Emulsifying property, antioxidant activity, and bitterness of soybean protein isolate hydrolysate obtained by Corolase PP under high hydrostatic pressure
2024-01-24HainingGuanChunmeiFengMinRenXiaojunXuDengyongLiuXiaoqinDiao
Haining Guan, Chunmei Feng, Min Ren, Xiaojun Xu, Dengyong Liu, Xiaoqin Diao
College of Food Science and Technology, Bohai University, Jinzhou 121013, China
Keywords: Soybean protein isolate High hydrostatic pressure Emulsif ication Antioxidant Bitter taste
ABSTRACT Enzymatic hydrolysis of proteins can enhance their emulsifying properties and antioxidant activities.However, the problem related to the hydrolysis of proteins was the generation of the bitter taste. Recently,high hydrostatic pressure (HHP) treatment has attracted much interest and has been used in several studies on protein modif ication. Hence, the study aimed to investigate the effects of enzymatic hydrolysis by Corolase PP under different pressure treatments (0.1, 100, 200, and 300 MPa for 1–5 h at 50 °C) on the emulsifying property, antioxidant activity, and bitterness of soybean protein isolate hydrolysate (SPIH). As observed,the hydrolysate obtained at 200 MPa for 4 h had the highest emulsifying activity index (47.49 m2/g) and emulsifying stability index (92.98%), and it had higher antioxidant activities ( 44.77% DPPH free radical scavenging activity, 31.12% superoxide anion radical scavenging activity, and 61.50% copper ion chelating activity). At the same time, the enhancement of emulsion stability was related to the increase of zeta potential and the decrease of mean particle size. In addition, the hydrolysate obtained at 200 MPa for 4 h had a lower bitterness value and showed better palatability. This study has a broad application prospect in developing food ingredients and healthy foods.
1. Introduction
Soy protein isolate (SPI) has been used in more than 6 000 different food products beacuse of its unique emulsifying and foaming properties[1]. The conversion of SPI into hydrolysates could be a novel idea with some specific bioactive activities[2]. Enzymatic hydrolysis is a safe, simple and economical method to enhance emulsifying and antioxidant activities of plant proteins[3]. The way could also increase solubility and surface hydrophobicity of plant proteins and enhance the adsorption of protein molecules at the oil-water interface[4]. Proteolytic active enzymes hydrolyze peptide bonds to produce peptides of different sizes[5]. Zhang et al.[6]also demonstrated that enzymatic hydrolysis was expected to obtain small soluble peptides and expose hydrophobic residues from proteins. Mild protein hydrolysis can be used to prepare hypoallergenic foods[7]. However,protein hydrolysis can also influence the sensory characteristics of proteins, especially the formation of bitterness, which restricts the use of protein hydrolysates as food ingredients. The bitterness of protein hydrolysates mainly ref lects the release of hydrophobic amino acid residues[8]. In this regard, it has been reported that exopeptidase treatment can reduce the bitterness of protein hydrolysates[9].
High hydrostatic pressure (HHP) treatment can cause changes in proteic conformation and structure, and has been used to improve the texture and taste of food[10]. In addition, the HHP process could induce partial unfolding of proteins, consequently, proteins exposure to the attack of proteolytic enzymes[11]. Gharibzahedia et al.[12]reported that high-pressure treatment could enhance physicochemical, functional and digestibility properties of protein hydrolysates prepared from legume crops. Zhang et al.[13]also indicated that enzymatic hydrolysis under HHP was beneficial for releasing antioxidant peptides. Some studies have noted that HHP treatment could enhance enzymatic hydrolysis of food-derived proteins and promote the production of bioactive peptides by using a large spectrum of enzymes[14].
Furthermore, Corolase PP (food-grade porcine pancreatic proteolytic preparation) is a complex mixture of trypsin,chymotrypsin, elastase, and carboxypeptidase that acts synergistically proteins leading to extensive degradation[15]. Jaiswal et al.[16]reported that compared with flavourzyme and alcalase, relatively higher degree of hydrolysis achieved by Corolase PP was due to very broad specificity. Some studies have shown that using Corolase PP to control enzymatic hydrolysis of SPI was a very useful tool that can produce hydrolysates having antioxidant and antihypertensive activities[17-18].To the best of our knowledge, there are few studies about the effects of enzymatic hydrolysis by Corolase PP during HHP treatment on the emulsifying and antioxidant properties of hydrolysates of SPI.
Therefore, the objective of this study is to evaluate the effects of HHP combined with Corolase PP treatments on the emulsifying characteristics and antioxidant activity. In addition, to better understand the potential use of soybean protein isolate hydrolysate(SPIH) as natural functional ingredient in food, the bitterness of hydrolysates was investigated. This research provided a novel perspective to obtain functional hydrolysates from SPI by using this technology in the food industry.
2. Materials and methods
2.1 Materials and reagents
Defatted soy flakes were obtained from the Harbin High-Tech Group (Harbin, Heilongjiang, China). The commercial enzyme complex Corolase PP (4 000 U/g) was purchased from Nanning Alvaropombo Biological Engineering Co. (Nanning, Guangxi,China). 1,1-Diphenyl-2-picryl hydrazyl (DPPH), phenazine methosulphate (PMS), nitroblue tetrazolium (NBT), and nicotinamide adenine dinucleotide (NADH) were purchased from Sigma Chemical Co. (St. Louis, MO). Other chemicals and reagents used were analytical grades.
2.2 Preparation of hydrolysates from SPI
SPI was prepared according to the procedure of Jiang et al.[19], and SPI was hydrolyzed by Corolase PP using a HHP instrument (HHP-400 MPa, Ren-He Electromechanical Engineering Co., Shenyang,China) with water as pressurizing medium according to our previous method[10]. Once the sealed samples were placed into the water chamber, water was heated from the initial temperature of 25 °C to the final temperature of 50 °C. The experiments were performed at different pressures (0.1, 100, 200, and 300 MPa) and 50 °C for different times (1–5 h). The values for the CUT (come up time) were about 0.5, 1.0, and 1.5 min for 100, 200 and 300 MPa, respectively,and decompression was almost instantaneous. After hydrolysis, the solution containing Corolase PP was heated in boiling water for 10 min to inactivate the enzyme, and then centrifuged at 10 000 ×gfor 10 min at 4 °C. The supernatants were freeze-dried and stored at 4 °C before further utilization.
2.3 Determination of emulsifying properties
Emulsifying activity index (EAI) and emulsifying stability index(ESI) were measured according to the method described previously by Wani et al.[20]with slight modifications. HHP enzymatic hydrolysates of SPI were dissolved respectively in borate buffer (0.1 mol/L, pH 6.5)to obtain a final protein concentration of 0.5 mg/mL. 2 mL of soybean oil was added to 8 mL of sample solution and homogenized at 16 000 ×gfor 2 min using an IKA T 18 Ultra-Turrax (IKA-Werke GmbH & Co., Staufen, Germany). Subsequently, aliquots (50 μL each) of prepared emulsions were taken from the bottom of the container at 0 and 10 min respectively and were dispersed in 5 mL of 0.1% sodium dodecyl sulfate solution, and absorbances were measured at 500 nm. The EAI and ESI were calculated according to the following equations:
Wherecwas the SPIH concentration (g/mL),φwas the oil volume fraction of the emulsion (V/V), andA0andA10were the absorbances at 500 nm after emulsification for 0 and 10 min, respectively.
2.4 Determination of zeta potential
The zeta potentials of emulsions with SPIHs obtained under different pressures were determined at room temperature using static light scattering with a Nano ZS series particle analyzer (Malvern Instruments Co. Ltd., Malvern, UK).
2.5 Determination of particle size distribution
The particle size distribution of emulsions with SPIHs obtained after 4 h of hydrolysis under different pressures was measured using static light scattering with a Malvern Master Sizer 2000 instrument(Malvern Instruments Co. Ltd., Malvern, UK). Before analysis, the emulsion was diluted to a suitable concentration with deionized water to avoid multiple scattering effects[21].
2.6 Determination of antioxidant activity
2.6.1 DPPH free radical scavenging activity
DPPH free radical scavenging activity was determined according to the method described previously by Bougatef et al.[22]with slight modifications. An aliquot of 2 mL sample solution was mixed with 2 mL of DPPH solution (0.2 mmol/L in 95% ethanol). The mixtures were reacted for 30 min at room temperature in the dark.The absorbance was measured at 517 nm using a spectrophotometer(Shimadzu UV-1800, Shimadzu Co., Kyoto, Japan). DPPH free radical scavenging activity was determined according to the following formula:
WhereAiwas the absorbance of the sample containing DPPH;Ajwas the absorbance of the sample containing 95% ethanol; andA0was the absorbance of the blank solution containing DPPH and 95%ethanol.
2.6.2 Superoxide anion radical scavenging activity
The superoxide anion radical scavenging activity was measured by the method of Kaur et al.[23]with slight modifications. Briefly, 0.1 mL of PMS solution (60 µmol/L PMS in 0.1 mol/L phosphate buffer) was mixed with 1 mL of NBT solution (0.156 mmol/L NBT in 0.1 mol/L phosphate buffer, pH 8.0), 1 mL NADH solution (0.468 mmol/L in 0.1 mol/L phosphate buffer, pH 8.0), 0.1 mL of sample solution(1 mg/mL), and subsequently incubated in a water bath for 5 min at 25 °C. After the reaction, the absorbance was measured at 560 nm.Superoxide anion radical scavenging capacity was calculated using the following equation:
WhereA0was the absorbance of the blank solution, andA1was the absorbance of sample solution.
2.6.3 Copper ion chelating activity
Copper ion chelating ability assay was investigated according to the procedure of Saiga et al.[24]with slight modifications. Briefly, 1 mL of CuSO4(2 mmol/L) was mixed with 1 mL of pyridine solution(100 mg/mL) and 0.02 mL of pyrocatechol violet (1 mg/mL). After adding 1 mL sample solution (1 mg/mL), the change of blue colour was monitored by determining the absorbance at 632 nm using a UV-visible spectrophotometer (Shimadzu UV-1800, Shimadzu Co.,Kyoto, Japan). The copper ion chelating ability by hydrolysates was calculated according to the following equation:
WhereA0was the absorbance of the blank solution, andAswas the absorbance of sample solution.
2.7 Bitterness evaluation
The bitterness evaluation of hydrolysates was determined by the method of Suhet al.[25]. The freeze-dried SPIHs obtained under HHP were reconstituted to 3% in water. Caffeine solutions of different concentrations were used as bitterness standards as follows:0, not bitter (no caffeine); 1, trace of bitterness (0.025%); 2, slightly bitter (0.05%); 3, bitter (0.1%); 4, very bitter (0.2%); 5, extremely bitter (0.3%).
The sensory assessment panel consisted of eight members who were required to be sensitive to bitterness and be able to identify differences in bitterness. Take a spoonful (about 3.5 mL) of the sample, kept in the mouth for 10 s, and then expectorated. The strength of bitterness was evaluated in comparision with the caffeine solutions. Water was served for palate cleansing before tasting the next sample. Sensory evaluation was performed in triplicate.
2.8 Statistical analysis
Statistical analysis was carried out according to the method described by Chen et al.[26]. For every batch of samples, all of the specific experiments were conducted three times (triplicate observations). The results were expressed as the mean values ±standard errors (SE). All data were analyzed using the General Linear Models procedure of the Statistix 8.1 software package (Analytical Software, St. Paul, MN, USA). The significance of the main effects was handled by analysis of variance (ANOVA) with Duncan’s multiple comparisons (P< 0.05).
3. Results and discussion
3.1 Emulsifying properties
EAI and ESI of HHP enzymatic hydrolysates of SPI are shown in Fig. 1. At various pressure conditions, the EAI and ESI were all increased with increasing hydrolysis time. Compared to non-hydrolysed SPI (21.75 m2/g), the EAI was significantly increased to 30.81, 36.95, 44.62, and 47.49 m2/g, respectively (P< 0.05) from 1 to 4 h of hydrolysis at 200 MPa. This phenomenon may be attributed to the fact that protein conformation was changed, and hydrophobic amino acid chains embedded in the protein were exposed[27].Furthermore, more polypeptides were produced with an increase of hydrolysis time, which contributed to the diffusion of hydrolysates at the oil-water interface and reduced the interfacial tension to improve the emulsifying activity[28]. In addition, compared to non-hydrolysed SPI (33.9%), the ESI of hydrolysates was significantly increased to 78.87%, 86.37%, 90.26%, and 92.98% (P< 0.05) from 1 to 4 h of hydrolysis at 200 MPa. A possible reason for the improvement of emulsion stability may be that the orderly arrangement of protein hydrolysates at the oil-water interface formed a viscoelastic film, which could protect the emulsion from being destroyed.Falade et al.[29]also reported that sweet potato protein hydrolysate obtained under HHP could quickly diffuse to the emulsion interface and form a thick viscoelastic film around the surface of each droplet,which might retard the coalescence of droplets and improve the stability of the emulsion. However, when the hydrolysis time reached 5 h, EAI and ESI were decreased, which might be attributed to the reduction of the protein chains size and a significant decrease in surface hydrophobicity[4]. A similar observation was also reported by Lopes-da-Silva et al.[30], who demonstrated that excessive enzymatic hydrolysis caused a further reduction in the emulsifying capability of soy protein hydrolysates.
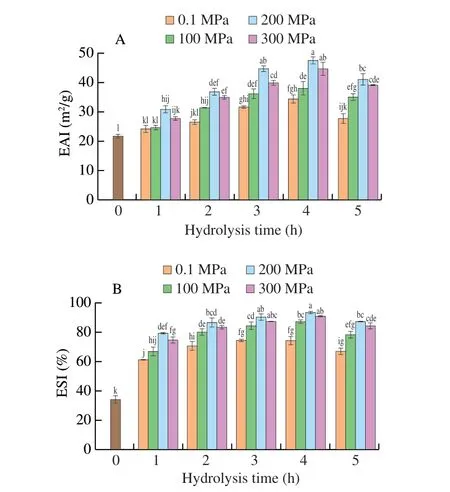
Fig. 1 EAI and ESI of SPIH obtained under different pressures as a function of hydrolysis time. Error bars represent standard error obtained from triplicate sample analyses. Different letters above the bars indicate significant differences (P < 0.05).
Furthermore, at atmospheric pressure (0.1 MPa), the EAI and ESI of hydrolysates were 34.32 m2/g and 74.03% respectively after 4 h-hydrolysis. After HHP treatment, significant increases in EAI and ESI were observed at 200 and 300 MPa, which were 47.49 m2/g and 92.98% for 200 MPa and 44.72 m2/g and 90.45% for 300 MPa,respectively (P< 0.05). This result may be attributed to the amount and structure of peptides produced by hydrolysis under appropriate pressure contributed to the improvement of emulsifying properties[29]. However, the EAI and ESI of SPIH obtained at 300 MPa were lower than at 200 MPa for the same hydrolysis time,which may be due to the protein aggregation or partial denaturation of enzymes under higher pressure conditions[31]. EAI and ESI of peptides were influenced by various factors, such as the molecular sizes, hydrophilicity/hydrophobicity, and the ability to diffuse to the interface surface and form a film to prevent droplets from gathering[32].Al-Ruwaihet al.[33]also found that the reason for the decrease in EAI and ESI of hydrolysates could be related to the small peptide fractions produced by the synergic effect of pressure and enzyme.Ahmed et al.[34]reported that the creation of small peptides could decrease the EAI and ESI of hydrolysates because of weak viscoelastic film formation at the oil-water interface, which was inadequate to resist the merger of adjacent droplets.
3.2 Zeta potential
The zeta potential reflects the potential stability of an emulsion system in light of its electrostatic interactions. Generally, the electrostatic repulsion caused by the same charge hinders droplet coalescence and improves the stability of the emulsion. Yan et al.[35]indicated that the higher electrostatic repulsion was attributed to the higher zeta potential, which could effectively inhibit the aggregation of emulsion droplets. Guzeyet al.[36]also demonstrated that the low zeta potential of emulsion droplets reduced the electrostatic repulsion among adjacent protein-coated droplets, which could not overcome various interactions, leading to droplet coalescence. In other words,emulsions prepared with higher absolute values had better stability.
The negative zeta potentials of the emulsion formed by all hydrolysates can be mainly attributed to the charge of the interface membrane. As displayed in Fig. 2, under different pressure conditions, the absolute zeta potential of SPIHs was increased significantly from 1 to 4 h of hydrolysis, and then significantly reduced at 5 h. Furthermore, the absolute zeta potential was enhanced by improving the pressure from 0.1 to 200 MPa (P< 0.05), and when the pressure rose to 300 MPa, the absolute zeta potential decreased.The phenomenon may be attributed to the fact that emulsions have different types of hydrolysates obtained under different pressures or hydrolysis time. In other words, the change in the absolute value of the zeta potential may be related to the different hydrolysates adsorbed on the surfaces of droplets[37]. The observation was in agreement with the result of the stability of the emulsion. However,Wang et al.[38]reported that rice bran protein hydrolysates pretreated at 100 MPa had a larger negative zeta-potential in the dispersion and showed better colloidal stability. The result was not consistent with our result, which may be due to different protein raw materials and processing methods.
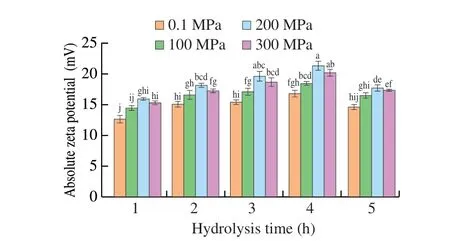
Fig. 2 Zeta potentials of SPIHs obtained under different pressures as a function of hydrolysis time. Error bars represent standard error obtained from triplicate sample analyses. Different letters above the bars indicate significant differences (P < 0.05).
3.3 Droplet size distributions
The distribution of droplet size is one of the important parameters to evaluate the stability of the emulsion. The authors generally agree that the smaller the droplet size was, the more stable the emulsion was[39]. The droplet size distribution of HHP enzymatic hydrolysates of SPI was shown in Fig. 3. With the increase of pressure, the distribution peaks of emulsions containing SPIHs shifted to the left in the direction of smaller sizes, and the emulsion with SPIH obtained under 200 MPa had the most leftward shift when particle diameter was less than 100 μm. Similar observations were reported by Ahmed et al.[34],who demonstrated that the high pressure (300 MPa) and alcalase(0.5%) treatment of lentil protein could produce smaller particle sizes compared to a control treatment (0.101 MPa). A possible explanation for this phenomenon was that the increasing susceptibility of the enzyme under higher hydrolysis pressure that breaked down the larger protein molecules into smaller fragments of peptides, and they could rapidly move to the interface during emulsification to form a protective layer against droplet accumulation[40]. Thus, the SPIH obtained at 200 MPa played a more important role in improving emulsion stability compared to those obtained at other pressures.
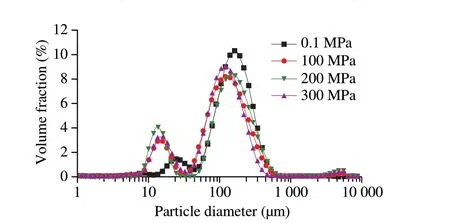
Fig. 3 Droplet size distribution of emulsions with SPIHs obtained after 4 h of hydrolysis under different pressures.
3.4 Antioxidant activities
3.4.1 DPPH free radical scavenging activity
DPPH is a stable free radical. Its ethanol solution is purple and exhibits maximum absorbance at 517 nm. Antioxidants deliver electrons or hydrogen atoms to DPPH, thus neutralizing its free radical[41]. When DPPH radicals react with an antioxidant, the purple DPPH solution will change to yellow, decreasing absorbance[42]. In the current study, the DPPH free radical scavenging ability of SPIHs obtained under different pressures (0.1–300 MPa) were evaluated. As presented in Fig. 4, all hydrolysates showed that DPPH free radical scavenging ability was improved with increasing hydrolysis time at the same pressure condition or increasing pressure at the same hydrolysis time. The highest DPPH free radical scavenging ability of hydrolysates after HHP treatment at 200 MPa was obtained at 5 h of hydrolysis (45.90%), but there was no significant change with hydrolysis for 3 (42.76%) and 4 h (44.77%) (P> 0.05). This finding may be explained by the fact that high-pressure induced the exposure of Corolase PP-susceptible peptide bonds in SPI[43], which resulted in the release of peptides with higher antioxidant activity, and the antioxidative peptides could access and capture the DPPH radical to inhibit its chain reaction[44]. Hemker et al.[32]noted that pressureassisted enzymatic hydrolysis could enhance the antioxidant activity of tilapia by-product protein hydrolysates. Zheng et al.[45]also found that black bean protein hydrolysates under different hydrolysis times showed excellent DPPH free radical scavenging ability.Furthermore, when pressure increased to 300 MPa, the DPPH free radical scavenging ability decreased to 44.32% after 5 h of hydrolysis,which had no significant difference with that at 200 MPa (P> 0.05).This phenomenon may be attributed to the aggregation of protein or partial denaturation of the enzyme at 300 MPa, leading to a slight decrease in antioxidative peptides[10]. Another reason may be that the content of hydrophobic peptides in the hydrolysate obtained at 300 MPa decreased, resulting in the reduction of the hydrophobic DPPH free radical scavenging activity[46]. Dong et al.[47]also reported that the DPPH free radical scavenging ability of peanut protein isolate hydrolysates depended mainly on the appropriate HHP treatment level.
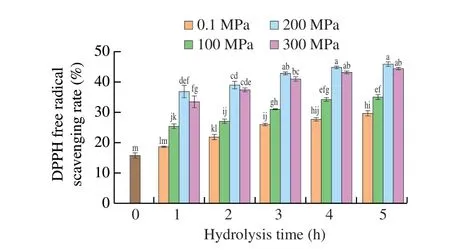
Fig. 4 DPPH free radical scavenging activity of SPIHs obtained under different pressures as a function of hydrolysis time. Error bars represent standard error obtained from triplicate sample analyses. Different letters above the bars indicate significant differences (P < 0.05).
3.4.2 Superoxide anion radical scavenging activity
The superoxide anion radical is active and can induce oxidative damage to lipid, protein and DNA in the body. The superoxide anion radical scavenging rate of SPIHs obtained at different pressures(0.1–300 MPa) followed a trend similar to the result of DPPH free radical scavenging ability (Fig. 5). After 5 h of hydrolysis, the scavenging ability of superoxide anion radical was enhanced from 23.72% at 0.1 MPa to 32.63% at 200 MPa, while a slight decrease was observed at 300 MPa (31.13%). Although the hydrolysate had the highest superoxide anion radical scavenging ability after 5 h hydrolysis at 200 MPa (32.63%), there was no significant difference compared with that at 4 h (31.12%) (P >0.05). A possible explanation for these findings was that hydrolysates obtained under different pressure conditions and increased hydrolysis time contained different kinds and amounts of peptides, which may terminate the radical chain reaction by trapping free radicals[48]. Suetsuna et al.[49]reported that enzymatic hydrolysis increased the exposure of tyrosine,proline, glutamic acid and leucine, and showed strong superoxide radical scavenging activity. Our previous study also demonstrated that antioxidant amino acids, such as tyrosine, methionine, lysine, histidine,phenylalanine, and cysteine were present in the SPIHs obtained at 200 and 300 MPa[10]. Another reason may be that these superoxide radicals were scavenged through electrons provided from hydrolysates after HHP treatment. This observation also agreed with the result of Zhang et al.[13],who showed that the chickpea protein isolates hydrolysates obtained at HHP have a higher capture rate of superoxide anion.

Fig. 5 Superoxide anion radical scavenging activity of SPIHs obtained under different pressures as a function of hydrolysis time. Error bars represent standard error obtained from triplicate sample analyses. Different letters above the bars indicate significant differences (P < 0.05).
3.4.3 The capacity to copper ion chelating
In addition to playing a vital role as an essential trace mineral,copper also has similar activity to iron in promoting oxidation.Zhang et al.[50]noted that hydroxyl radicals could be formed from superoxide anion and hydrogen peroxide in the presence of copper ion. Megías et al.[51]reported that copper could cause oxidative damage to DNA and low-density lipoprotein. Thus, the chelating ability of copper ions is an important index for evaluating antioxidant capacity. Copper ion chelating activity of SPIHs obtained at different pressures as a function of hydrolysis time was depicted in Fig. 6. All hydrolysates showed that the chelating ability of copper ions was improved with increasing hydrolysis time under different pressure conditions compared with non-hydrolyzed SPI. The hydrolysate obtained at 200 MPa after 5 h hydrolysis showed the highest ability to chelate copper ion (64.68%), but did not differ significantly from hydrolysis for 4 h (61.50%) (P >0.05). The finding may be explained by the fact that the copper ion chelating activity was enhanced with the increasing degree of hydrolysis (DH) of SPI. Our previous study found that the DH of SPI treated at 200 MPa for 4 h reached 30.6%,which was not significantly different from that 5 h (DH 31.4%)[10].Liu et al.[52]also noted chelation of copper ions increased with increasing DH.Hemker et al.[32]reported that the HHP accelerated the hydrolysis of fish protein, facilitated the release of free amino acids, and improved the antioxidant activity of protein hydrolysates. Peng et al.[53]reported hydrolysis of whey protein isolate enhanced Cu2+chelation, possibly due to the increased concentration of carboxylic and amino groups in the acidic and basic amino acids. Fan et al.[54]also indicated that acidic amino acids such as Asn and Glu played important roles in the chelation of metal ions through their side chains.
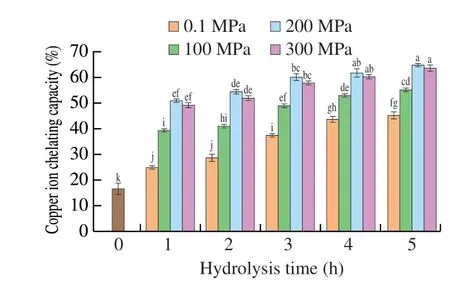
Fig. 6 Copper ion chelating capacity of SPIHs obtained under different pressures as a function of hydrolysis time. Error bars represent standard error obtained from triplicate sample analyses. Different letters above the bars indicate significant differences (P < 0.05).
3.5 Bitterness evaluation
The enzymatic hydrolysis of SPI by Corolase PP at high hydrostatic pressure could enhance its emulsifying property and antioxidant activity. However, one of the main problems related to enzymatic hydrolysis of SPI was the generation of bitterness[55].Cui et al.[56]reported that bitterness of milk protein hydrolysates increased with increasing DH. A possible reason was that bitter peptides chiefly composed of hydrophobic amino acid residues increased as the DH increased. The panel was invited to assess the bitterness of the hydrolysates under different pressures as a function of hydrolysis time based on a six-point scale, and the results were shown in Fig. 7. The results suggested that the bitterness intensity of SPIHs significantly increased (P< 0.05) with the increase of pressure and hydrolysis time. When the hydrolysis time reached 3 h, the bitterness value was the largest after HHP treatment at 200 MPa, but there were no significant differences (P> 0.05) with the treatment at 300 MPa. The finding may be illustrated by the fact that the Corolase PP activity was improved, and the protein structure occurred with exposure of more cleavage sites at 200 MPa, thus increasing the DH,exposing the bitter amino acids originally hidden in the internal of the protein struture to the surface of hydrolysed short peptide chains,and eventually enhancing the bitterness value[34]. Peñas et al.[57]reported that a dramatic decrease of DH occurred when the soybean whey proteins were treated at 300 MPa, and the bitterness decreased.Another reason may be that higher amounts of small peptides(< 3 kDa) were produced at 200 MPa than at 300 MPa[10]. Cho et al.[58]noted that the bitterness increased as the peptide molecular weight decreased from 3 000 to 2 000 Da. Schlegel et al.[8]also demonstrated that the bitterness of protein hydrolysates primarily reflected the release of low molecular weight peptides. Additionally, Belitz et al.[59]reported that the peptides with high content of hydrophobic amino acids could cause bitterness, and the bitterness of amino acids,dipeptides, and tripeptides was quantitatively related to their hydrophobicity. In terms of removing bitterness, it has been reported that the bitterness of protein hydrolysates could be reduced by the removal of bitter peptides[9]. Noguchi et al.[60]also reported that free aspartic and glutamic acids were useful for masking bitterness.However, in our study, the bitterness value decreased when the hydrolysis time was above 3 h, which may be attributed to the hydrolysis of some bitter peptides to free amino acids reduced the hydrophobicity and decreased their bitterness because of a higher DH. Sun et al.[55]also reported that a certain degree of hydrolysis could reduce the bitterness by splitting bitter peptides into free amino acids. Sensory analyses indicated that the SPIH obtained after 4 h of hydrolysis at 200 MPa had better palatability.

Fig. 7 Bitterness of SPIHs obtained under different pressures as a function of hydrolysis time. Error bars represent standard error obtained from triplicate sample analyses. Different letters above the bars indicate significant differences (P < 0.05).
4. Conclusion
Enzymatic hydrolysis using Corolase PP under HHP treatment affected the emulsifying properties and antioxidant activity of SPIHs. The EAI and ESI of SPIHs at different pressures were significantly improved, which were associated with the increase of absolute zeta potential and the decrease of mean particle diameter.At the same hydrolysis time, the EAI and ESI of SPIH obtained by 4 h of hydrolysis at 200 MPa were significantly higher than for other treatments (P< 0.05). In addition, the changes in DPPH free radical scavenging activity, superoxide anion radical scavenging activity, and copper ion chelating activity demonstrated that the SPIH obtained by 5 h of hydrolysis at 300 MPa had the highest antioxidant activity, but there were no obvious differences with hydrolysis for 4 h at 200 MPa(P> 0.05). Furthermore, the bitterness of the SPIH was the main factor affecting its practical application. We found that the bitterness value of the SPIH obtained after 4 h of hydrolysis at 200 MPa decreased and had better palatability. Overall, the results showed that the SPIH obtained by 4 h of hydrolysis at 200 MPa has good application prospects in developing food ingredients and healthy foods.
Declaration of conflicting interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Doctoral Research Foundation of Bohai University (05013/0520bs006); the Science and Technology Project of “Unveiling and Commanding” Liaoning Province(2021JH1/10400033); and the Scientific Research Project from Education Department of Liaoning Province (LJ2020010).
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango