Effects of prebiotics on the fermentation of traditional suancai of Northeast China
2024-01-24MingweiZhoXinyingCoYuzhengWuSioZouZhigoLiXinpingLinChofnJiLingDongSufngZhngChenxuYuHuipengLing
Mingwei Zho, Xinying Co, Yuzheng Wu, Sio Zou, Zhigo Li, Xinping Lin,Chofn Ji, Ling Dong, Sufng Zhng, Chenxu Yu, Huipeng Ling,
a National Engineering Research Center of Seafood, Collaborative Innovation Center of Provincial and Ministerial Co-construction for Deep Processing,Collaborative Innovation Center of Seafood Deep Processing, School of Food Science and Technology, Dalian Polytechnic University, Dalian 116034, China
b Department of Agricultural and Biosystems Engineering, Iowa State University, IA 50011, USA
Keywords: Bacterial microbiota Lactic acid bacteria Flavor Amino acid Taste
ABSTRACT Suancai is a traditional fermented vegetable widely consumed in Northeast China. In this study, different prebiotics were used to improve the quality of suancai. Four prebiotics (inulin (INU), xylooligosaccharide(XOS), galactooligosaccharide (GOS), and stachyose (STA)) were shown to reduce the pH value and increase the content of total titratable acidity (TTA) in suancai, while the contents of most organic acids were also increased. The addition of prebiotics had signif icant effects on the bacterial microbiota during the suancai fermentation process. All prebiotics were shown to contribute to the growth of Lactobacillus. The suancai sample with fructooligosaccharides (FOS) had the highest relative abundance of Lactobacillus. Besides,INU and XOS could increase the abundance of Weissella. To evaluate the quality of suancai fermented with prebiotics, profiles of volatile flavor compounds (VOCs) and free amino acids (FAA) were analyzed. The prebiotics affected the VOCs and FAA profiles via transforming the bacterial microbiota. In addition, the addition of prebiotics also changed the taste prof iles of the suancai samples. This study is among the f irst attempts to reveal the effects of different prebiotics on suancai fermentation, and the findings provide a foundation to develop new ways for improving the quality of suancai.
1. Introduction
Fermentation is one of the oldest methods for processing food to obtain desirable characteristics, such as extended shelf life and/or good organoleptic properties[1]. Fermented foods have been signif icant parts of the human diet since the start of human civilization for their pleasant f lavor and taste, texture, enhanced nutrition, and good keeping quality under ambient conditions[2]. Suancai is a well-known traditional fermented food widely consumed in Northeast China. In the past, every household made suancai according to the unchanged conventional method in the fall to prepare for the winter. The total annual household and industrial production of suancai in Northeast China has reached hundreds of thousands of tons. The procedure goes as follows: firstly, fresh cabbages are washed with tap water and drained, then they are stacked layer by layer in fermentation pots, and immersed in a low salt solution. The pots are then topped off with clean water, and the cabbages are subject to lactic acid bacterial (LAB) fermentation for approximately one month[3-4]until the suancai is ready to eat. Suancai is a great source of dietary f iber,vitamins, glutamine, and antioxidants called glucosinolates[5]. With this traditional approach, suancai is naturally fermented by the microorganisms attached to the surface of the cabbages, and/or in the environment, hence, inconsistency in quality has been a prevalent problem in industrial production of suancai[6]. As the demand for high-quality suancai products keeps rising, the need to address the inconsistency problem of suancai production is becoming ever more urgent.
