Inf luence of soaking Malus domeri (Bois) Chev. leaves on gut microbiota and metabolites of long-living elderly individuals in Hezhou city, Guangxi, China
2024-01-24HuiNieZhongyangGaoYangheLuoYajuanWangFeiyangWuGuangqingMuXiaomengWu
Hui Nie, Zhongyang Gao, Yanghe Luo, Yajuan Wang, Feiyang Wu,Guangqing Mu,, Xiaomeng Wu,
a School of Food Science and Technology, Dalian Polytechnic University, Dalian 116034, China
b Dalian Probiotic Function Research Key Laboratory, Dalian Polytechnic University, Dalian 116034, China
c Guangxi Key Laboratory of Health Care Food Science and Technology, Hezhou University, Hezhou 542899, China
Keywords: Longevity Malus domeri (Bois) Chev. leaves Gut microbiota Metabolomics
ABSTRACT To investigate the effects of drinking the soaking of Malus domeri (Bois) Chev. leaves on gut microbiota and metabolites of long-living elderly individuals in Hezhou city, Guangxi, China. It has been reported that longevity is closely related to metabolism and the gut microbiota. The 16S rRNA sequencing and liquid chromatography-mass spectrometry (LC-MS) were used to analysis fecal samples and explore the factors affecting longevity in the region. Interestingly, we discovered, that elderly individuals who had been drinking the soaking of M. domeri (Bois) Chev. leaves for a long time exhibited higher diversity of the gut microbiota than without drinking the soaking, notably. The proportions of Ruminococcaceae and Prevotella were decreased in those who did not drink this soaking. In addition, a total of 106 metabolites were characterized,and the people of long-lived people (> 90 years old) and elderly people (< 90 years old) who drinking soaking of M. domeri (Bois) Chev. leaves signif icantly altered the gut microbiota and upregulate d levels of haplopine, farnesol, genipic acid, momordicinin, 2-hydroxyestrone, hydroxyphenyllactic acid, caffeic acid,sophoraflavanone B, and soyasaponin I. We preliminarily determined that M. domeri (Bois) Chev. leaves consumption may be an important factor affecting longevity in this area.
1. Introduction
In recent years, health and longevity have attracted amounts of attention. The average age has increased with the development of society, and according to statistics, 1.2 billion people are expected to be over the age of 60 by 2025[1-2]. At the same time, the number of elderly people with a life span of more than 100 years old is gradually increasing in different regions, such as Bama[3-5], Zhongxiang[6], and Liuyang[7]. Hezhou is located in the eastern of Guangxi Province,China, and was named “World Longevity City” by the International Expert Committee on Population Ageing and Longevity (IECPAL)on 19thOctober, 2016. According to the Population Census of China in 2010, the ratio was 19.1 centenarians per 1 × 105persons[8], which was far exceeding than that estimated by the United Nations (7.5/100000)[6].Some people, widespreading in Hezhou city in Guangxi Province,have a habit of drinking soakingMalus domeri(Bois) Chev.leaves[9-10]. However, the effects of soakingM. domeri(Bois) Chev.leaves on human with few studies.
Longevity is a very attractive subject, and long-term research should be undertaken to explore the causes of longevity. Many scientists have studied the impact of genetics[11], food[7], geology[12],and atmosphere on longevity[13]. In the past decade, some researchers focused on the analysis of the gut microbiota of long-living humans[4,7]. Experts have pointed out that the microbiota species and composition are closely related to the occurrence of diseases in elderly individuals, and the diversity of the intestinal flora has been indicated to be one of the important reasons for longevity. Recently,the microbial community structure in the intestinal tract and host health are being widely studied. The microbiota can directly or indirectly affect host barrier function, immune regulation, aging and tissue development[14-15].
A survey of available research showed that comparative analyses have been able to identify signatures of longevity in the gut microbiota of individuals in China[7,16], Italy[17], India[1], and Japan[18].These studies jointly confirmed that the diversity of the intestinal flora affects the longevity of humans.
The gut microbiota structure in elderly individuals is considerably different from that in other people. Some studies identified,Bifidobacterium,Lactobacillus, and Ruminococcaceae species among the intestinal microorganisms of long-living elderly people (age >90 years old)[1,5,19], while a high abundance ofFaecalibacteriumwas observed in young adults[1]. At the same time, a relatively high level within Ruminococcaceae was identified in centenarians.
Differences in the intestinal flora affect the metabolism of the body[20]. For instance, short chain fatty acids (SCFAs) were benefits for host health, with corroborated SCFAs fabricants such asBacteroidesspp.,Clostridiumspp., andStreptococcusspp.products[21]; bile acids (BAs) facilitate lipid uptake, transport, and metabolism, and gut bacterial species involved in BAs metabolism wereBifidobacteriumandBlautiaspp.[22-23]. In addition, dietary habits are important factors affecting the structure of the intestinal microbiota. Differences in diets can certainly regulate metabolism,so that might be a factor of length of life span in different regions.Similarly drinking some soaking material liking has been a Chinese tradition for thousands of years. Meanwhile, most of the way of tea drinking is soaked directly with hot water. Similarly,M.domeri(Bois)Chev. leaves were drunk also using the same method, that was also called tea by Hezhou local inhabitants. Tea possesses anticancer,anti-inflammatory, antibacterial, and antioxidant effects due to the ingredient polyphenols, flavones and other compounds[24-25], and could alter gut microbiota composition of hypertensive rats caused by high-salt diet[26]. Additionally, the gut microbiota structure, alleviate inflammation, and the levels of SCFAs also could be influenced by drinking the soaking material with polyphenols, flavones and other compounds[27]. Importantly, it has been obtained a result that soaking ofM.domeri(Bois) Chev. leaves also contains numbers of polyphenols, flavones and other compounds in our preliminary study.
In order to gradually explore the mystery of longevity, we conducted interviews and surveys height, weight, diet and living habits of the residents living in Hezhou. After preliminary screening,28 of qualified fecal samples of local inhabitants were studied.Interestingly, we found that people in Hezhou have a habit of drinking the soaking ofM.domeri(Bois) Chev. Leaves for a long-term time.Hence, we analyzed gut microbiota and metabolites of elderly in different groups of whether drinking the soaking using 16S rRNA sequencing and liquid chromatography-mass spectrometry (LC-MS).The diversity of the gut microbiota of two groups and the metabolic differences associated with different flora would provide a reliable theoretical basis for explaining longevity of human in Hezhou.
2. Materials and methods
2.1 Soaking M. domeri (Bois) Chev. leaves process
The dried ofM. domeri(Bois) Chev. leaves (80–100 g) in boiled water (1.6–2.0 L), and the boiling water temperature was almost 100 °C.
2.2 Volunteer recruitment and fecal sample collection
Twenty-eight valid volunteers between 80 and 103 years old(8 males and 20 females) were recruited from Hezhou City, Guangxi Province, China. To avoid the interference of other factors including diet and lifestyle, the elderly population with a simple diet habit were obtained after preliminary screening, and their characteristics are summarized in Table 1. The groups were as follows: HZ1 (drinking the soakingM. domeri(Bois) Chev. leaves ) including YS (younger elderly people, age < 90 years old) and AS (long-lived people,age > 90 years old); HZ2 (not drinking soaking solution ofM. domeri(Bois) Chev. Leaves) including YN (younger elderly people) and AN(long-lived people). These volunteers were healthy and had not been using medication or antibiotics for three months prior to recruitment.Fecal samples were collected and stored at –80 °C.

Table 1 The details of subjects in each categorized groups analyzed in this study.
2.3 DNA extraction
Bacterial DNA was extracted from the 28 fecal samples using the E.Z.N.A.® Soil DNA Kit (Omega Biotek, Norcross, GA, USA). The concentration and purity of the extracted DNA were determined by NanoDrop2000 UV-visible spectrophotometry (Thermo Scientific,Wilmington, DE, USA), and the DNA mass was measured by 1%agarose gel electrophoresis. A polymerase chain reaction (PCR)thermocycler system (Gene Amp 9700, ABI, USA) was used to amplify the V3–V4 hypervariable regions of the bacterial 16S rRNA gene with the primers 338F (5’-ACTCCTACGGGAGGCAGCAG-3’)and 806R (5’-GGACTACHVGGGTWTCTAAT-3’). PCR was conducted using the following procedure: denaturation at 95 °C for 3 min, followed by 27 cycles of denaturation for 30 s at 95 °C,annealing for 30 s at 55 °C, and elongation for 45 s at 72 °C, and a final extension at 72 °C for 10 min. PCR assays were performed in triplicate using TransStart Fastpfu DNA Polymerase (TransGen AP221-02) in a 20 μL mixture. The reaction mixture contained 5 ×FastPfuBuffer (4 μL), 2.5 mmol/L dNTPs (2 μL), forward primer(5 μmol/L, 0.8 μL), reverse primer (5 μmol/L, 0.8 μL), FastPfuPolymerase (0.4 μL), bovine serum albumin (BSA, 0.2 μL), 10 ng of template DNA, and ddH2O to obtain a final volume of 20 μL.The PCR products were detected by 2% agarose gel electrophoresis,further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified using Quanti Fluor™-ST (Promega, USA)[28-29].
2.4 Illumina MiSeq sequencing
Purified amplicons were pooled in equimolar amounts and pairedend sequenced (2 × 300) on the Illumina MiSeq platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database.
2.5 Metabolic profiling by LC-MS analysis
Fifty milligrams of feces were crushed using a mixer mill (MM 400,Retsch, Germany) with a zirconia bead for 6 min at 50 Hz (–10 °C).The samples were extracted overnight at 4 °C using 400 μL of 80%methanol, and 2-chloro-L-phenylalanine was used as an internal standard. The samples were then centrifuged at 13 000 ×gfor 15 min at 4 °C. The supernatant was collected and passed through a 0.2 μm syringe filter (SCAA-104, 0.22 μm pore size; ANPEL, Shanghai,China) before UPLC-MS (Thermo, UHPLC-Q Exactive, USA) analysis.The analytical conditions were as follows: ACQUITY UPLC HSS T3(100 mm × 2.1 mm i.d., 1.8 μm; Waters, Milford, USA); solvent system, solvent A (water/acetonitrile, 95:5 (V/V); 0.1% formic acid)and solvent B (acetonitrile/2-propanol/water, 47.5:47.5:5.0 (V/V);0.1% formic acid); gradient program: 0–0.1 min 100%–95% A,0.1–2 min 95%–75% A, 2–13 min 75%–0% A, 13–13.1 min:0%–100% A, 13.1–16 min 100% A; flow rate 0.4 mL/min; temperature 40 °C; and injection volume 2 μL. The LC-MS was set up for mass range ofm/z70 tom/z1 050. The MS operating system parameters were as follows: negative ionization, spray voltage 2 800 V;heater temperature 400 °C; sheath gas flow rate 40 a.u.; aux gas flow rate 10 a.u.; capillary temp 320 °C. Quality control (QC) samples were prepared by mixing the extracts of all samples, and each QC sample was processed and tested in the same way as the analytical samples. For instrument analysis, a QC sample was inserted into 5–15 analytical samples to investigate the stability of the whole detection process.
2.6 Data analysis
Operational taxonomic units (OTUs) are uniform markers for a taxon (strain, genus, species, group, etc.) artificially set in phylogenetic or population genetic studies to facilitate analysis. To obtain information about the number of species, genera, etc., in a sample sequencing result, sequence clustering (cluster) is required. By clustering operations, the sequences are classified into many groups based on their similarity to one another, and one group is an OTU.OTUs of all sequences can be divided based on different similarity levels and are usually used for bioinformatic statistical analysis of OTUs at a 97% similarity level using UPARSE (version 7.0.1090 http://drive5.com/uparse/). To obtain the species classification information corresponding to each OTU, taxonomic analysis was performed at a 97% similarity level for representative OTU sequences using the RDP Classifier Bayesian algorithm. The community species composition of each sample was also counted at each taxonomic level, that is, domain, kingdom, phylum, class, order, family, genus,and the species, using Silva (Release138 http://www.arb-silva.de) and Greengenes (Release 13.5 http://greengenes.secondgenome.com/).Metabolomics was used with principal component analysis (PCA) and comparison with the Human Metabolome Database (HMDB)/Kyoto Encyclopedia of Genes and Genomes (KEGG).
3. Resulats
3.1 Gut microbiota changes in the two groups
3.1.1 Community richness and diversity
The rarefaction curve showed that the sequencing provided relatively comprehensive coverage of the bacterial diversity, the rarefaction curves tend to approach saturation, indicating that the sequencing data are reasonable[30]. The sequencing data of all the samples were reasonable as shown in Fig. 1A. It is well known that community richness reflects the number of different species in the gut. The results ofα-diversity including community richness expressing with Chao1 index and community diversity expressing with Shannon index was displayed in Fig. 1B and C. The values of Chao and Shannon indexes without significance (P< 0.05) in both HZ1 and HZ2 were obtained, so the elderly individuals drinking the soakingM. domeri(Bois) Chev. leaves or not possessed the similar intestinal flora on community richness and community diversity.The community richness and diversity of younger elder people and long-lived people are displayed in Fig. S1. The values of the Chao1 and Shannon indexes were not significantly different (P> 0.05)between YS and YN groups, and the values of the Chao1 and Shannon indexes were not significantly different (P> 0.05) between the AS and AN. The results ofβ-diversity analysis between HZ1 and HZ2 were expressed via PCA plot in Fig. 1D. As shown, the values of PC1 and PC2 were 34.99% and 21.61%, respectively. That is to say,the PCA plot indicated that the difference was existing in HZ1 and HZ2 on the gut microbiota, and parts of HZ1 sample overlapped with the HZ2.Therefore, the elderly individuals drinking the soakingM. domeri(Bois)Chev. leaves or not possessed differential intestinal flora.
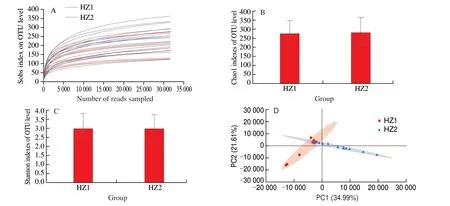
Fig. 1 (A) Rarefaction curve. Comparison of gut microbiota (B) Chao1 and (C) Shannon indexes. (D) PCA of long-living elderly microbial communities.
3.1.2 Microbial composition
Bacterial abundance at phylum/genera levels are shown in Fig. 2. At the phylum level (Figs. 2A–B), the gut microbiota in the two groups was dominated by 4 the predominant phylum, namely,Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteriota.In the groups, the relative abundances of Bacteroidetes and Actinobacteriota in HZ1 were higher than those in HZ2, but the relative abundance of Proteobacteria in HZ2 was higher than that in HZ1. Theα-diversity analysis shows in Figs. 2C–D, at the genus level, the relative abundances ofBacteroides,Ruminococcus,Clostridium_sensu_stricto_1,Bifidobacterium, andPrevotellawere substantially higher in HZ1 than HZ2. Meanwhile, the relative abundance ofEscherichia-ShigellaandAlistipesin HZ1 was substantially lower in these individuals in HZ2. As reported,Bifidobacteriumand Ruminococcaceae was considered as beneficial bacteria in the gut[31].That means drinking soakingM.domeri(Bois) Chev. leaves will increase the beneficial bacterial abundance. As Fig. 2E displayed,intestinal type analysis revealed the 2 groups were separated into 3 of the following: HZ1 and HZ2 are jointly showed the regnant bacteria families ofBacteroides, HZ1 with amount ofKlebsiellaand HZ2 with numbers ofEscherichia-Shigella. In Fig. 2F, the microbiotas at the genus level belonging to HZ2 showed an increasingEscherichia-Shigellaabundance and a decreasing abundance ofBifidobacterium,BacteroidesandRuminococcus. As Fig. S2 displayed, at the phylum level, the relative abundances of Bacteroidetes in the YS and AS groups were higher than those in the YN and AN groups, respectively.The relative abundances of Proteobacteria in the AN group were higher than AS group, and YS group were higher than YN group. At the genus level, the relative abundances ofEscherichia-Shigellawere decreased, and those ofBacteroides,Ruminococcus, andPrevotellawere increased, when younger elderly people and long-lived people both have the habit of drinking soakingM.domeri(Bois) Chev.leaves. That could mean drinking soakingM.domeri(Bois) Chev.leaves would improve the abundance of intestinal flora towards beneficial diversity development.

Fig. 2 Classification of the bacterial community composition across the HZ1 and HZ2. (A) Phylum level; (B) extended error bar plot showing the bacteria at the phylum level that had significant differences between the HZ1 and HZ2; (C) genus level; (D) extended error bar plot showing the bacteria at the genus level that had significant differences between the HZ1 and HZ2; (E) enterotype analysis at the genus level based on Bray-Curtis matrixes; (F) gut microbiota composition at the genus level.
Venn diagrams reflecting the difference of OTUs between the HZ1 and HZ2 groups are depicted in Fig. 3A. There were 681 and 713 OTUs in the samples of HZ1 and HZ2, respectively. The common OTUs belonging to HZ1 and HZ2 are 563, meanwhile,118 OTUs only in HZ1 and 150 OTUs only in HZ2. The LDA tool was used to analyze the microbial community at the level from the phylum to the genus. The 2 groups both possessing the LDA scores values of greater than or equal to 2 were confirmed by LEfSe in Fig. 3B. In HZ1 group, 8 groups of bacteria were enriched, namely, Ruminococcaceae, Aerococcaceae,Oribacterium,Anaerostignum,Ruminococcus,Granulicatella, Carnobacteriaceae and Erysipelotrichaceae_UCG-003. In HZ2, 11 groups of bacteria were enriched, namely,Escherichia-Shigella, Enterobacterales,Enterobacteriaceae, Proteobacteria, Gammaproteobacteria,Hungateiclostridiaceae,Hydrogenoanaerobacterium,Holdemania,Pyramidobacter, Lachnospiraceae and Oscillospirales. The relative abundance ofRuminococcus(Fig. 3C) andEscherichia-Shigella(Fig. 3D) in the HZ1 was significantly difference compared with the HZ2. Obviously, the significant difference obtained at the phylum level between HZ1 and HZ2, and this is more confirmation that drinking soakingM. domeri(Bois) Chev. leaves can affect intestinal flora.

Fig. 3 (A) Venn diagram. (B) Indicator bacteria with LDA scores of greater than or equal to 2 in bacterial communities associated with HZ1 and HZ2. Differentcolored regions represent different constituents (red, HZ1; blue, HZ2). (C) The relative abundance of Ruminococcus. (D) The relative abundance of Escherichia-Shigella. Significant correlation between the HZ1 and HZ2 groups; *P < 0.05; **P < 0.01.
PICRUSt2 (phylogenetic investigation of communities by reconstruction of unobserved states 2) program was used to predict our 16S rRNA data and analyze the data in the context of the Cluster of Orthologous Groups (COG) database (Fig. 4). We gained a relation between microbial COG database and the microbial functional features in the HZ1 and HZ2 group. The functional features included amino acid transport and metabolism, carbohydrate transport and metabolism, translation, ribosomal structure and biogenesis,transcription, cell wall/membrane/envelope biogenesis, inorganic ion transport and metabolism, energy production and conversion and so on.

Fig. 4 The microbial functional features in fecal samples.
3.2 Fecal metabolite analysis
A total of 106 metabolites (58 positively ionized metabolites and 48 negatively ionized metabolites) were identified in these groups (Fig. 5A). Among the 58 positively ionized metabolites,including 4 benzenoids, 19 lipids and lipid-like molecules, 1 lignans,neolignans and related compounds, 2 organic acids and derivatives,2 organic oxygen compounds, 7 organoheterocyclic compounds,4 phenylpropanoids and polyketides, and 19 unknown HMDB superclass. Among the 48 negatively ionized metabolites, including 1 alkaloids and derivatives, 3 benzenoids, 19 lipids and lipidlike molecules, 3 organic acids and derivatives, 1 organic nitrogen compound, 4 organic oxygen compounds, 6 organoheterocyclic compounds, 10 phenylpropanoids and polyketides, and 1 unknown HMDB superclass. Orthogonal partial least squares discriminant analysis (PLS-DA) is often used to intuitively show the classification effect of the model. PLS-DA is more sensitive than the other statistical methods to variables with low correlations and was used to identify potential markers of the different groups[32]. The separation of samples in the two groups in the chart with higher degree means classification effect possessing more significant difference. As shown in Fig. 5B, PC1 was 10.20%, and PC2 was 9.31%. The result showed that the metabolites of HZ1 and HZ2 were difference.
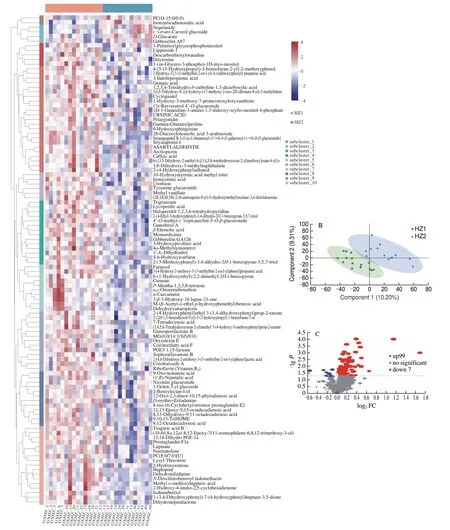
Fig. 5 (A) Heat map visualization. The level of each metabolite was normalized to the complete linkage hierarchical clustering. Each example is visualized in a single column, and each metabolite is represented by a single row. Red indicates high abundance, whereas metabolites with low relative abundance are shown in blue. (B) Orthogonal PLS-DA plot of group metabolites in comparisons of the HZ1 and HZ2 group. (C) The volcano plot shows the differential metabolite expression levels among the group.
To gain an in-depth understanding of the metabolite differences between HZ1 and HZ2, differential metabolite screening of all the 106 metabolites identified in accordance with the fold change were performed. The criteria for significant differences included a fold change of >1 or ≤1, and results are illustrated in Fig. 5C. A total of 106 differential metabolites including 99 upregulated and 7 downregulated were identified in HZ1 group. Therefore, drinking the soakingM. domeri(Bois) Chev. leaves or not could influence the human metabolite.
As shown in Fig. 6, 9 compounds (i.e. haplopine, farnesol,genipic acid, momordicinin, 2-hydroxyestrone, hydroxyphenyllactic acid, caffeic acid, sophoraflavanone B, and soyasaponin I) were detected and quantified in the HZ1 and HZ2 samples. These 9 compounds were clearly enriched in HZ1. We used Spearman’s to analyze the correlation between the gut microbiota (at the genus level) and metabolites, which appeared to be significantly correlated(Fig. 7). The abundance ofRuminococcus_gnavus_group was positively correlated with the soyasaponin I, genipic acid and caffeic acid concentration.Ruminococcus_torques_group,Romboustsia,Roseburia,Dorea,Klebsiella,FusicatenibacterandPrevotellawere positively correlated with soyasaponin I.Escherichia-Shigellawas negatively correlated with genipic acid, hydroxyphenyllactic acid,2-hydroxyestrone, haplopine, farnesol and momordicinin. Farnesol was positively correlated withBlautiaandRuminococcusbut negatively correlated withEscherichia-Shigella, and haplopine was positively correlated withBacteroidesbut negatively correlated withEscherichia-Shigella.
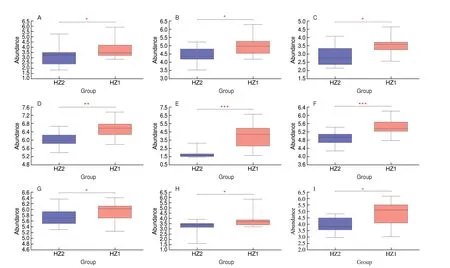
Fig. 6 The relative abundance of (A) 2-hydroxyestrone, (B) caffeic acid, (C) farnesol, (D) genipic acid, (E) haplopine, (F) momordicinin, (G) hydroxyphenyllactic acid, (H) sophoraflavanone B and (I) soyasaponin I. Significant correlation between the concentrate and forage groups, *P < 0.05; **P < 0.01; ***P < 0.001.

Fig. 7 Correlation analysis between fecal metabolites and fecal bacterial genus-level taxa (abundance in the top 30) in HZ1 and HZ2 group. Each column in the figure represents a sample, each row represents a metabolite, and the color indicates the relative amount of metabolites expressed in the group;Blue indicates that the metabolite is expressed at high levels, and red indicates lower expression. Significant correlation between the concentrate and forage groups,*P < 0.05; **P < 0.01, ***P < 0.001.
KEGG enrichment and pathway analysis (Fig. 8) and the detailed information was listed in Table 2. Four metabolic pathways were enriched (P≤ 0.05) between the HZ1 and HZ2 groups, including terpenoid backbone biosynthesis,N-glycan biosynthesis, drug metabolism-other enzymes and ascorbate and aldarate metabolism.
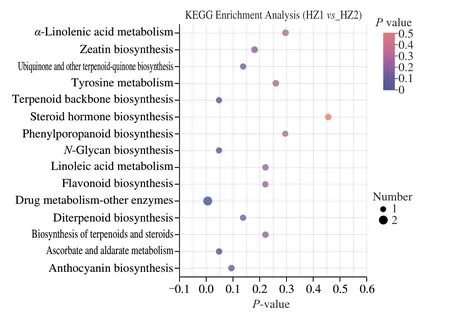
Fig. 8 KEGG enrichment analysis of identified different metabolites of HZ1 and HZ2. Generally, P-value less than 0.05 is considered as a significant enrichment term. The size of the bubble in the figure represents the number of metabolites enriched into the pathway.
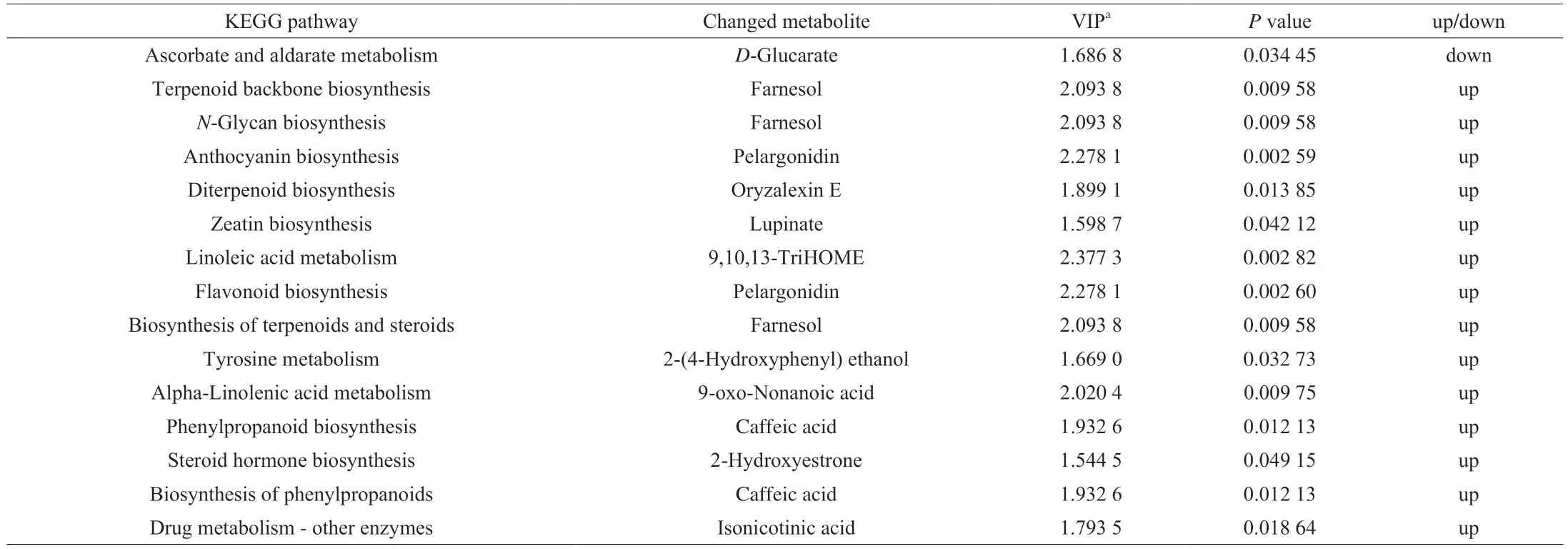
Table 2 KEGG pathway enrichment of the changed metabolites for HZ1 versus HZ2.
4. Discussion
Changes in diets can certainly regulate body metabolism. The diversity and richness of intestinal species play an important role in maintaining intestinal health. The diversity of the gut microbiota was higher, which may have kept the local residents healthy and allowed them to live long lives, which may be closely related to their living environment and eating habits[33]. There is a significant difference in intestinal microbial species and abundance between long-lived elderly people and other individuals[33]. The diversity and richness might represent a reasonable explanation for the longevity of people in Hezhou, Guangxi, China. Theα-diversity metrics of the HZ1, AS and YS groups were higher than those in AN and YN groups of HZ2,respectively. This result means that the species richness and diversity of the HZ1 (AS and YS) were higher than those of the HZ2 (AN and YN), respectively.Ruminococcusspecies, with a higher richness in the HZ1 (AS and YS) than HZ2 (AN and YN), can produce SCFAs,reducing the generation of intestinal inflammation[34-35]. In this study,at the phylum level, drinking the soakingM. domeri(Bois) Chev.leaves resulted in high abundances of Bacteroidota and Proteobacteria and a low abundance of Firmicutes, which was similar to a report that healthy long-living elderly individuals had a higher abundance of Bacteroidetes[36]. At the genus level, drinking the soakingM. domeri(Bois) Chev. leaves could increase the abundances ofBacteroides,Ruminococcus,Ruminococcus_gnavus_group, andRuminococcus_torques_group, and decrease the abundance ofEscherichia-Shigella.As reported,Escherichia-Shigellaspecies are human pathogens[37],which are less abundant in the gut microbiome of healthy aging people[38], whileRuminococcusspecies are a common longevity signature[32]. These consistent conclusions further confirm our hypothesis that drinking the soakingM. domeri(Bois) Chev. leaves could benefit human health.
Notably, these metabolomic data showed obvious differences between the HZ1 and HZ2 groups. Drinking the soakingM. domeri(Bois) Chev. leaves significantly altered the gut microbiota and upregulate haplopine, farnesol, genipic acid,momordicinin, 2-hydroxyestrone, hydroxyphenyllactic acid, caffeic acid, sophoraflavanone B, and soyasaponin I. To gain an in-depth discussion of the metabolite differences between long-lived people and younger elderly who drinking or not drinking the soakingM. domeri(Bois) Chev. leaves, we found that 9 compounds were clearly enriched in AS and YS groups (Fig. S3 and Fig. S4). The compounds of 2-hydroxyestrone, haplopine and hydroxyphenyllactic acid were significant differences in YS and YN groups, while genipic acid and soyasaponin I were significant differences in AS and AN groups. Haplopine exhibit antioxidant and anti-inflammatory can inhibited the mRNA expressions of inflammatory cytokines IL-6[39-40].Farnesol is a C15organic isoprenol synthesized by plants and mammals with antioxidant, anti-inflammatory, and neuroprotective activities[41]. Momordicinin as antidiabetic agent using studies[42].Genipic acid can inhibit the growth ofTrichophyton menrugruphytes,Chlorella vulgarisandTetruhymena gelleii[43]. Zhao et al.[44]studied on the effect of leaf ofLycium barbarumL. (LLB) on type 2 diabetic mellitus (T2DM) rat, the metabolomics could reverse T2DM upregulate caffeic acid. 2-Hydroxyestrone as anti-proliferative metabolites can inhibit the proliferation of MCF-7 cells[45]. As report that the variation of the amounts of hydroxyphenyllactic acid indicate that the supplementation induced changes to the activity of gut microbiota[46]. Sophoraflavanone B, a known prenylated flavonoid,exhibit antibiotics againstStaphylococcus aureus[47]. Soyasaponin I is believed to be beneficial for human health, and some studies shown that soyasaponin I has anti-carcinogenic, hepatoprotective and antiviral activities[48]. Therefore, according to the analysis of metabolites we conjecture drinking the soakingM. domeri(Bois)Chev. leaves could promote human health.
In brief, we can definitively conclude that drinking the soakingM. domeri(Bois) Chev. leaves are correlated with the health of individuals in Hezhou city. In this study, there were many special taxa of Lachnospiraceae, Desulfovibrionaceae,Deinococcus,Verrucomicrobia and Streptococcaceae were identified in the intestines of elderly individuals in Hezhou city. We combined microbiome and metabolomic analyses to identify associations between the specific bacterial genera and metabolites in the soakingM. domeri(Bois) Chev. leaves. This study provides a new understanding of the role of the intestinal flora of elderly individuals in longevity in Hezhou.
5. Conclusions
In this study, 28 valid volunteers and younger elderly were recruited from Hezhou city. The 16S rRNA results showed that drinking soaking ofM.domeri(Bois) Chev. leaves can increase Ruminococcaceae andPrevotellaand decreaseEscherichia-Shigella.A total of 106 fecal metabolites were detected and annotated drinking soaking ofM.domeri(Bois) Chev. leaves and upregulated levels of haplopine, farnesol, genipic acid, momordicinin, 2-hydroxyestrone,hydroxyphenyllactic acid, caffeic acid, sophoraflavanone B, and soyasaponin I. The gut microbiota and metabolites were different of long-lived people and younger elderly who do or do not drink the soaking ofM.domeri(Bois) Chev. Indeed, age has a certain effect on intestinal flora and metabolites, but the intervention of drinking the soaking ofM.domeri(Bois) Chev. would play a more important leading role on intestinal flora and metabolites. Therefore, we preliminarily determined thatM.domeri(Bois) Chev. leaves consumption may be an important factor affecting longevity in this area.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (32072193) and the Natural Science Foundation of Guangxi, China (2020GXNSFBA297083).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.26599/FSHW.2022.9250110.
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango