via the UPR in T1DM[31]. The proteomic results of islets indicated an important role of AAMP-N in ameliorating UPR associated with ER stress.As shown in Fig. 3G, AAMP-N re-established ER protein folding homeostasis and promoted β-cell survival via activating the three UPR signaling pathway, including ATF6, PERK, and IREα/XBP1 in the islets of T1DM mice. In addition, the protein expression levels of p-JNK, cleaved PARP and cleaved caspase-3 were significantly increased in STZ-treated mice islets, but these effects were reversed by AAMP-N treatment (Fig. 3H), suggesting that ER stress-induced cell death was markedly reversed by AAMP-N treatment. These results suggested that AAMP-N protected structural integrity of islets and restored insulin secretion probably via activating the UPR signaling pathway.
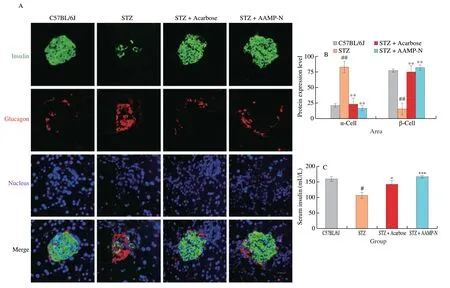
Fig. 3 AAMP-N protected islet function via UPR signaling pathway in T1DM mice. (A) Representative double-immunofluorescence images showing insulin(green), glucagon (red) staining, and the nucleus were counterstained with DAPI (blue). Scale bar, 100 μm. (B) β-Cell positive area was calculated based on insulin-positive areas compared to total islet area. α-Cell area was calculated based on glucagon-positive areas compared to total islet area. Quantification of total islet area was calculated based on insulin- and glucagon-positive areas. (C) Insulin levels in serum. (D) Venn diagram analysis of islet proteomics. (E) GO analysis of islet proteomics. (F) KEGG analysis of islet proteomics. (G) Representative Western blotting images and quantification of UPR signaling pathway in islets.(H) Representative Western blotting images and quantification of apoptosis markers in islets. Full-length PARP (PARP-FL), cleaved PARP (PARP-CL),full-length capspase-3 (capspase-3-FL), cleaved capspase-3 (capspase-3-CL).
3.4 AAMP-N promoted insulin synthesis
We further determined how the AAMP-N contributed to the insulin secretionin vitrodue to the benefits observedin vivo.Compared to control cells, AAMP-N and positive control KCl exhibited insulin release at basal glucose in primary β-cell and Min6 cells (Fig. 4A). Proinsulin is cleaved into insulin and C-peptide.AAMP-N increased C-peptide content (Fig. 4B), and also dominantly enhanced the protein expression of insulin (Fig. 4C). Next, we analysed how AAMP-N enhances proinsulin production.Ins1andIns2genes reside on different chromosomes in mice, andIns1gene is a defective gene that comes from a reverse-transcribed partially processed mRNA ofIns2[32].Ins1gene knockout prevented from diabetes, whereasIns2gene knockout accelerated diabetes[33].AAMP-N upregulated mRNA expression ofIns2notIns1(Fig. 4D). Various insulin transcription factors bind to DNA elements of the insulin promoter to activate insulin transcription, such as the transcription factor MafA binds to the C1 element, and Pdx1 binds to the A element, NeueoD1 binds to the E element,HNF4αbinds to the HBE element[34]. AAMP-N significantly upregulated the expression of insulin transcription factors ofMafa,Neurod1,Pdx1andHnf4α(Fig. 4E). These results suggested that AAMP-N promoted insulin synthesis via increasing transcription factors.
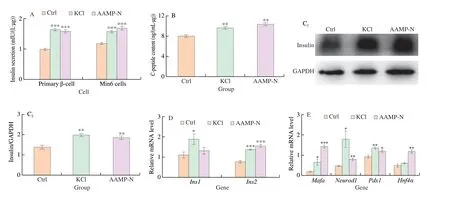
Fig. 4 AAMP-N promoted insulin synthesis via upregulating the expression of insulin transcription factor in vitro. (A) Insulin levels in primary β-cell and Min6 cells. (B) C-peptide content in Min6 cells. (C) Representative Western blotting images and quantification of insulin protein in the Min6 cells. (D) Fold changes in the mRNA levels of insulin in the Min6 cells, determined by qPCR. (E) Fold changes in the mRNA levels of typical insulin transcription factors in the Min6 cells,determined by qPCR. All data was expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, vs. control.
3.5 AAMP-N promoted insulin secretion
Insulin was released through granule exocytosis after stimulation.Exocytosis, a key process in insulin secretion, included intracellular insulin secretory vesicle transport and insulin secretory vesicle fusion with the plasma membrane[35]. Increases in cytosolic Ca2+directly stimulate vesicle transport within insulin-producing β-cells[36].Compared with the fluorescence intensity before stimulation, the treatment with AAMP-N in Min6 cells increased clear Ca2+-dependent fluorescence transients (Fig. 5A). Intracellular Ca2+concentration increased significantly after Ca2+influx (Fig. 5B). Ca2+signaling is closely related to CamkII, a key feed-forward stimulator of β-cell Ca2+signals[37]. As expected, AAMP-N phosphorylated CamkII increasing intracellular Ca2+influx (Fig. 5C). Except CamkII, cAMP also promoted Ca2+influx through voltage dependent Ca2+channels and promoted Ca2+mobilization from intracellular Ca2+stores[38].Compared to control cells, the treatment with AAMP-N increased the levels of cAMP (Fig. 5D). Elevation of cAMP levels led to activation of PKA, which phosphorylated and upregulated insulin granule transport after the treatment with AAMP-N (Fig. 5E). Fusion of the insulin secretory vesicle and the plasma membrane involves VAMP2. After the treatment of AAMP-N, we analyzed whether VAMP2 played a role in membrane fusion using siRNA transfection.The levels of insulin secretion increased by AAMP-N was also significantly reduced after transfection with the siRNA targeting VAMP2 (Fig. 5F), suggesting that AAMP-N promoted vesicle fusion through VAMP2 to release insulin. Collectively, these results supported that AAMP-N promoted insulin secretion via increasing insulin secretory vesicle transport and its fusion.
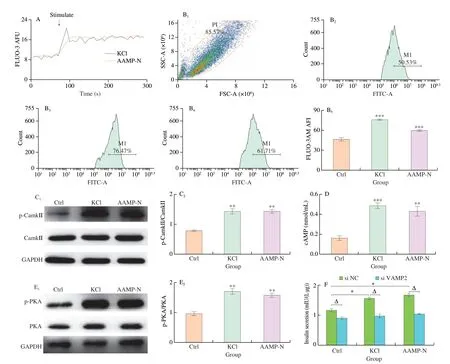
Fig. 5 AAMP-N promoted granule exocytosis to secrete insulin in vitro. (A) Ca2+ influx was analysed by using flow cytometry in Min6 cells. (B) Intracellular Ca2+ concentration was analysed by using flow cytometry. B1, Min6 cells; B2, Ctrl; B3, KCl; B4, AAMP-N; B5, intracellular Ca2+ concentration. P1 means the population of Min6 cells. M1 represents the selected range of high fluorescence intensity. (C) Representative Western blotting images and band density of phosphorylation level of CamkII in the Min6 cells. (D) cAMP levels in Min6 cells. (E) Representative Western blotting images and quantification of phosphorylation level of PKA in the Min6 cells. (F) Insulin levels in Min6 cells by transfecting the siRNA targeting VAMP2. NC was a negative control, meaning the group treated with non-targeted siRNA. All data was expressed as mean ± SEM. *P < 0.05, **P < 0.01, vs. control. ΔP < 0.05, vs. the group with control siRNA.
4. Discussion
T1DM is characterized by a lack of insulin due to the autoimmune destruction of β-cells, which causes uncontrolled blood glucose homeostasis[39]. Therapeutic approaches to stop this destructive process, protect pancreatic islets and enhance insulin secretion are being pursued. Natural products, particularly of mushroom origin,play an imperative role in the antidiabetic dietary supplements[10,20,40].Here, T1DM mouse model was induced by injected intraperitoneally with STZ, a glucosamine-nitrosourea compound, which could induce strong β-cells destruction mostly by causing damage to cellular DNA and proteins through covalent modifications[41]. T1DM is characterized by sustained hyperglycaemia, hypoinsulinemia,accompanied by severe diabetic symptoms, such as weight loss,polyphagia, polydipsia and polyuria. Polysaccharides improved T1DM mainly by stimulating insulin secretion[42-43]and β-cell proliferation[10,44], alleviating immune imbalances[45-47]and oxidative stress[44,48-49]. AAMP-N significantly alleviated symptoms of T1DM,which could be the result of maintaining glucose homeostasis. High levels of ketones were the result of lacking insulin secretion from the β-cells[50]. AAMP-N significantly decreased ketone body levels in serum and urine, indicating it possibly enhanced insulin secretion in T1DM mice.
Increased insulin secretion is the result of improved islet function and mass[8,10]. AAMP-N significantly restored damaged islets and increased the ratio of islet β-cell area to total islet area, consistent with our previous work of AAMP-N carried out indb/dbmice[20].ER is the first compartment of the secretory pathway, the core of protein synthesis equipped to achieve proper folding and processing of insulin. Toxicity of STZ causes to ER stress, which increases the levels of unfolded/misfolded insulin, ultimately leading to β-cell death in islets[51]. In response to ER stress of islets, cells triggered an adaptive UPR signaling pathway to sustain insulin correct fold and secretion[51-52]. Hence, we speculated that AAMP-N could protect islets and increase insulin secretion by responding to UPR.UPR is a dynamic cellular response, which provides signals that may cause an apoptotic outcome when key adaptive mechanisms fail[53]. A previous study indicated that cyanidin-3-glucoside (C3G)significantly suppressed the PERK/eIF2α/ATF4/CHOP pathway to maintain normal ER morphology in retinal pigment epithelial cells[54].However, our results found AAMP-N significantly reversed the increased apoptosis induced by STZ, which indicated that AAMP-N likely increased ER capacity and led β-cells toward a prosurvival fate rather than a proapoptotic one in islets via preserving the expression of key UPR components. Consistent with our results, it has been found that administration of tauroursodeoxycholic acid (TUDCA)reduced β-cell apoptosis and preserved insulin secretion via restoring expression of UPR mediators in T1DM mice islets[53]. In addition,highly secretory β-cells maintain function properly via an active and well-balanced UPR[53]. Thus, the expression level and activity of UPR related proteins in normal islets is higher than that in destroyed islets,including as ATF6[53], IRE1α[55-56], XBP1[53,57], and PERK[58-60]. We speculated that the different functions induced by UPR signal could be related to different pathological conditions and specific tissues.
In T1DM, exhaustion of β-cells in compensating increased insulin requirement results in subsequent β-cell dysfunctions, which could be restored by stimulating insulin neogenesis and secretion[61].AAMP-N enhanced insulin biosynthesis by upregulating critical insulin transcription factors, which may be the root cause of increased insulin secretion. Ca2+depletion leads to T1DM-triggered β-cell death, and extracellular Ca2+influx triggers the transport of insulin[6].AAMP-N contributed to a rise in cytosolic Ca2+concentration through activation of CamkII and cAMP/PKA, which could lead to increased intracellular insulin secretion vesicle transport. SNARE is the molecular basis of insulin exocytotic activity. VAMP, a SNARE anchored to synaptic vesicles and secretory granules, plays a very important role in insulin exocytosis. There are four identified VAMPs, including VAMP2, VAMP3, VAMP7 and VAMP8 in granule secretory vesicles[62]. VAMP2, a structural protein of the synaptic vesicle membrane, is closely related to the secretion of insulin in β-cells[63]. VAMP3, a tetanus neurotoxin (TeNT)-sensitive SNARE, regulates transferrin transport[64], but has no effect on insulin secretion[65]; VAMP7 is involved in vesicle transport between the inner body and the lysosome; VAMP8 is involved in vesicle transport between early and late endosomes found in the trans Golgi network and other organelles[62]. Therefore, we evaluated the effect of AAMP-N on VAMP2-mediated insulin exocytosis. We found the release of insulin by AAMP-N was dependent on VAMP2 after using siRNA of VAMP2. Insulin secretion pathway includes insulin synthesis, transport and release. Currently, studies on the mechanism of polysaccharides regulating insulin secretion pathway mainly focused on insulin synthesis[10,66], but the regulation of the latter two processes is not clear. Our results suggested that AAMP-N enhanced insulin secretion by increasing Ca2+-induced insulin transport and VAMP2-mediated insulin release, which provides a new idea for subsequent studies on the mechanism of polysaccharide-promoting insulin secretion.
5. Conclusions
In summary, mannogalactoglucan from mushrooms protected pancreatic islets via restoring UPR and promoted insulin secretion in T1DM mice. Our results highlighted that mannogalactoglucan could be used as the potential natural functional food for prevention and treatment of pancreatic dysfunction in T1DM.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research was funded by the National Natural Science Foundation of China (32371341, 31872674), the Scientific and Technologic Foundation of Jilin Province (20230202050NC), and the Fundamental Research Funds for the Central Universities (CGZH202206).
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango