In situ direct reprogramming of astrocytes to neurons via polypyrimidine tract-binding protein 1 knockdown in a mouse model of ischemic stroke
2024-01-24MengYuanYaoTangTianwenHuangLiningKeEnHuang
Meng Yuan ,Yao Tang ,Tianwen Huang ,Lining Ke,,En Huang,
Abstract In situ direct reprogramming technology can directly convert endogenous glial cells into functional neurons in vivo for central nervous system repair.Polypyrimidine tract-binding protein 1 (PTB) knockdown has been shown to reprogram astrocytes to functional neurons in situ.In this study,we used AAV-PHP.eB-GFAP-shPTB to knockdown PTB in a mouse model of ischemic stroke induced by endothelin-1,and investigated the effects of GFAP-shPTB-mediated direct reprogramming to neurons.Our results showed that in the mouse model of ischemic stroke,PTB knockdown effectively reprogrammed GFAP-positive cells to neurons in ischemic foci,restored neural tissue structure,reduced inflammatory response,and improved behavioral function.These findings validate the effectiveness of in situ transdifferentiation of astrocytes,and suggest that the approach may be a promising strategy for stroke treatment.
Key Words:astrocyte; in situ direct reprogramming;ischemic stroke;miR-30 based shRNA;neuron;polypyrimidine tract-binding protein 1;transdifferentiation
Introduction
Ischemic stroke is one of the leading causes of death and disability worldwide.Occlusion of a cerebral artery and blood flow in the brain leads to neuronal loss and the loss of corresponding neural function,and brain deficit syndrome(Campbell and Khatri,2020).The high incidences of disability and death due to ischemic stroke are a serious burden on human life and health (Ma et al.,2021).Currently,there is no ideal treatment for stroke except for acute intravenous thrombolysis and endovascular intervention.Unfortunately,the thrombolysis window (3–4.5 hours) and the endovascular intervention window (6–24 hours) are narrow,and the effects on recovery are limited (Xiong et al.,2022).Because neuronal loss is the key feature of ischemic stroke and neurons are unable to self-regenerate (Zhao et al.,2022;Hu et al.,2023;Ramanathan et al.,2023),cell replacement therapy could be a promising strategy to repair the damaged tissue and restore neurological function in stroke.
Transplantation of neural stem cells into the brain is the classic approach for stem cell therapy for brain injury repair (He et al.,2023).Initially,fetal tissue was transplanted into Parkinson’s disease patients,but the resource of human fetal tissue was limited and faced ethical issues (Lindvall et al.,1988).The successful isolation and culture of embryonic stem cells has made it possible to obtain robust stem cells for cell therapy and avoid the use of human fetal tissue.The advent of induced pluripotent stem cell (iPSC) reprogramming technology(Yamanaka,2007) allows for the generation of patient-specific neural stem cells throughin vitrostem cell differentiation.Cell therapy using iPSCs has been applied to ischemic stroke,and studies have shown that the grafted cells can somewhat restore neuronal function (Bono et al.,2022;Zhao et al.,2022).The use of human iPSCs avoids the risk of graft rejection and ethical concerns related to embryonic stem cells (Huang and Zhang,2019;Palma-Tortosa et al.,2021).However,there are some huge challenges in transplanting externally reprogrammed cells into the central nervous system,including overcoming immune rejection and functional integration.Therefore,scientists have explored the potential of direct reprogramming technology,which converts endogenous glial cells into functional neuronsin vivofor central nervous system repair (Qian and Fu,2021).Previous studies reported that overexpression of NeuroD1 directly reprogrammed astrocytes into new functional neurons and restored the lost neuronal function in mouse models of Alzheimer’s disease (Guo et al.,2014) and ischemic injury (Chen et al.,2020;Chen and Li,2022).Recent studies proposed a simple strategy to directly convert astrocytes into functional dopaminergic neurons in the substantia nigra via depleting RNA-binding protein polypyrimidine tract-binding protein 1 (PTB) in astrocytes(Chen et al.,2020;Maimon et al.,2021).This approach restored the compromised motor function in a mouse model of Parkinson’s disease (Qian et al.,2020).Furthermore,local transient injection of antisense oligonucleotides(ASOs) targeted to PTB mRNA in the substantia nigra also reprogrammed astrocytes to dopaminergic neurons and recovered motor function in Parkinson’s disease mice (Maimon et al.,2021).Another study showed that using an adenoassociated virus (AAV) expressing CasRx and two guide RNAs targeting PTB mRNA converted Müller glia cells into retinal ganglion cells and alleviated the symptoms associated with retinal ganglion cell loss (Zhou et al.,2020).These studies strongly indicate that astrocyte-to-neuron reprogramming by PTB depletion has promising therapeutic potential for neurodegenerative diseases and neuronal injuries including stroke.
In the present study,we applied AAV-PHP.eB-GFAP-shPTB to knockdown PTB in astrocytes in an endothelin 1 (ET-1)-injected ischemic mouse model to explore whether GFAP-shPTB leads to transdifferentiated neuronsin situ.We aimed to understand whether this approach will be a promising treatment strategy for stroke.
Methods
Vector and virus production
The AAV-PHP.eB-GFAP-miR30-based small hairpin RNA(shRNA) vector was constructed by our team (Figure 1A).AAVPHP.eB is a variant of AAV9 with enhanced central nervous system tropism and more efficient transduction (Chan et al.,2017).The shRNA sequences that were embedded within the microRNA (miR)-30 encoding region were driven by the GFAP-Pol II promoter and were mainly expressed in astrocytes(Chang et al.,2013).The shPTB sequence: 5′-GCG TGA AGA TCC TGT TCA ATA-3′,and shCon (control) sequence: 5′-CAA CAA GAT GAA GAG CAC CAA-3′,were the same as in a published study (Qian et al.,2020).The shRNA sequence embedded in the miR-30 backbone was produced by custom DNA synthesis and then inserted between theNotI andXbaI sites of pAAV.GFAP.eGFP.WPRE.hGH (Addgene,Watertown,MA,USA,#105549).The pUCmini-iCAP-PHP.eB plasmid (Challis et al.,2019) (Addgene,# 103005,RRID: Addgene_103005)was used to produce AAV-PHP.eB virus by OBiO Technology(Shanghai,China).
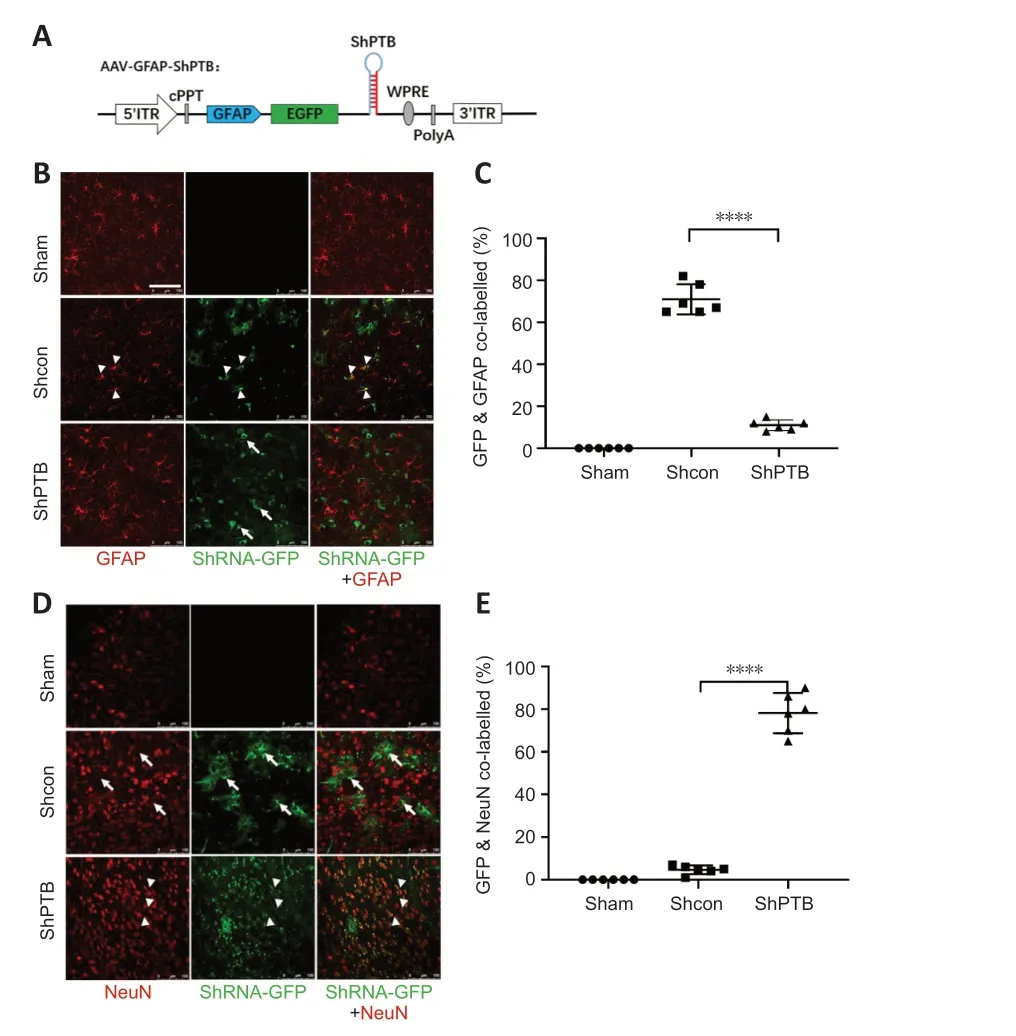
Figure 1 | In situ astrocyte-to-neuron direct reprogramming via PTBP1 knockdown.
Animals
Two-month-old male wild-type FVB mice,weighing 25–30 g,were purchased from Speifu Biotechnology Co.,Ltd.(Beijing,China;License No.SCXK (Jing) 2019-0010).The behavior tests were involved in this study and only male mice were selected for the data stability.The mice were housed in the laboratory animal room,with the room temperature maintained at 24± 2°C and the humidity at 45–65%.The drinking water was fresh,filtered water.All animal protocols were approved by the Ethics Committee of Fujian Medical University (No.FJMUIACUC2020-0091) on December 25,2020.The entire experimental process was conducted in accordance with the guidelines of the Animal Ethics Committee of Fujian Medical University,and the number of experimental animals used and the suffering of experimental animals were minimized in the experiment.All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines (Percie du Sert et al.,2020).
Establishment of a mouse model of ischemic injury and virus injection
Six FVB mice were anesthetized with 1.25% Avertin (0.6 mL/20 g;Sigma-Aldrich,St.Louis,MO,USA) by intraperitoneal injection.The mice were then injected with 15 µL of 0.1 µg/µL ET-1 (GLPBIO,Montclair,CA,USA;Cat# GC30485,n=3,ischemic stroke [IS] group) or phosphate-buffered saline (PBS;n=3,sham group) at 1.5 µL/min into the cerebral cortical projection area (anterior-posterior (AP): +0.2 mm;mediallateral: +1.5 mm;dorsal-ventral: +1.6 mm) corresponding to left forelimb motor function using a stereotaxic instrument(RWD Life Science,Shenzhen,Guangdong,China).
At 9 days post-injection (dpi),the mice were evaluated for the ischemic stroke model.For virus injection,99 mice were allocated randomly (Sham group,IS+shCon group,and IS+shPTB group;n=33 mice/group).Ten days after ET-1 injection,PBS,AAV-PHP.eB shCon and shPTB (5 × 1011particles/mL,0.5 µL/min,total 5 µL) were injected in the same area in mice.After AAV injection,six mice were processed to test the effect of the AAV at 60 dpi.Immunostaining for all treated groups was performed at 7,14,40,60 dpi.Gait analysis tests for all treated groups were performed at -10,-1,20,36,and 60 dpi.
Immunofluorescence staining
Mice were anesthetized with 1.25% Avertin and perfused transcardially with PBS and 4% paraformaldehyde.Mice were decapitated and the brains were fixed in 4% paraformaldehyde and cryoprotected as previously described (Huang et al.,2017).Free-floating serial coronal sections (40 µm thickness)around the cerebral cortical projection area (AP -1.8 mm to 2 mm) were collected.Sections were blocked with 10% donkey serum solution for 2 hours and then incubated with primary antibodies for 24 hours at 4°C.The primary antibodies were as follows: rabbit anti-ionized calcium-binding adaptor molecule 1 (Iba1;1:500,Abcam,Cambridge,UK,Cat# ab178846,RRID:AB_2636859),rabbit anti-GFAP (1:500,Abcam,Cat# ab7260,RRID: AB_305808),rabbit anti-neuronal nuclear protein(NeuN;1:250,Abcam,Cat# ab177487,RRID: AB_2532109),rabbit anti-vesicular glutamate transporter 1 (VGLUT1;1:200,Abcam,Cat# ab180188,RRID: AB_2868428),rabbit anti-COUPTF-interacting protein-2 (Ctip2;1:200,Abcam,Cat# ab28448,RRID: AB_1140055),rabbit anti-CD68 (1:500,Abcam,Cat#ab125212,RRID: AB_10975465),rabbit anti-microtubuleassociated protein 2 (MAP2;1:500,Abcam,Cat# ab32454,RRID: AB_776174),and rabbit anti-CD31 (1:50,Abcam,Cat#ab28364,RRID: AB_726362).For single-or double-labeling immunofluorescence,samples were incubated with secondary donkey anti-rabbit IgG H&L Alexa Fluor® 594 (1:1000;Abcam,Cat# ab150080,RRID: AB_2650602),donkey anti-mouse IgG H&L Alexa Fluor® 488 (1:1000,Abcam,Cat# ab150113,RRID:AB_2576208),or both at room temperature for 1 hour.Images were acquired under a Leica TCS SP5 X confocal microscope(Wetzlar,Germany).Some images were processed with threedimensional (3D) reconstruction imaging with Leica imaging software.For quantification of the infarction area,the area of brain tissue loss indicated by loss of NeuN-positive cells in AP: +0.2 mm coronal sections was delineated and the lesion area was calculated using ImageJ 1.53e version software(National Institutes of Health,Bethesda,MD,USA;Schneider et al.,2012).Quantification of the integrated optical density of immunofluorescence was processed by ImageJ software.
Gait analysis
Mice were trained prior to recording for at least 1 week,and the training was conducted by the same person who performed the final animal testing.Automated quantitative gait analysis with the CatWalk gait analysis system (ZS-BT/S,Zhongshidichuang Science and Technology Development Co,Beijing,China) was performed 1 day before ET-1 injection and at 7,14,40 and 60 dpi by researchers blinded to the groupings.CatWalk system application was described in detail in a previous study (Kappos et al.,2017).Mice were individually placed into the walkway and allowed to move freely in both directions.Three dynamic parameters(movement speed,swing speed and walk cycle) were collected and assessed with CatWalk gait analysis and processed for statistical analysis.
Statistical analysis
No statistical methods were used to predetermine sample sizes;however,our sample sizes were similar to that reported in a previous publication (Chen et al.,2020).No animals or data points were excluded in the analysis.The above experiments were carried out by researchers blinded to the mice groupings.All collected experimental data were processed with GraphPad Prism (version 8.0.2 for Windows;GraphPad Software,Boston,MA,USA,www.graphpad.com).Two-tailedt-tests were used to compare the results between two groups,and one-way analysis of variance with Bonferronipost hoctest was used to compare the results between different groups.Differences were considered statistically significant at 0.05.
Results
ShPTB knockdown in situ directly reprograms astrocytes to neurons
We constructed a set of AAV-PHP.eB viral vectors (Chan et al.,2017) with GFAP promoter-driven miR30-based shRNA (Chang et al.,2013),which were mainly expressed in astrocytes (Figure 1A).Then,PBS,AAV-shCon and AAV-shPTB were injected into the cortex of three mice for 60 days to observe whether infected astrocytes were converted to neurons.No green fluorescent protein (GFP) expression was observed in the sham group,and the shCon and shPTB groups had similar GFP expression,indicating viral infection.Most GFP-positive cells in the shCon group were colabeled with GFAP (approximately 70%;Figure 1BandC) and few overlapped with NeuN (Figure 1DandE),whereas most GFP-positive cells in the shPTB group were colabeled with NeuN (approximately 78%;Figure 1BandC) and few overlapped with GFAP (Figure 1DandE).These data indicated that the shPTB-infected astrocytes were converted to neurons whereas shCon-infected cells retained astrocyte characteristics,indicating thatin situastrocyteto-neuron direct reprogramming was achieved via PTBP1 knockdown.
Establishment and evaluation of an ischemic stroke model in FVB mice
After validating the AAV-PHP.eB-GFAP-shPTB effect,which indicated astrocyte-to-neuron reprogramming in the mouse brain,we examined the effect of direct reprogramming in an ischemic stroke model.Initially,we established an ischemic stroke model in FVB mice by ET-1 injection.At 9 dpi,NeuN immunostaining showed that there were severe tissue defects and neuronal loss in the ischemic stroke group (ET-1 injection)compared with the sham (PBS injection) group (Figure 2A–E).We next stained the tissue with microglia-specific markers Iba1 and GFAP.Compared with those in the sham group,the optical density of staining in microglia and astrocytes were increased around the ischemic foci in the ischemic stroke group (Figure 2D).The above results suggested that there was neuronal loss and gliosis in the ischemic foci after ET-1 injection.Compared with those in the sham group,the number of NeuN-positive cells decreased significantly (P<0.01;Figure 2E) and Iba1-positive cells increased significantly(P<0.01;Figure 2F) in the ischemic stroke group.To observe whether behavior was affected in the ischemic model,a gait analysis system (Figure 2G) was used to measure the motor coordination of the mice at 9 dpi.Compared with those in the sham group,the overall movement speed was reduced(P<0.001;Figure 2H),the swing speed of the left forelimb was reduced (P<0.0001;Figure 2I),and the walk cycle was prolonged (P<0.001;Figure 2J) in the ischemic stroke group,indicating that there was a motor deficiency in the model.
In situ astrocyte-to-neuron reprogramming in the mouse model of ischemic stroke
Next,we examined the effect of direct reprogramming in the mouse model of ischemic stroke.The experimental design for brain histology and animal behavior testing is shown inFigure 3A.There was no GFP expression in the sham group injected with PBS,whereas the shCon and shPTB groups showed obvious green fluorescence,indicating AAV infection (Figure 3BandC).We next investigated the time course of shPTBmediated astrocyte-to-neuron reprogramming around the injected stroke foci.Most GFP-positive cells were colabeled with GFAP in both the shCon and shPTB groups (Figure 3BandD) and few were colabeled with NeuN (Figure 3CandE) around the ischemic foci within 40 dpi.However at 60 dpi,47% of the GFP-positive cells in the shPTB group were colabeled with NeuN (Figure 3C–E) and fewer were colabeled with GFAP (Figure 3BandD),whereas the GFP-positive cells in the shCon group were colabeled with GFAP (Figure 3BandD)and few were colabeled with NeuN (Figure 3CandE).These data indicated that the AAV-shPTB infected astrocytes were reprogrammed to neurons at around 60 dpi in the mouse model of ischemic stroke.
Characteristics of transdifferentiated neurons from shPTBmediated in situ reprogramming
The previous experiment at 60 dpi (Figure 1) suggested that many GFP-positive astrocytes were reprogrammed to neurons in the shPTB group;therefore,60 dpi was used for further experiments.To confirm the neuronal characteristics of the converted cells from the shPTB-mediated astrocyte reprogramming,immunostaining with the neuronal markers NeuN and MAP2 was performed.A proportion of GFPpositive cell bodies were round with polar structure and were colabeled with NeuN in the shPTB group (Figure 4A).The 3D reconstruction imaging further supported the colocalization of GFP and NeuN (Additional Video 1andAdditional Figure 1A).In contrast,the GFP-positive cells in the shCon group were mainly colabeled with GFAP,and few were colabeled with NeuN (Figure 4A).The GFP-positive cells in the shPTB group were also colabeled with MAP2-stained nerve fibers,which is consistent with neuronal axons,whereas GFP-positive cells were not colabeled with MAP2 in the shCon group (Figure 4B).These results suggested that GFP-positive cells in the shPTB group had neuronal characteristics,with NeuN-positive neuronal nuclei and MAP2-positive nerve fibers,but those in the shCon group did not.To further explore the subtypes of the GFP-positive cells,the cortical neuronal marker Ctip2 and a specific marker of glutamatergic neurons (vesicular glutamate transporter 1,VGLUT1;Chen et al.,2020) were applied.GFPpositive cells in the shPTB group were colabeled with Ctip2(Figure 4C),which was localized in the nucleus,suggesting that the cells had the characteristic of neurons in deep cortical layers (layers V–VI;Chen et al.,2020).Additionally,GFPpositive cells were colabeled with VGLUT1 in the shPTB group(Figure 4D).The colocalization of GFP and VGLUT1 was further supported by 3D reconstruction imaging (Additional Video 2andAdditional Figure 1B).These data indicated that the shPTB-mediated reprogrammed neurons had characteristics of neurons in deep cortical layers and glutamatergic neurons.
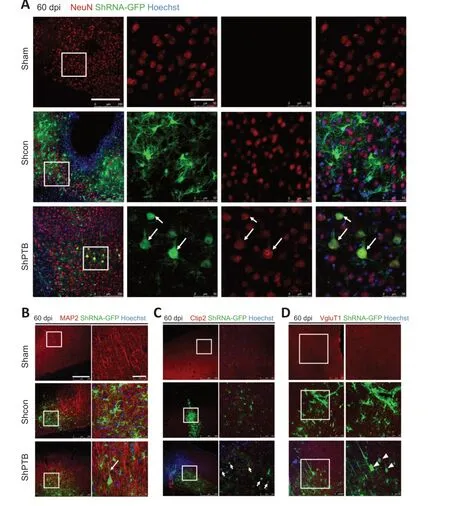
Figure 4 |Characteristics of neurons from shPTB-mediated in situ reprogramming in ischemic foci at 60 dpi.
Vascular remodeling and reactive glial cells are ameliorated around cerebral ischemic foci after in situ reprogramming
Brain vasculature and vascular remodeling play a critical role in stroke regeneration (Liu et al.,2014).To observe the vascular changes around the ischemic foci afterin situreprogramming at 60 dpi,immunostaining with a vascular endothelial cell marker,CD31,was performed.CD31-positivetissue had clear luminal structures in the sham group (Figure 5A).The morphology of CD31-positive vasculature was compromised and luminal structures were lost in the shCon group.Compared with that in the shCon group,the CD31-positive tissue around the ischemic foci in the shPTB group had a noticeable improvement in the lumina,suggesting that the compromised blood vessels were restored and remodeled in the shPTB group.
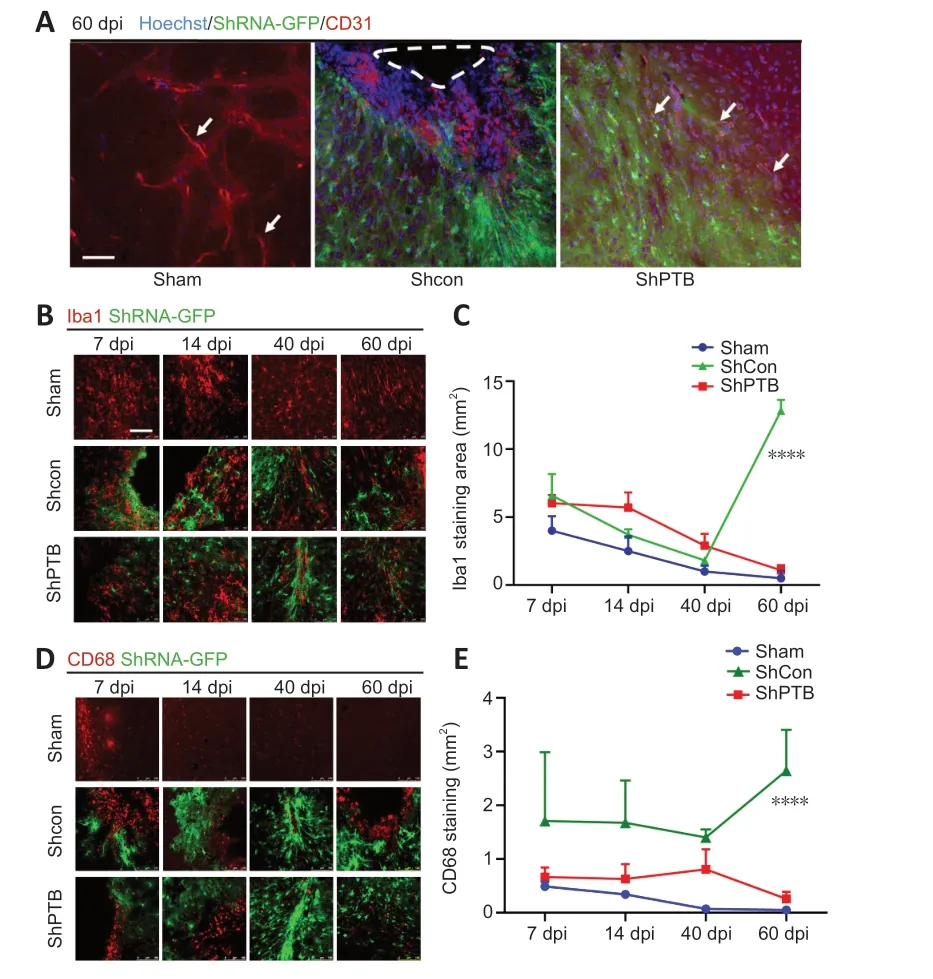
Figure 5 |Vascular remodeling and reactive microglia in ischemic foci after in situ reprogramming.
Reactive glial cells are a pathological hallmark of neuronal injury (Kiaie et al.,2022).To observe the effect of reactive glial cells around the ischemic foci with shPTBin situreprogramming,Iba1 immunofluorescence was performed.The Iba1-positive area decreased over time in the sham group(7 dpivs.60 dpi,P<0.05) and shPTB group (7 dpivs.60 dpi,P<0.001),whereas the Iba1-positive area in the shCon group was significantly increased at 60 dpi compared with the previous time points (Figure 5BandC).Similar to the Iba1 staining pattern,the number of cells stained with CD68,an activated microglia marker (lysosomal marker in phagocytes;Hopperton et al.,2018),showed a downward trend in the sham group and shPTB group and was increased in the shCon group.There was a significant increase (P<0.0001) at 60 dpi in the shCon group compared with that in the sham and shPTB groups (Figure 5DandE),indicating that the shPTB treatment significantly reduced the microglial reaction.
Brain tissue repair in ischemic foci after in situ reprogramming
We focused on the ischemic injury core to observe neuronal recovery after viral injection.The ischemic injury core(approximately AP 0.2 mm) was observed under a lowpower microscope.In the shCon group,the number of NeuNpositive neurons gradually decreased and the infarction area increased with time.In the shPTB group,the number of NeuN-positive cells gradually increased and the infarction area decreased with time (Figure 6A–C).Notably,there was a significant increase in the number of NeuN-positive neurons and infarction area in the shPTB group compared with those in the shCon group at 60 dpi.Immunofluorescent imaging of the infarction area from the series slides (AP 0.7 mm to-0.3 mm) demonstrated that the tissue defect area of the ischemic foci was reduced and restored after shPTB treatment(Figure 6D).These data suggested that shPTB-mediatedin situastrocyte-to-neuron reprogramming contributed to neuronal regeneration and brain tissue repair in the stroke model.

Figure 6 |Brain tissue repair in ischemic foci after in situ reprogramming.
Functional rescue of behavioral deficiencies after in situ reprogramming
With indications of neuronal regeneration and brain tissue repair in the injury foci after shPTB-mediated astrocyteto-neuron reprogramming,we next investigated whether shPTB treatment can rescue behavioral deficiencies caused by ischemic injury.Behavioral tests for motor function were carried out using automated quantitative gait analysis with the CatWalk gait analysis system.This system was used to detect the motor coordination of mice,including movement speed,swing speed and walk cycle.We tested the behavior of mice after virus injection in each group.The movement speed analysis showed that compared with that at -10 dpi,the movement speed at -1 dpi was decreased significantly in the shCon and shSTB groups (P<0.001;Figure 7AandAdditional Video 3),indicating that there was deficient behavior in the stroke model.The movement speed in the shCon and shPTB groups increased at later time points,and that of the shPTB group showed larger increases.There was a significant difference (P<0.05) between the shCon and shPTB groups at 36 dpi,indicating that the deficiency in movement speed was ameliorated with shPTB treatment (Additional Video 3).The results of swing speed (Figure 7B) and walk cycle (Figure 7C) analysis demonstrated a similar pattern as movement speed.Compared with that at -10 dpi,the swing speed and walk cycle at -1 dpi were significantly decreased(P<0.001).The swing speed and walk cycle in the shCon and shPTB groups improved over time from 20 dpi.There were significant differences in swing speed and walk cycle (bothP<0.01) between the shCon and shPTB groups at 36 dpi.These data indicated that the shPTB-mediatedin situreprogramming contributed to motor function recovery in this stroke model.
Discussion
The present study demonstrated thatin situdirect reprogramming to neurons was achieved via PTB knockdown by AAV-PHP.eB -GFAP-shPTB in a mouse model of ischemic injury.The shPTB-mediated reprogrammed cells possessed neuronal characteristic markers.The results suggest that direct reprogramming repaired the brain tissue defects in ischemic foci,restored the compromised motor function,promoted vascular remodeling,and reduced reactive microglia.
In the present study,we showed that AAV-PHP.eB-GFAP-shPTB was initially colabeled with GFAP in the cortex of ET-1-injected mice before 40 dpi,and the shPTB-infected cells were later colabeled with NeuN,MAP2,Ctip2 and VGLUT1 at 60 dpi.This indicated that GFAP-shPTB-mediated direct reprogramming to neurons was achieved by 60 dpi,and the transdifferentiated cells possessed the characteristics of functional neurons.At 60 days after AAV-shPTB injection,the number of NeuN-positive cells was markedly increased in the ischemic foci.Tissue defects and neuronal loss were observed in the ischemic foci in the shCon group,which were improved in the shPTB treatment group.These data suggest that AAV-shPTB-infected astrocytes in the ischemic foci were converted to neurons,which repaired the neural tissue defects and restored behavioral function.Additionally,microglia were decreased in the shPTB group compared with the shCon group.Microglia in ischemic foci may play a protective role by removing debris.Here,we consider that the microglia indicated tissue damage and that the decreased microglia was a marker for recovery of neural tissue with shPTB treatment.
Substantial neuronal death is a main pathological feature of ischemic stroke,and the inability of neurons to selfregenerate in the adult brain is a key obstacle to effective stroke treatment.A promising strategy in stroke treatment is replenishing lost neurons in injured areas by stem cell-and progenitor cell-based therapies (George and Steinberg,2015).To overcome the deficiency in endogenous neurogenesis,scientists tried to transplant external stem cells into brain tissue for neuronal transdifferentiation in injured areas.Following the discovery of iPSCs,it soon became possible to produce a variety of neuronal subtypes for cell replacement and bypass the immune rejection and ethical issues faced by embryonic stem cells (Goldman,2016).Efforts in autologous cell therapies with iPSC-derived neural stem cells in neurological disorders have achieved promising results.However,there are still some limits to iPSC-derived cell therapy (Lei et al.,2019).For example,the transplanted neural stem cells are not fully adapted to the host’s environment and are vulnerable to immune attack.The therapy also faces potential risks of tumorigenesis(iPSCs induced by oncogene),the expensive burden ofin vitroclinical-grade stem cell transdifferentiation,issues with long-term storage,and instability between different cell batches.To overcome the limits of external iPSC-derived cell transplantation,in vivoengineered neurogenesis emerged recently by transdifferentiating internal glial cells to generate neurons throughin situdirect reprogramming technology(Lei et al.,2019).Astrocytes are prevalent in the brain and can self-regenerate.Astrocytes share primitive progenitor cells with neurons (Kriegstein and Alvarez-Buylla,2009) and are ideal candidates for being reprogrammed into neurons to replenish lost neurons in brain injuryin vivo(Wang et al.,2021c).In light of these properties of astrocytes,previous studies introduced neural transcript factors,such as NeuroD1(Guo et al.,2014),Asc1 (Ueki et al.,2015),and Sox2 (Niu et al.,2015),into astrocytes and successfully converted astrocytes into functional neurons.This direct reprogramming did not need a pluripotent intermediate state (usually iPSC)and therefore is simpler,faster and more efficient.It can be appliedin vitroorin situ.The latter has unique advantages for tissue repair especially in stroke (Wang et al.,2021a).Chen et al.(2020) applied AAV-NeuroD1 to astrocytes in a mouse model of ischemic stroke and demonstrated that NeuroD1 overexpression converted astrocytes to functional neuronsin situand restored the loss of neuronal function.Previous studies also demonstrated that astrocyte-toneuron direct reprogramming restored function in other models of neurological disorders such as Parkinson’s disease(Qian et al.,2020) and spinal cord injury (Puls et al.,2020).Direct reprogramming has the advantages of autologous cell origin,no immunogenicity and low tumorigenesis risks.Another advantage of shPTB-mediated reprogramming is its subtraction-based approach (Qian and Fu,2021).In contrast to overexpression such as NeuroD1 by viral vector,PTB knockdown by shRNA can be achieved with a shorter vector and ASO technique (Maimon et al.,2021),which are more easily applied in the clinic.ASO technology has progressed markedly and an ASO nusinersen has successfully entered the market for spinal muscular atrophy (Finkel et al.,2017).Compared with permanent downregulation with an AAV vector,ASO enables dose adjustment in administration and can be produced in large quantities and with stable quality control.ASO-mediated PTB knockdown forin situreprogramming would be more clinically applicable for regenerative therapy for stroke and other neurological disorders.
It is important to note that other recent studies reported results that conflict with those of the present study (Wang et al.,2021b;Chen et al.,2022;Hoang et al.,2022;Xie et al.,2022).These studies reported that neither clustered regularly interspaced short palindromic repeats (CRISPR)/CasRx nor shRNA knockdown of PTB could convert astrocytes into neurons or transform Müller glia into retinal ganglion cells(Wang et al.,2021b;Xie et al.,2022).Different from the above studies,which all used genetically modified mice (i.e.,flox-CRE system),our study investigated shPTB on wild-type FBV mice.It is reasonable thatin situreprogramming should be more efficient in wild-type than in genetically modified mice(Xu et al.,2022).The viral quality,dose and promoter strength might also be factors for concern (Xu et al.,2022).The AAVPHP.eB vector used in this study was previously reported to be more efficient than AAV-9 (Chan et al.,2017),which might affect reprogramming efficiency.Our data showed that AAVGFP-shCon was mainly expressed in GFAP-positive cells and sparsely in neurons,whereas AAV-GFP-shPTB was clearly expressed in neurons and less in GFAP+cells at 60 dpi.One study reported that the neurons were not converted from astrocytes and that the GFAP-positive cells may have been due to the leaky expression of AAV-GFAP into neurons,which then made the faulty impression that neurons were converted from the GFAP-expressing astrocytes (Wang et al.,2021b).Our data cannot be explained by leaky expression of AAVGFAP.Although our study could not directly confirm that neurons were transdifferentiated from astrocytes,we think that the histological findings and behavioral improvements in the model mice support this conclusion.Normally,most neurons die in the ischemic foci and there should be few neurons remaining if the PTB-mediated reprogramming failed.However,there were many neurons colabeled GFP in the ischemic foci at 60 dpi with AAV-shPTB treatment.Most importantly,our tissue staining and behavioral test indicated that the AAV-shPTB treatment repaired neural tissue and boosted motor function in the ischemic model.If the shPTB failed to reprogram astrocytes to neurons,it could not rescue the damaged tissue and behavioral function.Furthermore,a recent study performed two-photon live imaging in astrocytic lineage-tracing mice and provided solid evidence for the achievement of astrocyte-to-neuron reprogramming (Xu et al.,2022).Nevertheless,we cannot confirm that the neurons were transdifferentiated from astrocytes through GFPpositive cells driven by the GFAP promoter.In view of other studies,there are three possible origins of transdifferentiated neurons from GFAP-shPTB direct reprogramming (Figure 7D): 1.The neurons transdifferentiated from astrocytes with GFAP promoter;2.Viral leakage to neurons occurred.The infected degenerating neurons can be easily induced into neural progenitor cells after PTB knockdown and later were differentiated into varied brain cells that contributed to brain functional recovery (Fan et al.,2022);3.The reactive astrocytes dedifferentiated into stem cells.PTB knockdown transdifferentiated the reactive astrocytes to varied brain cells and the brain function was restored (Zhang et al.,2022).
In summary,we applied AAV-PHP.eB-GFAP-shPTB to an ET-1-injected mouse model of ischemia.The findings suggest that GFAP-PTB knockdownin situdirectly reprogrammed GFAPpositive cells to neurons expressing neuronal characteristic markers.This reprogramming repaired defective neural tissue and restored behavioral function in the ischemic model.Compared with other cell transplant therapies,in situreprogramming has the advantages of efficiency,autologous origin,no ethical issues and low risk of tumorigenesis.The findings support direct cellular reprogramming as a promising therapeutic strategy in neural regenerative therapy for stroke.
Author contributions:MY,LK and EH designed the research project.MY,YT and TH performed the experimental work.MY,LK,EH analyzed data and wrote the manuscript.EH supervised the project.All authors approved the final version of this manuscript.
Conflicts of interest:The authors declare no competing interests.
Data availability statement:All data relevant to the study are included in the article or uploaded as Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Johannes Boltze,University of Warwick,UK.
Additional files:
Additional Figure 1:NeuN-positive cells colabeled with GFP-positive cells in shPTB-mediated in situ reprogramming in ischemic foci at 60 dpi.
Additional Video 1:NeuN-positive cells colabeled with GFP-positive cells in shPTB-mediated in situ reprogramming in ischemic foci at 60 dpi.The 3D reconstruction imaging for colabeling of GFP-and NeuN-positive cells in AAVshPTB infected FVB cortex in stroke model at 60 dpi,corresponding to Figure 4A.3D reconstruction imaging indicated the co-labeling of NeuN-and GFPpositive cells.
Additional Video 2:VGLUT1-positive cells colabbeled with GFP-positive cells in shPTB-mediated in situ reprogramming in ischemic foci at 60 dpi.The 3D reconstruction imaging for colabeling of GFP-and VGLUT1-positive cells in AAV-shPTB infected FVB cortex,in stroke model at 60 dpi,corresponding to Figure 4D.3D reconstruction imaging indicated the colabeling of VGLUT1-and GFP-positive cells.
Additional Video 3:The gait analysis test in ET-1 injected mice.The behavior test by gait analysis system in the sham group vs.shCon group at–1 dpi and shCon vs.shPTB group at 36 dpi(corresponding to Figure 7).The deficiency behavior function in ischemic model was ameliorated with shPTB treatment compared with shCon treatment.
杂志排行
中国神经再生研究(英文版)的其它文章
- Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice
- Conformational dynamics as an intrinsic determinant of prion protein misfolding and neurotoxicity
- Exploring the synergy of the eyebrain connection: neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation
- Pathogenic contribution of cholesteryl ester accumulation in the brain to neurodegenerative disorders
- Cognition and movement in neurodegenerative disorders:a dynamic duo
- Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease: searching far and wide
