Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice
2024-01-24GolnoushMirzahosseiniTauheedIshrat
Golnoush Mirzahosseini,Tauheed Ishrat
Stroke is a significant leading cause of death and disability in the United States (Tsao et al.,2022).Approximately 87% of strokes fall into the ischemic category,mainly caused by arterial blockage (Jayaraj et al.,2019).Although the only FDA-approved effective medication is tissue plasminogen activator (tPA),it should be administrated within 4.5 hours of ischemic stroke.Furthermore,tPA has been an integral part of managing acute ischemic stroke.However,it increases the development of intracerebral hemorrhage (Goncalves et al.,2022).Therefore,discovering a novel neuroprotective drug is a high clinical need.In recent years,there has been a significant increase in the utilization of thrombectomy procedures.
Neurotrophins (NTs) have been shown to contribute to several neurological diseases,including stroke,Alzheimer’s disease,and traumatic brain injury.NTs are produced in a proform and then cleaved to a mature form.
NTs include nerve growth factor,brain-derived neurotrophic factor,neurotrophin-3,and neurotrophin-4 (Huang and Reichardt,2001).NTs interact with two main receptors,p75 neurotrophin receptor (p75NTR) and tyrosine receptor kinase.p75NTRbelongs to the tumor necrosis factor receptor family.It is expressed in many cell types and plays a pivotal role in several cell functions,including apoptosis,survival,differentiation,and migration.During the developmental stage,p75NTRis extensively expressed in both the central and peripheral nervous system.However,as neurons mature into adulthood,their expression is downregulated.This receptor is highly upregulated in several neurological diseases,including stroke and dementia.p75NTRactivation triggers the activation of multiple signaling pathways,such as c-Jun N-terminal kinase-p53-Bcl-2 associated X protein(JNK-p53-Bax) (Mirzahosseini et al.,2023b).Previous reports have shown that downregulation of p75NTRmitigates neuroinflammation and promotes neurogenesis.
LM11A-31 is a novel p75NTRligand that is hydrophilic and orally bioavailable.This compound is in phase 2 clinical trials for mild to moderate Alzheimer’s disease (NCT03069014).This nonpeptide ligand can cross the blood-brain barrier(BBB).Researchers have shown that LM11A-31 effectively improves several brain diseases,including cerebral ischemia and pneumoniae meningitis.It has been shown that modulation of p75NTRusing LM11A-31 significantly reduced neuroinflammation,cholinergic degeneration,inflammation,and improved memory in mouse models of Huntington’s disease and Alzheimer’s disease (Simmons et al.,2016).Recently,our
research illustrated that LM11A-31 improves BBB permeability and motor function and reduces brain injury (Nasoohi et al.,2023).Suppressing p75NTRresulted in decreased brain damage and improved motor function in traumatic brain injury,mitigating Tau’s phosphorylation in the pR5 model(Sebastiani et al.,2015).Moreover,LM11A-31 reduces apoptosis in oligodendrocyte cells and preserved myelinated axons in a mouse model of spinal cord injury (James et al.,2017).The basis of the therapeutic approach for this compound lies in its ability to modulate the dimerization,activation,and interaction of NT receptors with co-receptors,utilizing a distinct mechanism compared to naturally occurring peptides (Meeker and Williams,2015).Our hypothesis is that modulation of p75NTReither pharmacologically or genetically enhances the functional outcome of ischemic stroke in mice.In this study,we utilized two distinct stroke models:intraluminal filament and photothrombotic models (Mirzahosseini et al.,2023a).The choice of stroke models depends on the specific research objectives.The intraluminal filament model closely simulates human ischemic stroke but carries the risk of hemorrhage with various types of sutures.This model has a higher mortality and infection risk than the photothrombotic model.Moreover,it induces severe and prolonged neurological deficits,making it challenging to assess behavioral function.The photothrombotic model is minimally invasive,with a high survival rate (Salman et al.,2023).It is known for its high reproducibility and minimal variation in infarction size.Moreover,we utilized two time points,including 24 and 72 hours to assess the impact of p75NTRmodulation at both early and subacute stages post-stroke in mice.We induced ischemic stroke using an intraluminal filament model for 24 hours to understand the early mechanisms post-stroke.Subsequently,we opted to induce ischemic stroke using the photothrombotic model for 72 hours to investigate the potential role of p75NTRmodulation in the subacute stage post-stroke.In this study,due to previous reports highlighting behavioral dysfunction and small body shape in p75NTRknock-out homozygous mice,we employed p75NTRheterozygous mice to induce ischemic stroke (Sebastiani et al.,2015).We administrated 50 mg/kg LM11A-31 at 1 hour following intraluminal stroke in wild-type mice.
Cerebral edema and hemorrhagic transformation(HT) are significant complications of ischemic stroke,leading to extended hospital stays for stroke patients.Around 3–40% of patients who suffer from ischemic stroke may develop HT.Also,using tPA and endovascular therapy has been shown to exacerbate HT (Mirzahosseini et al.,2023a).
We found that pharmacological modulation of p75NTRusing LM11A-31 or genetically knocking down p75NTRin mice significantly reduces cerebral edema,infarction,and HT following ischemic stroke in mice.Our results align with prior research indicating that knocking down p75NTRcan have a neuroprotective effect,reducing infarction in stroke (Qin et al.,2022).This research showed that administration of LM11A-31 reduced inflammation,including tumor necrosis factor-α/nuclear factor (NF)-κBp65 following stroke in mice.p75NTRactivation triggers the production of NF-κB signaling,and NF-κBp65 has been demonstrated to have detrimental effects on cerebral ischemia.NF-κB activation initiates neuronal damage and stimulates microglial cells,which can produce inflammatory markers.
Our evidence indicated that p75NTRmodulation decreases cell apoptosis by reducing Bax and increasing Bcl-2 protein expression.Bax and Bcl-2 are proteins with pro-apoptotic and anti-apoptotic roles,respectively.The interplay between Bcl-2 and Bax,regulating survival and death signals,ultimately determines the fate of cells (Singh et al.,2019).Additionally,we found that administration of LM11A-31 or genetically knocking down p75NTRinhibits the protein expression of JNK/RhoA following ischemic stroke in mice.JNK is a target of RhoA,known as stress-activated kinase,belonging to the mitogen-activated protein kinase family.JNK activation results in apoptosis,neurodegeneration,and ischemia-reperfusion processes.
In this study,inhibition of p75NTRresulted in upregulation of Akt signaling following ischemic stroke in mice.It has been demonstrated that the neuroprotective effect of Akt operates through phosphorylation and inactivation of downstream signaling pathways such as glycogen synthase kinase 3β (GSK3),Bcl-2,JNK,and apoptotic p53.Moreover,genetically knocking down p75NTRdownregulated the protein level of GSK3 following stroke.GSK3 has been shown to reduce neuroinflammation and ischemia/reperfusion injury in brain endothelial cells.We did not observe a significant difference in the protein level of extracellular signal-related kinase 1/2 (ERK1/2)following administration of LM11A-31 in mouse models of ischemic stroke.
Stroke disrupts the level of tight junction proteins,and there have been reports indicating that upregulation of p75NTRimpairs BBB permeability.BBB impairment leads to the production of inflammatory markers in the ischemic brain.Our results illustrated that the genetic knocking down of p75NTRimproved BBB integrity by increasing the protein expression of ZO-1 and occludin.ERK1/2,NF-κB,interleukin-1β,and tumor necrosis factor-α are among the markers that regulate the expression of occludin and ZO-1 (Rochfort and Cummins,2015).
Furthermore,the data showed that genetically knocking down p75NTRimproves sensory motor function following ischemic stroke in mice.Speed and pressure-related parameters were significantly improved in p75NTR+/–mice compared to the wildtype mice following ischemic stroke.These results indicate that knocking down p75NTRresulted in less brain injury than the wild-type mice.
Taken together,this is the first report illustrating the role of pharmacological modulation of p75NTRusing LM11A-31 or genetically knocking down p75NTRto decrease the cerebral infarction,edema,HT,cell apoptosis,and promote neuroprotection following intraluminal filament and photothrombotic model in mice.The evidence indicates that modulation of p75NTRshows a promising approach in advancing stroke therapy.The summary of the hypothesis and findings has been illustrated inFigure 1.The findings illustrated in the study underscore the potential mechanisms and neuroprotective effects associated with p75NTR,shedding light on its role in neuronal survival following ischemic stroke.Additionally,discovering combinatorial approaches,including integrating p75NTRmodulation with existing therapeutic agents,may yield a synergist effect and improve the outcome of ischemic stroke.
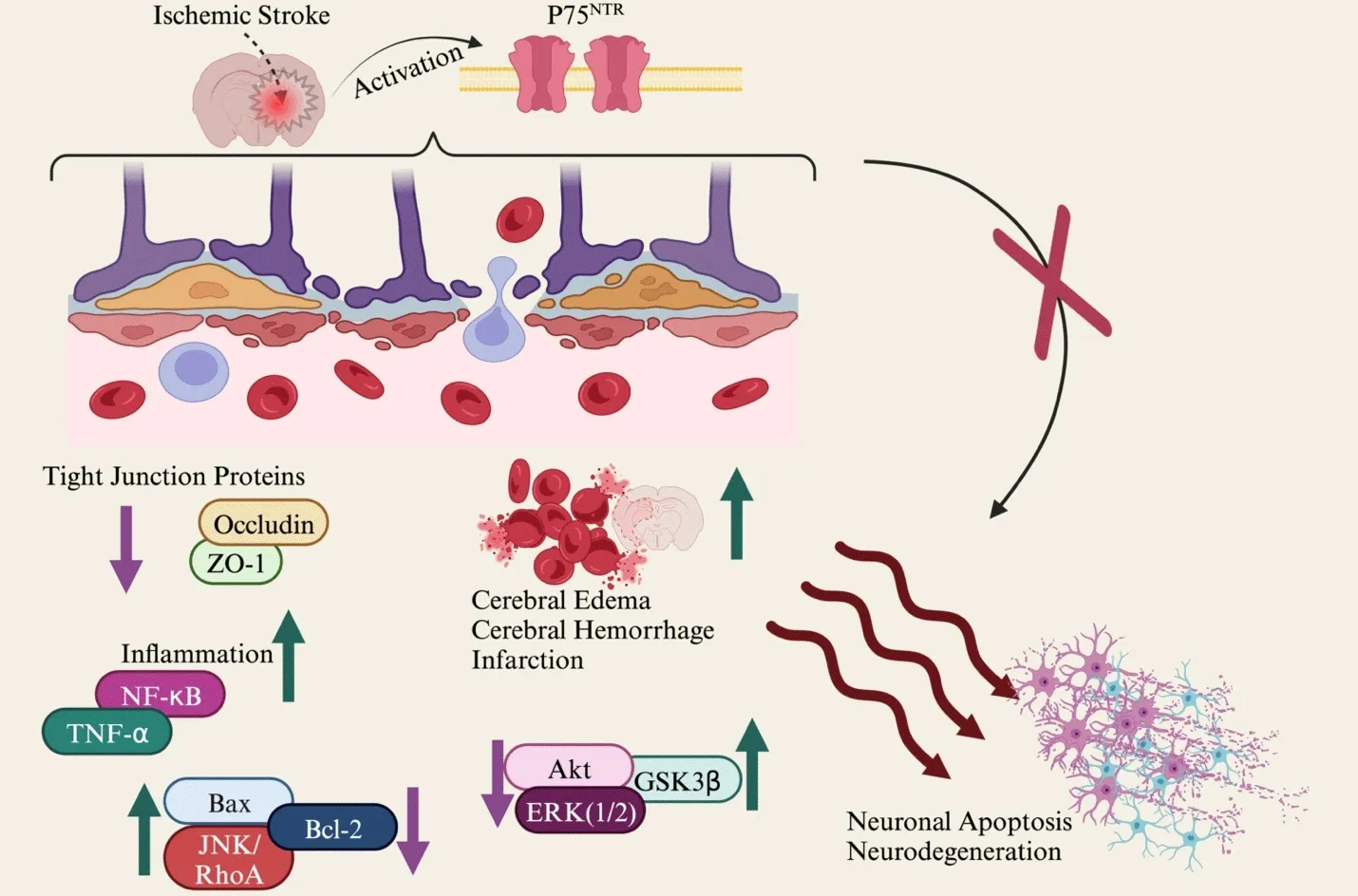
Figure 1 |The research hypothesis of p75NTR modulation in ischemic stroke in mice.
Our findings suggest that LM11A-31 can be a potential therapeutic agent for addressing ischemic stroke.However,it is crucial to conduct a long-term study to determine how modulating p75NTRaffects cognitive function in mice poststroke.Additionally,the role of p75NTRmodulation should be investigated in females post-stroke.
This work was supported by the UTHSC Bridge funding award(E073005058 Bridge Support-2022)and the National Institute of Health(R01-NS097800 and R56 NS127924-01)to TI.
Golnoush Mirzahosseini,Tauheed Ishrat*
Department of Anatomy and Neurobiology,College of Medicine,The University of Tennessee Health Science Center,Memphis,TN,USA(Mirzahosseini G,Ishrat T)
Pharmaceutical Sciences,College of Pharmacy,The University of Tennessee Health Science Center,Memphis,TN,USA (Mirzahosseini G,Ishrat T)
Neuroscience Institute,The University of Tennessee Health Science Center,Memphis,TN,USA (Ishrat T)
*Correspondence to:Tauheed Ishrat,PhD,tishrat@uthsc.edu.
https://orcid.org/0000-0002-9869-1342(Tauheed Ishrat)
Date of submission:October 16,2023
Date of decision:November 18,2023
Date of acceptance:December 6,2023
Date of web publication:January 8,2024
https://doi.org/10.4103/1673-5374.392860
How to cite this article:Mirzahosseini G,Ishrat T(2024)Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice.Neural Regen Res 19(10):2093-2094.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Conformational dynamics as an intrinsic determinant of prion protein misfolding and neurotoxicity
- Exploring the synergy of the eyebrain connection: neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation
- Pathogenic contribution of cholesteryl ester accumulation in the brain to neurodegenerative disorders
- Cognition and movement in neurodegenerative disorders:a dynamic duo
- Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease: searching far and wide
- Music as medicine for traumatic brain injury: a perspective on future research directions