Plenty of studies confirmed that LAB are the principal microorganisms playing key roles in suancai fermentation[7-9].Lactobacillus,Pediococcus,Leuconostoc,Weissella, andEnterococcuswere the dominant genus found in suancai, and were highly correlated with the volatile flavor compounds (VOCs) and physicochemical characteristics[3,7-8]. Therefore,LAB were commonly used as starters in industrial suancai fermentation to improve the quality of suancai products[10-11]. However, the inoculation of single LAB has been reported to cause serious acidification and reduce the bacterial diversity, and negatively affect the flavor and quality of fermented vegetables[10]. Prebiotics are non-digestible food ingredients that can beneficially affect the host by selectively stimulating the growth and activity of the microflora, such as fructooligosaccharides (FOS), inulin (INU), xylooligosaccharides(XOS), galactooligosaccharides (GOS), and stachyose (STA).Prebiotics are frequently used as dietary fiber additives in functional foods[12]for their ability to enhance the viability of healthy gut bacteria[13]. Many fruits and vegetables rich in dietary fibers contain prebiotics. As selectively fermented dietary ingredients, prebiotics can stimulate the growth and/or activity of certain types of bacteria(e.g.,BifidobacteriumandLactobacillus) to bring health benefits to consumers[14-16], and may also be achieved by increasing the low organic acid content of the product[17]. Besides, prebiotics are also used to improve textural attributes of foods, such as the reduction in fat content of sausages[18], the reduction of sodium and fat content in processed cheese[13]. The effects of prebiotics on food quality and sensory properties depended on the type and dosage of prebiotics, as well as the nature of the food matrix[19]. In this study, it was reasoned that the addition of prebiotics can improve suancai quality by promoting the growth of key LAB during suancai fermentation. Few reports can be found on the effects of prebiotics on the fermentation of suancai, we aimed to fill this knowledge gap. Therefore, this work investigated the effects of different prebiotics on the bacterial diversity of microbiota and quality characteristics of suancai. The findings provided a foundation for developing new ways to improve the quality and consistency of industrial suancai production.
2. Materials and methods
2.1 Sample preparation
Cabbages (Brassica rapa pekinensis) were bought from the local market in Dalian, Liaoning Province, China. The prebiotics, including FOS, INU, XOS, GOS, and STA, provided by Zhejiang Yano Biotech Company (a company for R & D of crude drug synthesis), were used in this study as additives for suancai fermentation. Cabbages were washed thoroughly with tap water and put into a sterilized jar (2.5 L)with 6% salt and/or 2% prebiotics according to the mass of cabbage(m/m). The jar was then topped off with tap water and sealed with caps, with cabbages fully submerged. Six different batches were performed with the following methods: 1) Control group (without adding prebiotics, CON), 2) fermentation with FOS (FOS),3) fermentation with INU (INU), 4) fermentation with XOS (XOS),5) fermentation with GOS (GOS), and 6) fermentation with STA(STA). Each batch was performed in triplicate. All sealed jars were placed in a big container and fermented at (15 ± 1) °C for 30 days.The day 30thsamples were collected and stored at -20 °C for subsequent analyses.
2.2 Physicochemical analysis
2.2.1 Determination of pH, total titratable acidity (TTA), and nitrite content
The pH was detected by a pH meter (METTLER TOLEDO FE28,Greifensee, Switzerland). Each sample was titrated to light pink using 0.1 mol/L NaOH with 1% phenolphthalein-ethanol solution as an indicator to determine its TTA. The nitrite content of each sample was determined according to GB 5009.33-2016.
2.2.2 Determination of organic acids profile
The profile of organic acids for each sample was determined by HPLC (Agilent 1220 Infinity II system, Agilent Technologies Inc.,Palo Alto, CA, USA) with a ZORBAX-SB C18column (250 mm ×4.6 mm, 5 μm). The determination method was performed as our previous study[10]. Samples were eluted isocratically at ambient temperature with a mobile phase (0.01 mol/L KH2PO4) at a flow rate of 1 mL/min. Elution was performed with a gradient of 95:5. After that,a UV detector was used to detect the elute at 210 nm. Each injection was 20 μL, and the sample was vortexed before each injection. The organic acids contained in each sample included citric acid, tartaric acid, succinic acid, lactic acid, acetic acid, and malic acid.
2.3 Microbial diversity analysis
Genomic DNA of suancai samples was extracted using MagPure Soil DNA LQ Kit (Angen Biotech, Guangdong, China).The concentration and integrity of DNA was determined by NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA) and agarose gel electrophoresis.The V3-V4 region of the 16S rRNA gene was amplified with 343F (5’-TACGGRAGGCAGCAG-3’) and 798R(5’-AGGGTATCTAATCCT-3’) as the primers[20]. The PCR was performed as follows: 94 °C for 5 min; 26 cycles of 94 °C for 30 s,56 °C for 30 s and 72 °C for 20 s; 72 °C for 5 min. The amplified DNAs were analyzed by gel electrophoresis, then purified with Agencourt AMPure XP beads (Beckman Coulter Inc., Brea, CA,USA), and subject to a new round of PCR. The final amplicon was then quantified using the Qubit dsDNA assay kit. Equal amounts of purified amplicon were pooled for subsequent sequencing on the Illumina NovaSeq platform (Illumina Inc., San Diego, California,USA) by OE Biotech Co., Ltd. (Shanghai, China)[7].
2.4 Bioinformatic analysis
Raw sequencing data were presented in FASTQ format. Pairedend reads were then preprocessed to detect and cut off ambiguous bases (N) using Trimmomatic software[21]. It also used a sliding window trimming method to truncate low-quality sequences with a mean quality score below 20. Paired-end reads were assembled by FLASH software after trimming[22]. Assembly parameters were: a minimum overlap of 10 bp, the maximum overlap of 200 bp, and the maximum mismatch rate of 20%. Sequences were further denoised as follows: reads with ambiguous, homologous sequences or less than 200 bp were discarded. 75% of the base reads onQ20were retained.Chimeric reads were then detected and removed. These two steps were implemented using QIIME software (version 1.8.0)[23]. Using Vsearch software[24], the clean reads were deprived and clustered to generate operational taxonomic units (OTUs) with a similarity cutoff of 97%. Representative read for each OTU was selected by the QIIME software package. All representative reads were annotated with the RDP classifier (confidence threshold of 70%) and blasted against Silva database Version 123[25].
2.5 VOCs and E-tongue analysis
Suancai samples were placed in a headspace sampling bottle with 50 mg/L cyclohexanone as the internal standard. The bottle was then placed in a 60 °C water bath for 20 min. After stabilization,divinylbenzene/carboxyl/polydimethylsiloxane (DVB/CAR/PDMS)SPME fiber (Supelco, Inc., Bellefonte, PA, USA) was inserted into the headspace to extract VOCs for 20 min. The fiber was then removed and inserted into the GC-MS 7890A/5977A (Agilent Technologies Inc., Palo Alto, CA, USA) with an elastic capillary vessel column(HP-5MS, 30 m × 0.25 mm i.d., 0.25 μm film thickness) for VOCs analysis. The oven temperature gradient was performed as follows:35 °C for 5 min, and then at 3 °C/min to 50 °C for 3 min; raised to 150 °C at 5 °C/min; raised to 250 °C at 20 °C/min, and subsequently held for 5 min[8]. The mass spectra were acquired with a source temperature of 230 °C, under a 70 eV ionization potential. The mass scan range ism/z15 tom/z300. The injector was operated in splitless mode at a flow rate of 1 mL/min. By comparing the GC peak area with the internal standard, the semi-quantitative analysis of VOCs was performed. The E-tongue analysis was performed by a TS-5000Z type taste analysis system (Insent Inc., Kanagawa, Japan), following the method previously reported[7].
2.6 Determination of amino acids content
The amino acids content for each sample was determined by an amino acid analyzer (AAA, LA8080, Hitachi High-Technologies Co., Tokyo, Japan) according our previous study[10]. The samples were filtered through a 0.22 μm water filtration membrane and mixed with 3 mL acetone. After that, each tube was vortexed for 5 min to precipitate the proteins. After centrifugation at 10 000 ×gat 4 °C for 10 min, the supernatant was collected and blown dry with nitrogen to get the amino acid sample. Subsequently, the amino acid sample was redissolved in 2 mL 0.02 mol/L hydrochloric acid, filtered through a 0.22 μm filter membrane, transferred to the bushing, and put into the amino acid analyzer. The injection volume was 20 μL. The amino acids in the samples were determined by comparing retention times with a mixed amino acid standard solution, type H (Wako Pure Chemical Industries, Ltd., Osaka, Japan).
2.7 Statistical analysis
The graphs were drawn using Excel 2016, STAMP v2.1.3[26],and Origin v8.5 (Origin lab Corporation, Ma, USA). OTU flower and circus plots were created using the variable R package. The significance of the difference in one-way ANOVA in physicochemical properties, organic acid, VOCs and free amino acid was determined by SPSS v13.0 software (SPSS Inc., Chicago, IL, USA). The principal components analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were generated by SIMCA v1.0.1 (Umetrics AB,umeå, Sweden). Spearman’s rank correlations between the bacterial community and free amino acids were calculated using SPSS v13.0 software (SPSS Inc. IL, USA).
3. Results
3.1 Physicochemical properties of suancai fermented with different prebiotics
The physicochemical properties of suancai fermented with different prebiotics are shown in Fig. 1. The pH of suancai fermented with prebiotics ranged from 3.21 to 3.45. Compared with the control(CON), the pH of suancai fermented with prebiotics was lower(P< 0.05), except for FOS (Fig. 1). The TTA of suancai fermented with prebiotics was higher than the control (P< 0.05), except for FOS(Fig. 1). Among the samples, the TTA of suancai fermented with INU was the highest ((8.25 ± 0.18) g/L), followed by that with XOS((7.13 ± 0.57) g/L). As shown in Fig. 1, the addition of prebiotics led to reduction of nitrite in suancai samples than the control (P< 0.05).After fermentation, the contents of nitrite in all suancai samples were lower than the national standard level (20 mg/kg).
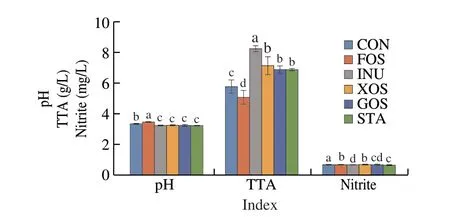
Fig. 1 Changes of physicochemical properties of suancai fermented with different prebiotics. TTA, total titratable acid. All analyses were conducted in triplicate and the average values are presented. Different letters in the same column indicate a significant difference (P < 0.05).
3.2 The organic acids profile of suancai fermented with different prebiotics
The profiles of organic acid of suancai fermented with prebiotics are shown in Fig. 2. The addition of prebiotics, except for XOS,resulted in a significant increase (P< 0.05) in lactate (Fig. 2A).The lactate content of suancai fermented with STA was the highest((7.79 ± 0.06) g/L), followed by that with GOS ((6.81 ± 0.05) g/L).Compared with CON, the acetate contents of suancai fermented with FOS, INU, GOS, and STA were increased (P< 0.05), while that fermented with XOS was decreased (P< 0.05) (Fig. 2B). As shown in Fig. 2C, the addition of all prebiotics significantly increased the content of citrate (P< 0.05), while that of suancai fermented with INU was the highest ((0.66 ± 0.02) g/L). The content of the succinate in suancai fermented with prebiotics was higher than that of CON(P< 0.05) (Fig. 2D) as well, with the highest value observed in the sample with FOS ((1.51 ± 0.04) g/L). The addition of XOS significantly increased the content of malate (P< 0.05) (Fig. 2E).Futhermore, the content of tartrate in all samples with prebiotics was also significantly increased (P< 0.05) (Fig. 2F).
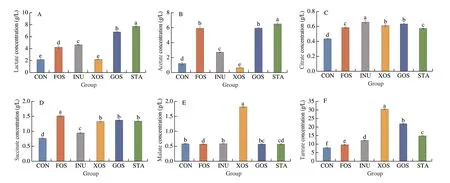
Fig. 2 Changes in organic acid of suancai fermented with different prebiotics. (A) Lactate; (B) Acetate; (C) Citrate; (D) Succinate; (E) Malate; (F) Tartrate. All analyses were conducted in triplicate and the average values are presented. Different letters in the same column indicate a significant difference (P < 0.05).
3.3 Bacterial diversity in suancai fermented with different prebiotics
After quality control processing of the suancai samples sequenced by Illumina NovaSeq, the amount of clean tags data ranged from 72 554 to 77 542, and the amount of valid tags data ranged from 61 582 to 72 079. The bacterial alpha indexes of suancai fermented with different prebiotics are shown in Table 1. The Chao1 and Observed species indexes reflected the OTU amount of the microbiota in samples. The addition of prebiotics had no significant effects on the Chao1 and Observed species in the bacterial microbiota in the suancai samples (Table 1). The Shannon index, the Simpson index, and the PD whole tree reflected the diversity of the microbiota in samples.The addition of INU or XOS appeared to increase the Shannon index of the suancai microbiota (Table 1), while the addition of GOS or STA appeared to have no effect on the Shannon index, and the addition of FOS appeared to decrease it. The addition of prebiotics appeared to have no significant effects on the PD whole tree of the suancai microbiota (Table 1). The Goods coverage of all samples was 0.99, indicating the majority of the bacterial phylotypes present in samples had been recovered (Table 1). The Venn diagram of OTUs of samples is shown in Fig. 3A. A total of 351 OTUs was shared by all samples. Suancai samples fermented with GOS had the highest OTU numbers (532), while the OTU numbers of suancai fermented with FOS, STA, INU, and XOS were all lower than that of CON (Fig. 3A).

Table 1 Alpha diversity index of suancai fermented with different prebiotics.

Fig. 3 The OTUs venn plot (A), circus plot (B), and relative abundance at genus level (C) of suancai fermented with different prebiotics.
3.4 Bacterial community structures of suancai fermented with different prebiotics
The OTUs were annotated and blasted to identify their taxonomy assignments. The circus plot is shown in Fig. 3B. The microbiota of the prebiotic suancai was composed of more than 20 phyla,including Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria,Epsilonbacteraeota, Gemmatimonadetes, etc., but more than 95%of the annotated reads belonged to Firmicutes and Proteobacteria(Fig. 3B). At the genus level, a total of 343 genera were detected in all suancai samples. As shown in Fig. 3C,Lactobacillus,Lactococcus,Weissella,Pediococcus,Serratia,Citrobacter, andKlebsiellawere observed as dominant genera (> 1%). The suancai samples with different prebiotics were clustered in diverse groups, indicating that the addition of prebiotics could affect the bacterial community structures of the microbiota (Fig. 4A) in unique ways, and the suancai sample with different prebiotics had different biomarkers (Fig. 4B).The linear discriminant analysis (LDA) coupled with effect size measurements (LEfSe) results showed thatPediococcus,Leuconostoc,Lactococcus,Achromobacter,Tepidimicrobium,Solirubrobacter,andSinibacilluswere biomarkers of the CON sample (Fig. 4B).Lactobacillus,Herbaspirillum,Acinetobacter, andSerratiawere the biomarkers of the FOS sample, whileMethylophiluswas the biomarker of the GOS sample (Fig. 4B).RomboutsiaandKlebsiellawere the biomarkers of the INU sample.Weissella,Citrobacter,Chryseobacterium,Taibaiella,Hafnia_Obesumbacterium,Mucilaginibacter,Pseudoxanthomonas, andXanthomonaswere the biomarkers of the XOS sample (Fig. 4B). Compared with the control, the addition of prebiotics could increase the relative abundance ofLactobacillus(Fig. 4C). The addition of prebiotics decreased the relative abundance ofLactococcus,Pediococcus, andLeuconostoc. The relative abundance ofWeissellawas higher in suancai samples fermented with FOS,INU, and XOS, respectively (Fig. 4C).
3.5 Volatile compounds analysis in suancai with different prebiotics
One of the most critical appeals of suancai for consumers is its unique aroma associated with the VOCs. After fermentation,a total of 35 VOCs were identified, including 6 nitriles, 3 esters,5 acids, 10 alcohols, 1 ether, 3 phenols, 2 ketones, 1 aldehyde, and 4 isothiocyanates (Fig. 5A and Supplementary Table S1). A PLS-DA model was constructed to discriminate samples with different VOCs profiles, and theR2XandR2Yof the model were 0.999 and 0.783,respectively.Q2of the model was 0.609, suggesting that the model was well fitted for analysis and prediction of VOCs characteristics of various suancai samples. The principal component analysis (PCA)was also used to show the differences between the VOCs profiles of various suancai samples with different prebiotics. As shown in Fig. 5B,suancai samples with different prebiotics were clustered into different groups, respectively, indicating that the VOCs profiles were affected by the prebiotics. The concentration of 5-cyano-1-pentene (C03) and benzenepropanenitrile (C06) in suancai fermented with STA increased significantly. The (2-isothiocyanatoethyl)-benzene (C35) decreased upon the addition of prebiotics except for STA. The concentration of 2-ethyl-1-hexanol was higher in suancai fermented with prebiotics than that in CON (Fig. 5A). The variable importance of projection(VIP) plot showed that important microorganisms (VIP > 1.0)related to VOCs in suancai during the fermentation includedLactococcus,Lactobacilli,Weissella,Pediococcus, andKlebsiella(Fig. 5C).

Fig. 5 Heatmap (A) and PCA analysis (B) of volatile flavor compounds (VOCs) of suancai fermented with different prebiotics. (C) VIP plot of microorganisms related VOCs was analyzed based on PLS modeling. The number of volatile flavor compounds in (B) was in correspondence with Supplementary Table S1.
3.6 Amino acid content of suancai fermented with different prebiotics
Amino acids profiles of various suancai samples are shown in Fig. 6A. The total amino acid content in suancai was significantly increased by addition of prebiotics (Fig. 6B), with the GOS leading to the highest total amino acid content, followed by the FOS. Among the amino acids, Thr, Glu, and Ala were the primary ones in all suancai samples (Fig. 6B). The effects of prebiotics on total amino acids were also analyzed via PCA. As shown in Fig. 6B, there were significant differences among the samples of suancai fermented with different prebiotics. The relationships between bacteria and amino acids of suancai fermented with different prebiotics are shown in Fig. 6C.As the dominant genus,Lactobacilluswas positively correlated to amino acids, with a significant correlation with Thr, Pro, Phe and Cys.Lactococcuswas significantly and positively correlated with Gly, Cys, and Met (Fig. 6C).Klebsiellawas significantly and positively correlated with Ser, Met, Ile, Tyr, Phe, Lys, His, and Arg.Other than these three, bacteria correlated closely with the amino acids of suancai fermented with different prebiotics (VIP > 1) also includedPediococcus, hgcl-clade,Stenotrophomonas,Citrobacter,Leuconostoc,Saccharomonospora,Bacillus,Weissella, andSerratia(Fig. 6D).
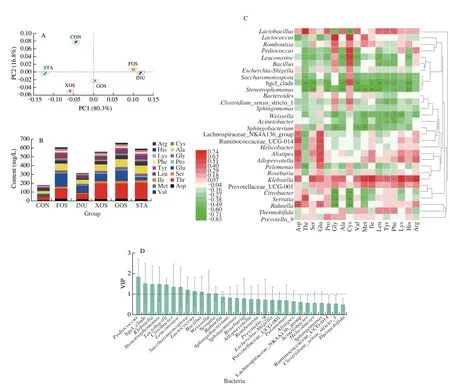
Fig. 6 PCA analysis (A), content (B), heatmap of Spearman rank correlation with bacteria (C), and VIP plot of microorganisms related to amino acid (D) of suancai fermented with different prebiotics. VIP plot was analyzed based on PLS modeling. *P < 0.05, **P < 0.01.
3.7 Sensory evaluation
E-tongue profiles of suancai samples fermented with different prebiotics are shown in Fig. 7. The addition of prebiotics could affect the taste profiles of suancai. After fermentation, the suancai of INU and STA groups had higher sourness scores than the CON and the other groups (Fig. 7). It was also shown that the addition of FOS, INU, GOS, and STA could substantially reduce the bitterness score (P< 0.05). The addition of FOS could significantly reduce the astringency score (Fig. 7). Aftertaste-A score reflects the aftertaste of astringency, and aftertaste-B score reflects the aftertaste of bitter taste. The addition of all prebiotics could considerably increase the aftertaste-B score, while the addition of INU, XOS, and STA could significantly increase the aftertaste-A score (Fig. 7). The STA appeared to significantly enhance the umami score, while the GOS appeared to dramatically improve the richness score, both are important taste attributes for consumers. As expected, the addition of prebiotics had no significant effect on the saltiness scores of suancai samples (Fig. 7).

Fig. 7 E-tongue profiles of suancai fermented with different prebiotics.
4. Discussion
In this study, the bacterial diversity, organic acid, amino acid,and VOCs in suancai fermented with different prebiotics were investigated using Illumina NovaSeq sequencing, HPLC, AAA,and GC-MS. The addition of prebiotics can contribute to the safety characteristics of suancai. The prebiotics addition could inhibit the accumulation of nitrite[9], and the content of all the suancai fermented with different prebiotics far below the national limit of 20 mg/kg(Fig. 1). As fermented vegetable food, the pH and TTA are important indexes to evaluate the characteristics of food fermented by lactic acid bacteria[11,27]. The addition of prebiotics decreased the pH and increased the TTA of suancai except for FOS (Fig. 1). The acidity of suancai is controlled by a variety of organic acids. The contents of organic acid in suancai, including lactate, acetate, citrate, succinate,malate, and tartrate, were improved respectively by adding the prebiotics (Fig. 2). As one of the critical factors affecting the quality and taste of suancai, acidity is also closely related to the bacterial composition of fermented food[28]. Lactic acid, an essential product of LAB, was one of the primary organic acids in suancai (Fig. 2A)and possibly had a positive effect on the organoleptic characteristics of suancai because lactic acid confers a softer taste and increases the microbial stability of fermented products[11]. Acetic acid is a by-product of lactic acid fermentation and ethanol fermentation by heterofermentative bacteria or yeast[29]. Suancai fermented with STA significantly increased lactic acid and acetic acid (Figs. 2A and B),which may be explained by its higher abundance of LAB (Fig. 3C).Consequently, the sour taste of suancai fermented with STA was significantly higher than the CON (Fig. 7). Citrate is a crucial metabolic intermediate of the tricarboxylic acid cycle and is used as a pleasant sour taste. In this study, the citrate content of suancai was the least (Fig. 2C). Astringency and bitterness were involved in succinate, malate, and tartrate[30]. The samples of suancai fermented with XOS significantly increased the content of malate and tartrate(Fig. 7), leading to a high taste of bitterness and astringency[31].Prebiotics are substances that exert beneficial physiological effects on the host by modulating the composition and activity of the microbiota[32]. In this study, the prebiotics had a significant impact on the bacterial community diversity and structures of suancai (Figs.3C and 4A). Alpha diversity analysis showed that the addition of INU and XOS increased the diversity of the bacterial community in suancai, while GOS and STA had no noticeable effect (Table 1).Inversely, the addition of FOS decreased the diversity of the bacterial community in suancai (Table 1). At the phylum level, Firmicutes and Proteobacteria are the dominant phyla in prebiotic suancai(Fig. 3B), which was also obtained by previous studies[8,10]. In this study, LAB is found to be the dominant microorganisms in suancai,which was consistent with many studies[7,10-11]. Among them,Lactobacillus,Lactococcus,Weissella,Pediococcus, andLeuconostocwere the leading LAB in suancai fermented with different prebiotics(Fig. 3C).Lactobacillus(65.19%–87.82%) was the dominant genus in all suancai samples (Fig. 3C), which is inconsistent with the findings on other prebiotic products[33-34]. LAB is the “preferred target organism” of prebiotics[35]. Previous studies reported that some prebiotics, such as GOS and INU, can increaseLactobacillus[15,36].Thus, the genusLactobacilluswere the most favored one in this study(Fig. 4C). The addition of FOS, INU, and XOS contributed to the growth ofWeissella. For the other LAB, the prebiotic addition led to a decrease in abundance. Therefore, the addition of prebiotics could affect the diversity of LAB during the fermentation of suancai.
VOCs play an essential role in consumer acceptance of suancai[8].The VOCs of fermented food during fermentation was associated with bacteria[37-39]. In this study,Lactococcus,Lactobacillus,Weissella,Pediococcus,andKlebsiellawere identified as important bacteria(VIP value > 1.0) associated with VOCs in suancai fermented with different prebiotics (Fig. 5C). The prebiotics could affect the profiles of VOCs in suancai by affecting the diversity of the bacterial community during fermentation. The distribution of VOCs in suancai with different prebiotics is quite different (Fig. 5A). Previous studies on suancai or other fermented foods have shown thatLactobacilluscan increase the concentration of alcohols, nitriles, terpenes and organic acids[3,40-41]. The concentrations of 3-methyl-2-butenenitrile(C01), 5-cyano-1-pentene (C03), and benzenepropanenitrile (C06)were increased with the addition of different prebiotics, especially the addition of STA (Fig. 5B). The alcohols, including 1-pentanol (C15),3-hexen-1-ol (C16), and 2-heptanol (C17), were also increasing with the addition of different prebiotics (Fig. 5B). This may be because of the high abundance ofLactobacillusin STA fermented suancai(Fig. 3C). In previous study, a high abundance ofLactobacillussignificantly enhanced the amount of 2-heptanol in red beetroot fermentation[42]. In cruciferous vegetables, various isothiocyanates(ITC) are formed from glucosinolates by the action of myrosinase.The compound of (2-isothiocyanatoethyl)-benzene (C35) is the most abundantly investigated aromatic ITC in cruciferous vegetables and then its concentration in suancai was high (Fig. 5B). The nitriles like benzenepropanenitrile (C06) also came from the enzymolysis of glucosinolates[43]. In addition to their own flavor, organic acids can also react with alcohols to form esters, which are helpful for the aromatic components of food[44]. The methyl formate (C07)was observed in suancai fermented with GOS and INU (Fig. 5B).In addition, fermentation with prebiotics also increased amino acid concentrations (Fig. 6B). Significant differences were found between suancai samples fermented with different prebiotics (Fig. 6A). Amino acids contribute directly to the taste of suancai[45]. Among them, Asp and Glu are umami amino acids and umami were considered to be the essential flavor of Chinese cooking[46]. Fermentation of suancai with all prebiotics increased the abundance of Asp and Glu (Fig. 6B). The taste of umami of suancai fermented with prebiotics increased but was not significant except for STA (Fig. 7). The high content of Arg and Glu (Fig. 6B) may be due to lower pH in suancai, which is consistent with previous studies that Glu and Arg are responsible for combating acid stress[47]. Many microorganisms can produce proteolytic enzymes to generate amino acids. The prebiotic addition could increase the concentration of amino acids by adjusting the microbiota.Some genera, such asLactobacillus,Lactococcus,Pediococcus,Leuconostoc, andKlebsiella, were positively correlated with amino acids (Fig. 6C) and identified as the crucial bacteria (VIP value >1.0) associated with amino acids in suancai fermented with different prebiotics (Fig. 6D).
5. Conclusion
Prebiotics are selectively fermented substances that can stimulate the growth of the potentially health-promoting microorganisms. In this study, the effects of prebiotics (i.e., FOS, INU, XOS, GOS, and STA) on the microbiota and the quality characteristics of suancai were investigated. The addition of prebiotics had significant effects on microbiota during suancai fermentation, which further affected the characteristics of suancai products, such as reduce the pH, increase the organic acids content and reduce the nitrate content. The addition of all the prebiotics could increase the quantity ofLactobacilluscomparing to the control. The sample with FOS had the highest abundance ofLactobacillus. In addition, the suancai fermented with INU and XOS showed a higher abundance ofWeissella. The generaLactococcus,Lactobacillus,Weissella,Pediococcus,andKlebsiellawere important microorganisms related to VOCs and free amino acids, key contributors to the appealing flavor of suancai. In turn,the prebiotics affected the VOCs profiles as well as taste scores of suancai samples. This study is among the first of such work to reveal the effects of different prebiotics on suancai fermentation, which provides guidelines for improving industrial suancai production.
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was supported by National Natural Science Foundation of China (31901809) and the Doctoral Research Start-up Fund of Dalian Polytechnic University (6102072007).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.26599/FSHW.2022.9250114.
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango