Differences in the effects and action modes of gut commensals against dextran sulfate sodium-induced intestinal inf lammation
2024-01-24DingwuQuZhennanGuSaisaiFengLeileiYuFengweiTian
Dingwu Qu, Zhennan Gu, Saisai Feng, Leilei Yu, Fengwei Tian,
Hao Zhanga,b,c,d, Wei Chena,b,c, Qixiao Zhaia,b,*
a State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, China
b School of Food Science and Technology, Jiangnan University, Wuxi 214122, China
c National Engineering Research Center for Functional Food, Jiangnan University, Wuxi 214122, China
d Wuxi Translational Medicine Research Center and Jiangsu Translational Medicine Research Institute Wuxi Branch, Wuxi 214122, China
Keywords: Gut commensals Dextran sulfate sodium (DSS) colitis Intestinal barrier Immunoregulation
ABSTRACT Inflammatory bowel disease (IBD) is a complex relapsing inflammatory disease in the gut and is driven by complicated host-gut microbiome interactions. Gut commensals have shown different functions in IBD prevention and treatment. To gain a mechanistic understanding of how different commensals affect intestinal inflammation, we compared the protective effects of 6 probiotics (belonging to the genera Akkermansia,Bifidobacterium, Clostridium, and Enterococcus) on dextran sulfate sodium (DSS)-induced colitis in mice with or without gut microbiota. Anti-inf lammatory properties (ratio of interleukin (IL)-10 and IL-12) of these strains were also evaluated in an in vitro mesenteric lymph nodes (MLN) co-culture system. Results showed that 4 probiotics (belonging to the species Bif idobacterium breve, Bif idobacterium bif idum, and Enterococcus faecalis) can alleviate colitis in normal mice. The probiotic strains differed in regulating the intestinal microbiota, cytokines (IL-10, IL-1β and interferon (IFN)-γ), and tight junction function (Zonulin-1 and Occludin). By constrast, Akkermansia muciniphila AH39 and Clostridium butyricum FHuNHHMY49T1 were not protective. Interestingly, B. breve JSNJJNM2 with high anti-inf lammatory potential in the MLN model could relieve colitis symptoms in antibiotic cocktail (Abx)-treated mice. Meanwhile, E. faecalis FJSWX25M1 induced low levels of cytokines in vitro and showed no benef icial effects. Therefore, we provided insight into the clinical application of probiotics in IBD treatment.
1. Introduction
Inflammatory bowel disease (IBD), a manifestation of gut mucosal damage and local ulceration, is thought to be driven by multiple factors (e.g., unhealthy lifestyles, host inherited susceptibility, altered mucosal immune responses, and unbalanced gut microbiome)[1-2]. Altered diversity and perturbations in gut microbiota have been implicated in patients with IBD. Recent studies have observed a reduction in beneficial microbial species (e.g.,Bif idobacteriumspp.,Lactobacillusspp.,Roseburia intestinalis, andFaecalibacterium prausnitzii), along with increasedRuminococcus gnavus,Fusobacterium nucleatum, pathogenicEscherichia coli,and enterotoxigenicBacteroides fragilis, in patients with active colitis[3-6]. Breech in the epithelial barrier caused by unbalanced gut bacteria triggers secondary inf lammation that is responsible for colitis initiation and continuation. Manipulation of the gut microbiome, such as fecal microbiota transplantation, is an emerging treatment modality for human colitis[7]. A better understanding of the transferable microbes and their modes of action is needed to develop more efficient microbiome-targeted therapies for IBD.
Moreover, several commensal bacteria have shown several potential mechanisms of action related to the remission of colitis.Oral administration ofBifidobacteriumandLactobacillusstrains can prevent experimental colitis by restoring bowel integrity and gut microbiota in mice and reducing the clinical appearance of mucosal inflammation in patients with IBD[8-10].Clostridium butyricumprotects the intestinal barrier function by activating the Akt/mTOR signaling pathway in a mouse model of colitis[11]. Extracellular vesicles derived fromAkkermansia muciniphilaand their enrichment in the intestinal tracthave been shown to alleviate dextran sulfate sodium(DSS)-induced acute colitis in mice[12-15]. In addition,Enterococcus faecaliscan modulate inflammation through mitogen-activated protein >kinase (MAPK) signaling pathways, and secreted antigen A (SagA)fromE. faeciumis sufficient to protect the host againstSalmonellapathogenesis by enhancing pathogen tolerance[16-20]. The protective properties of these microorganisms, including intestinal microbiome restoration, immunoregulation, reinforcement of anti-inflammatory responses, and improvement of the gut barrier, seem to be strain-specific and their major action modes against colitis are unclear.
Production of mucosal proinflammatory cytokines, such as interferon (IFN)-γ, interleukin (IL)-1β, and IL-12, correlates with the endoscopic activity of patients with IBD[21]. A study has shown that cytokines, such as IFN-γ, drive IBD pathogenesis through disruption of the adherens junction protein[22]. Prevention strategies targeting IL-10, a predominantly anti-inflammatory cytokine, have been reported to relieve DSS-induced colitis by downregulating nitric oxide and reactive oxygen species production[23]. A probiotic mixture(Lactobacillus reuteri,Bacillus coagulans,Bifidobacterium longum,andC. butyricum) significantly promoted the expression of IL-10 and improved gut barrier function in a colitis mouse model[24]. Lactobacilli,Bifidobacteria, andC. butyricumcan activate macrophages, resulting in different IL-10 secretion[25-27]. Administration of probiotic strains with IL-10 inducing ability could relieve inflammatory immune responses, including rheumatoid arthritis, allergic rhinitis, and T-cellmediated colitis[28-30]. The above studies have documented a high correlation between the IL-10 inducing ability of gut commensals and its function in the management of pathological inflammation.
Multiple probiotic strains with different functions are reported to alleviate the symptoms of IBD. To obtain a mechanistic understanding of how different beneficial bacteria affect gut inflammation, we herein compared 6 probiotic strains for their capacity to prevent acute colitis in mice with or without gut microbiota and link strain-specificin vitroimmune modulation capacity to their function.
2. Materials and methods
2.1 Bacterial strains
A. muciniphilaAH39 (Akk),C. butyricumFHuNHHMY49T1(Cb),B. bifidumFHNFQ11M4 (B120),B. bifidumFBJ1M4 (B278),B. breveJSNJJNM2 (B97), andE. faecalisFJSWX25M1 (B113) were obtained from the Culture Collection of Food Microbiology, Jiangnan University (Wuxi, China). All strains were isolated from the feces of healthy individuals.
2.2 Animal experiments
Six-week-old male C57BL/6J mice (WT mice) from Shanghai Laboratory Animal Center were housed in a specific pathogen-free room and fed ad libitum. The mice were killed by inhaling 100% CO2at the end of the experiment. All animal experiments were carried out according to the European Community guidelines (Directive 2010/63/EU). This experiment was approved by the ethics committee of Jiangnan University, China (JN No. 20210315c1100425[015] and 20210430c1000625[104]).
2.2.1 Experimental design I
Eighty mice were divided into 8 groups (Table 1). The clinical symptoms of inflammation and body weight were evaluated daily. On day 8 after DSS (35−50 kDa; MP Biomedicals) treatment, all mice were sacrificed.

Table 1 Animal experiment design I.
2.2.2 Experimental design II
Eighty mice were divided into 8 groups (Table 2). Mice were administered with an antibiotic cocktail (Abx) via oral gavage to deplete intestinal microbes in the 1stweek[31-32]. Fecal samples were collected after Abx treatment to ensure depletion of intestinal microbes.
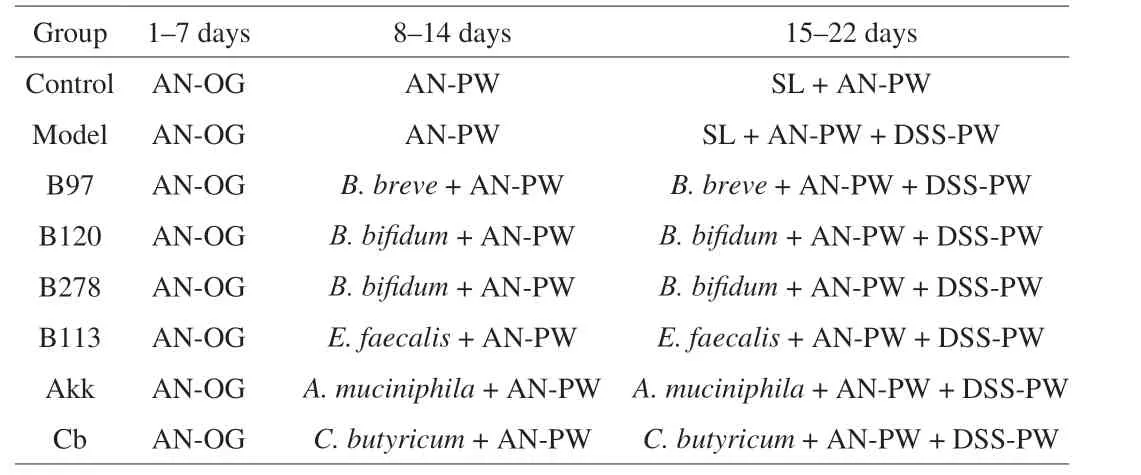
Table 2 Animal experiment design II.
2.2.3 16S rRNA gene sequencing
The 16S rRNA sequencing was conducted as previously described[33]. Briefly, total DNA was extracted from fecal samples using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany)and amplified using 314F and 806R primers, followed by normalization and barcoding. Operational taxonomic unit (OTU)picking was performed using the QIIME2 software package.β-Diversity was analyzed using hierarchical clustering and principal coordinate analysis (PCoA). LEfSe was performed online (https://www.microbiomeanalyst.ca/).
2.2.4 Histopathological studies and cytokine assays
Colonic tissues (0.5 cm) fixed in 4% formalin solution were embedded in paraffin and sectioned at a thickness of 5 mm. The tissue sections were stained with hematoxylin and eosin (H&E) and observed using light microscopy[1]. The IL-10, IL-1β, IFN-γ, and immune globulin A (IgA) levels in the intestinal homogenates and IgG1 levels in the serum were detected using ELISA kits (R&D Systems, USA).
2.2.5 Determination of gut permeability
After fasting for 6 h, all mice were orally administered with DX-4000-FITC at a dose of 500 mg/kg BW. After 1 h, 100 μL of blood samples was taken from mice and centrifuged at 3 500 r/min for 20 min. Fluorescence intensities of serum were detected using a fluorescence microplate (SpectraMax; Molecular Devices, San Jose, CA, USA) at excitation wavelength of 485 nm and emission wavelength of 535 nm.
2.2.6 Quantitative real-time PCR (qPCR)
Total mRNA from colon tissues (20 mg) was extracted using TRIzol and converted to cDNA using RevertAid First Strand cDNA Synthesis Kit (Vazyme Biotech Co., Ltd., Nanjing, China). The primers for the targeted and reference genes were designed, and the qPCR was performed as previously described[34].
2.3 In vitro immune-modulation evaluation
In vitroimmune-modulation evaluation of the different strains was performed according to the methods reported by Verma et al.[35].Briefly, leukomonocytes (1 × 106cells) from mesenteric lymph nodes(MLN) of mice were co-cultured with the test strains at a 1:10 ratio.1 mmol/L anti-CD3 (eBioscience, USA), and anti-CD28 (eBioscience,USA) were added to activate T cells. To prevent bacterial growth,0.15 mg/mL gentamycin was added. After 3 days, IL-10 and IL-12 p70 levels in the culture supernatants were detected using ELISA kits (R&D Systems, USA).
2.4 Statistical analysis
Statistical analysis and data visualization were performed using GraphPad Prism (version 9.1). Differences between the two groups were assessed using thet-test or the non-parametric Mann-Whitney test. Error bars indicate ± SD.*P< 0.05;**P< 0.01;***P< 0.001;****P< 0.000 1.
3. Results
3.1 Oral administration of gut commensals (Bifidobacterium spp. and E. faecalis) ameliorated disease symptoms in DSS-induced acute colitis mice
Treatment with DSS decreased the body weight and colon length in mice compared to that in control mice. Mice pre-treated withBifidobacteriumspp. andE. faecalisshowed a weight loss reduction and colon shortening compared to DSS-treated mice. Contrarily, oral administration ofA. muciniphilaandC. butyricumdid not improve these symptoms (Figs. 1A and B). Histopathologically, DSS treatment resulted in ulceration of the epithelial layer, extensive inflammatory cell infiltration into the lamina propria (LP), and thickened submucosa in the colon. However, these structural lesions were significantly improved by the administration of all probiotic strains(Fig. 1C).Specifically, pre-treatment withB. breveandE. faecalismarkedly preserved the colonic expression of tight junctions (TJs; Zonulin-1(ZO-1) and Occludin) in DSS-treated mice. All groups treated with gut commensals decreased the FITC-dextran serum concentration when compared with the DSS group (Figs. 1D-F). Increased levels of IL-1β and IFN-γ in colon tissues and decreased levels of IL-10 in colon and ileum tissues were observed in the DSS group compared to the control mice (P< 0.05). Test strains other than B113 significantly downregulated the colonic secretion of IFN-γ and upregulated the ileal level of IL-10 in colitis mice. Moreover, B113 treatment significantly induced the level of IFN-γ in the ileum homogenates of mice compared to the DSS group (Figs. 1G-L).

Fig. 1 Effects of gut commensals on DSS-induced acute colitis in WT mice. (A) Body weight loss after DSS treatment. (B) Colon length. (C) H&E staining photos of colonic mucosa. Scale bars = 100 μm. (D-E) Relative expression of TJs. (F) Serum level of DX-4000-FITC. (G-I) Level of IL-1β, IFN-γ, and IL-10 in colon homogenates. (J-L) Level of IL-1β, IFN-γ, and IL-10 in ileum homogenates. n = 10. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.000 1. The same below.
3.2 Commensal bacteria differed greatly in restoring the gut microbiota dysbiosis caused by DSS exposure in WT mice
The overall structure of the gut microbiota changed significantly after acute DSS exposure (Fig. 2). DSS treatment decreased Shannon index,Evenness, and Observed_otus. AdministeringBifidobacteriumspp.andC. butyricumrecovered these indices relative to the DSS group(Figs. 2A-C). Treatment withE. faecalisincreased the Observed_otus compared to the DSS-treated mice (Fig. 2C). Based on the PCoA, the gut microbial community structure significantly shifted after exposure to DSS.E. faecalistreatment seemed to restore the disorder of the gut flora induced by DSS exposure. The gut community structures were further altered in theA. muciniphilagroup compared to that in the DSS group (Fig. 2D).

Fig. 2 Effects of gut commensals on the microbial community in DSS-induced acute colitis mice. (A-C) α-Diversity index of the intestinal microbiota.(D) β-Diversity of the intestinal microbiota.
At the phylum level, the relative abundance of Bacteroidetes and Patescibacteria markedly decreased, whereas that of Proteobacteria and Firmicutes increased in the DSS group compared to the control group. After 2 weeks of administration ofBifidobacteriumspp.,Firmicutes and Verrucomicrobia substantially decreased and the Bacteroidetes proportion increased (Fig. 3A). The administration ofE. faecalisincreased the Bacteroidetes/Firmicutes (B/F) ratio (Fig. 3B),and decreased the proportion of Proteobacteria, which was similar to that observed in the control group.On the other hand, treatment withA. muciniphilahad the opposite effect (Fig. 3C). A total of 15 significant features at the family level were identified using linear discriminant analysis (LDA) (P< 0.05, LDA value > 4). The relative abundance of 3 families (Peptostreptococcaceae, Bifidobacteriaceae,and Rikenellaceae) were recovered in all intervention groups compared with the DSS group.E. faecalistreatment also restored 4 families (Muribaculaceae, Bacteroidaceae, Deferribacteraceae, and Desulfovibrionaceae) (Fig. 3D).
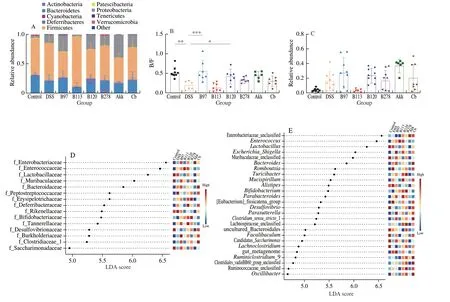
Fig. 3 Effects of gut commensals on microbiome features in DSS-induced acute colitis mice using LEfSe analysis. (A) Histogram of microbial composition at the phylum level. (B) Ratio of B/F. (C) Relative abundance of Proteobacteria in each group. (D-E) Linear discriminant analysis at the family and genus level.
At genus level, LEfSe analysis revealed a total of 26 significant features (P< 0.05, LDA value > 4) (Fig. 3E). Fourteen OTUs were significantly increased and 12 OTUs were significantly decreased by DSS treatment. Of these OTUs,Faecalibaculum,Bifidobacterium,Butyricimonas,Candidatus Soleaferrea, andRomboutsiawere markedly reversed in B97 (P< 0.05). Oral administration ofE. faecalissignificantly reduced the abundance ofRomboutsiaandEscherichia-Shigella. The B120 group had an enriched abundance ofBifidobacterium,Intestinimonsa, andNegativibacilluscompared to the DSS group (Fig. S1). The B278 group had a decreased abundance of 4 OTUs and increased the abundance of 4 OTUs compared with the DSS group. An increased level ofEnterobacterand decreased levels of 9 OTUs (e.g.,Turicibacter,Desulfovibrio,Romboutsia,andEscherichia-Shigella) were observed in the Akk group when compared with DSS group. Administration ofC. butyricummarkedly enhanced the proportion of 10 OTUs and suppressed the proportion of 2 OTUs (EnterorhabdusandTuricibacter) compared to the DSS group (Fig. S1).
3.3 Oral administration of Bifidobacterium but not E. faecalis ameliorated DSS-induced inflammation in the microbiota-depleted mice
WT mice were depleted of all detectable commensals through oral administration of the Abx for 7 days (data not shown). Mice pre-treated with B97 but not with B113 showed improvement in colon shortening, splenectasis, and weight loss induced by DSS in Abx-treated mice (Figs. 4A-C). Furthermore, B97 and Cb treatment markedly decreased inflammatory infiltration and mucosal structural damage, and increased villus length. B113 and Akk treatments had no protective effects against intestinal damage and inflammation (Fig. 4D).Administration of both strains decreased gut permeability but failed to upregulate the expression of TJs in comparison to the DSS group (Figs. 4E-G). Moreover,Bifidobacterium,C. butyricum, andE. faecalisdownregulated the levels of the cytokines (IL-1β and IFN-γ) in the colonic tissues of colitis mice (Figs. 4H-J).

Fig. 4 Effects of gut commensals on DSS-induced acute colitis in Abx-treated mice. (A) Loss of body weight in Abx-treated mice after DSS treatment. (B) Ratio of spleen weight and body weight in Abx-treated mice after DSS treatment. (C) Colon length. (D) H&E staining photos of colonic mucosa. Scale bars = 100 μm.(E-F) The relative expression of TJs. (G) The level of serum DX-4000-FITC. (H-L) The content of IL-1β, IFN-γ, IL-10, IL-12 and IgA in colon homogenates.(M) The level of IgG in serum.
3.4 Bifidobacterium but not E. faecalis induced the production of IL-10 in MLN cells
Three Bifidobacterium strains specifically induced secretion of the IL-10 in MLN cells, and B97 had the strongest induction ability.However,E. faecalisexhibited silent immunostimulatory effects.Neither strain induced the production of the pro-inflammatory cytokines (IL-12 and IL-17) (Fig. 5).
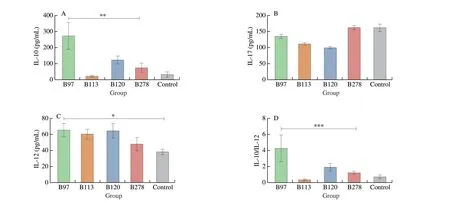
Fig. 5 Effects of Bifidobacterium and E. faecalis on cytokines secretion of MLN cells. Data are quantified from 3 independent experiments.
4. Discussion
Changes in the intestinal microbiota and cytokine microenvironment in the gut-associated tissue impact the development of colitis[36].Multiple commensal strains with different functions have been reported to regulate the intestinal inflammatory response and alleviate colitis[8,37]. Nonetheless, comparative studies on the modulatory mechanisms of test strains and guidelines for the use of probiotics are limited. In this study, we observed thatBifidobacteriumspp.andE. faecaliscould alleviate DSS-induced colitis symptoms in normal mice. However, administration ofA. muciniphilaandC. butyricumstrainsfailed to relieve the disease symptoms.B. breveJSNJJNM2, which has strong IL-10-producing activity, still exhibited potential functions in Abx-treated mice. In addition, the effects of these strains were different on the adjustment of gut immunity,epithelium barrier, and microbial composition.
The protective effects ofBifidobacteriumspp. andE. faecalisagainst acute colitis in WT mice may be explained as follows. First,it can be attributed to their modulatory effects on the composition of the gut microbes. In the present study, DSS treatment caused gut microbiome dysbiosis, as shown by the reducedα-diversity index and B/F ratio(Fig. 2). Dysbiosis of gut microbes may contribute to the imbalance in the interplay between commensal microbes and immunological niches and the loss of functional genes[4]. AllBifidobacteriumspp.strains relieved the change in gut microbiota diversity caused by DSS, similar to those discussed in previous studies[38]. InterventionwithB113 restored the gut microbial community and decreased the abundance of phylum Proteobacteria(Fig. 2). The abundance of Proteobacteria is suggested to be a microbial signature of gut microbial dysbiosis and a potential diagnostic criterion for gut disease and microbial dysbiosis[39-40].At the genus level, DSS exposure led to an increase in the levels ofParabacteroides,Bacteroides,Enterococcus,RomboutsiaandEscherichia-Shigella(P< 0.05) and a decrease in the levels ofLactobacillus,Faecalibaculum,Bifidobacterium,ButyricimonasandCandidatus Soleaferrea. B97 treatment increased the relative abundance of beneficial short chain fatty acid-producing bacteria,such asFaecalibaculum,Bifidobacterium, andButyricimonasand restored the commensal bacteria to a normal level. A recent study has shown thatFaecalibaculumproduces short-chain fatty acids (SCFAs)that control protein acetylation and protect mice from intestinal tumor cell proliferation[41].B. bifidumstrains synergize with immune checkpoint inhibitors to reduce intestinal inflammation and tumor burden in mice[42].In addition, the relative abundances ofEscherichia-ShigellaandRomboutsiaare positively correlated with colitis and autoimmune diseases[43-44].In this study, oraladministration ofE. faecalissignificantly altered the structure of the intestinal microbiota and showed significant inhibitory effects on harmful bacteria (especially the two genera mentioned above), which may be part of the protection mechanism[45].
Second, we observed thatB. breveandE. faecalisincreased the expression of TJs in the colonic epithelium. These results concurred with previous reports that revealed the ability of probiotic strains to enhance the physical barrier (TJs and mucin)[46-47]. Bacterial-mediated stimulation of toll-like receptor (TLR)-2 regulates the expression and localization of specific protein constituents of TJs[48]. We also observed that theB. bifidumstrains failed to adjust TJ function.Similarly, another study reported that the enhancement of the TJ barrier byB. bifidumwas strain-specific[49]. Reduced expression of TJ proteins and increased gut permeability in patients with IBD to exposure of luminal antigens and virulence factors to the intestinal LP and induction of secondary inflammatory response[3]. The polysaccharideofL. plantarumcan attenuate gut permeability in mice by enhancing the expression of TJs and the differentiation of goblet cells[50]. Targeting the gut TJ barrier using probiotic bacteria can prevent or treat colitis in a mouse model.
Third, the tested strains exhibited different patterns of immune regulation in the colon and ileum tissues of mice (Fig. 2). IL-10 has emerged as a critical suppressor of the immune response in macrophages and other antigen-presenting cells. Administration of all strains markedly improved the secretion of IL-10 in colon and ileum tissues (Figs. 1I and L), which may be crucial for the reduction of intestinal inflammation[51]. For instance, probiotic yeast andL. curvatuspromote regulatory dendritic cell IL-10 production and attenuate DSS-induced colitis in mice[9,52]. Cell polysaccharides ofB. bifiduminduced the production of IL-10, which has been shown to suppress experimental colitis[35]. In our study, B97 and B278 markedly decreased the production of IFN-γ in colon tissues, which may be due to the inhibitory effect of IL-10[53]. Meanwhile, IFN-γ induces the production of nitric oxide as a toxic effector molecule in mice with colitis[54]. Inhibition of these inflammatory cytokines helps alleviate intestinal inflammation.
Without relieving colitis-associated symptoms, administration ofA. muciniphilaresulted in the normalization of the gut microbiome,ameliorated the severity of colonic inflammation, and downregulated the expression of IFN-γ in WT mice, which was similar to previous reports[13,32]. Another study has shown that the anti-inflammatory abilities ofA. muciniphilastrains in murine colitis are strainspecific[15]. Significantly,A. muciniphilatreatmentaggravated the structural damage of colon mucosa in Abx-treated mice, which is closely linked with the controversial effects ofA. muciniphilain manipulating gut barrier function. Given thatA. muciniphilautilize mucin as the sole carbon and nitrogen source in the intestinal tract,its proliferationcontributes to the disruption of the mucous layer and exacerbates the inflammatory infiltration of LP due to excessive mucin degradation[55-56]. Similarly,C. butyricumtreatment failed to improve weight loss and colon shortening, but exerted intestinal barrier enhancement in DSS-treated mice with or without the gut microbiome (Figs. 2F and 4G). Several reports have shown thatC. butyricumtreatmentcan ameliorate acute colitis and restore gut microbiota disorders caused by DSS in WT mice[11,57]. IL-10 production from macrophages is required for protection against colitis byC. butyricum[26]. We noted that theC. butyricumstrain failed to induce the upregulation of IL-10 in the colon and ileum of WT mice,which may limit its role in relieving intestinal inflammation.
Finally, we observed thatB. breveB97, but not other test strains,alleviated colitis symptoms in pseudo germ-free mice (Fig. 4). This may be closely related to the effects ofB. breveon the regulation of immune homeostasis. ProbioticB. brevehas been reported to induce IL-10-producing Tr1 cells via the TLR2/MyD88 pathway in the colon[30].B. brevemodulated T cell polarization towards Th2 and Treg cell-related responses in human peripheral blood mononuclear cells and in a murine DSS colitis model[58]. Meanwhile, monocolonization of mice withB. fragilisenhanced the expression of IL-10 and protects against DSS-induced colitis[59]. We assayed the immunomodulatory effects of the test strains in MLN co-culture models. Interestingly,strain B97 induced the highest levels of IL-10 compared with B120 and B278, andE. faecalishad little effect (Fig. 5). Various research groups have uncovered the strain-specific immunoregulatory properties ofBifidobacterium. Verma et al.[35]reported a co-culture model of MLN and screened out the anti-inflammatory strainsB. bifidumPRI1 andL. helveticusHY7801, which can suppress inflammation damage in experimental rheumatoid arthritis or T cell transfer model of colitis. A previous study showed that bifidobacteria performed strain-specific functions to induce the production of pro- and anti-inflammatory cytokines in cell cultures and differed markedly in the prevention of acute colitis[8]. However, lactobacilli and bifidobacteria induce macrophages to secrete IL-10 differently.This ability was related to the activation of the signaling pathways mediated by MyD88 and TLR2[25]. Moreover, the effectiveness of probiotics in immunomodulation usually differs among individuals.Probiotic induction of IL-10 and IL-12 production by macrophages is flexibly converted by costimulation with microbial TLR ligands[60].E. faecaliscould not ameliorate DSS-induced gut inflammation in the microbiota-depleted mice (Fig. 4), which indicated that the lack of interaction with the gut microbes may limit the protective effect of tested probiotics, especiallyE. faecalis, against acute colitis. This is in line with the report thatE. faecaliscan modulate the balance of intestinal microecological and thereby protect the mice from experimental colitis[20]. Furthermore, we observed that none of the tested strains restored the mRNA expression of TJ and intestinal permeability compared to the DSS group. In conjunction with our previous experiment that administered 4 antibiotics irreversibly damaged TJ function and increased gut permeability[32]. This may explain why some probiotics are ineffective in the clinical recurrence of IBD and antibiotic-associated diarrhea[61-62].
5. Conclusion
In summary, our study showed that tested probiotic strains exert different action modes against DSS-induced colitis by regulating the gut microbiota, epithelium barrier, and immune homeostasis.A. muciniphilaAH39 andC. butyricumFHuNHHMY49T1 failed to offer protection against DSS-induced colitis.B. breveJSNJJNM2 andE. faecalisFJSWX25M1 alleviated acute DSS-induced colitis by regulating gut microbiota, promoting IL-10 production, and enhancing epithelial barrier function. Interestingly, when mice were depleted of all detectable commensals,B. brevebut notE. faecalisshow colitis-alleviating effects in mice. Furthermore,B. breveinduced IL-10 production in MLN cells, butE. faecalisdid not. The protective effect ofE. faecalisstrains might be dependent on interactions with the gut microbiota, therefore providing a theoretical foundation for the clinical application of probiotics in IBD treatment.
Conflicts of interest
Qixiao Zhai is an associate editor and Wei Chen is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.The authors declare no conflict of interests.
Acknowledgements
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20200084); The National Natural Science Foundation of China (U1903205 and 31972971); and Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.26599/FSHW.2022.9250100.
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango