Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease: searching far and wide
2024-01-24GiuliaDematteisLauraTapellaDmitryLim
Giulia Dematteis ,Laura Tapella ,Dmitry Lim
Alzheimer’s disease (AD) is the most frequent form of dementia in elderly people and is an incurable disease with an exponentially growing number of cases.Extracellular deposition of amyloid-β (Aβ)plaques and intraneuronal formation of neurofibrillary tangles represent neuropathological hallmarks of AD.A high failure rate of clinical trials,testing drugs aimed at either removal of Aβ or normalization of neuronal functions,suggests that the amyloid cascade hypothesis is not able to explain the complexity of AD pathogenesis,and the role of non-neuronal cells of the central nervous system,such as astroglial cells,should be considered.A significant advance in AD research has been the understanding that the pathogenesis,at a cellular level,starts much earlier than the appearance of clinical symptoms.Cellular remodeling includes alteration of protein synthesis,folding,and degradation,dysregulation of calcium homeostasis and signaling,mitochondrial alterations accompanied by bioenergetic deficit,and accumulation of reactive oxygen species.These alterations have been exploited to formulate numerous AD hypotheses,including the amyloid cascade and the inflammatory,vascular,and infectious factors,which,traditionally,have been developed and interpreted through the lens of neuronal dysfunction,while alterations of glial cells,such as astrocytes have been largely overlooked (Verkhratsky et al.,2019;Lim et al.,2023).Recently,dysregulation of inter-organellar communication,in particular,the mitochondria-endoplasmic reticulum (ER) interaction,both in neurons and non-neuronal cells,came to age as a potential cause of cellular dysfunction in AD and other diseases,such as Parkinson’s disease (Paillusson et al.,2016;Area-Gomez and Schon,2017;Lim et al.,2021).
ER-mitochondria contact sites (MERCS) represent important signaling platforms,coordinating numerous cellular activities such as phospholipid and steroid biosynthesis,ER stress/unfolded protein response transduction,apoptosis,mitochondrial functions and dynamics,formation of autophagosomes,and protein synthesis.Last but not least,MERCS house a molecular super complex composed of inositol-1,4,5-trisphosphate receptor (IP3R),glucose-regulated protein 75 (Grp75) and a porin voltage-dependent cation channel 1 (VDAC1),implicated in direct ERmitochondria Ca2+transfer,warranting a low-affinity mitochondrial Ca2+uptake by the mitochondrial Ca2+uniporter (Lim et al.,2021).ER-mitochondrial Ca2+flux drives mitochondrial energetics and regulates apoptosis,thereby defining cell fate.Enhanced ERmitochondria interaction was found in whole brain preparations and neurons from AD mouse models and patients’ brains,suggesting its association with AD pathogenesis (Paillusson et al.,2016;Area-Gomez and Schon,2017).These alterations were linked to increased cholesterol and phospholipid biosynthesis,and mitochondrial energetic failure.However,the molecular mechanisms and causative role of the augmented ER-mitochondrial interaction in ADrelated cell dysfunction and pathogenesis remain unknown (Lim et al.,2021a,2023).
In this Perspective,we first discuss our recent findings about cause-effect relationships between ADrelated astrocytic cellular dysfunction and the ERmitochondria interaction;next,we briefly discuss the parameters that define ER-mitochondrial interaction,which have to be considered while assessing MERCS(dys)function,last,we critically assess methods used to investigate ER-mitochondrial interaction and point to the gaps in MERCS methodology.
Cellular dysfunction in AD astrocytes and its relation to ER-mitochondrial interaction:Astrocytes,the main homeostatic cell in the central nervous system,during AD pathogenesis,undergo complex morphological and functional changes.Astroglial atrophy and asthenia are found at preclinical and prodromal stages of AD.In a mouse AD model,3×Tg-AD mice,reduced complexity of the cytoskeletal network,as judged by glial fibrillary acidic protein staining,is found as early as at 1 month of mouse age.Neuroimaging studies on human AD brains show a significant decrease in glial glucose metabolism at the early stages of the disease.Concomitantly with the deposition of aggregated Aβ,astrocytes assume reactive phenotype.Astroglial reactivity likely has a neuroprotective role and delays neuronal degeneration.However,at the terminal AD stage,when the neuroprotective capability of reactive astrocytes is exhausted,astroglial paralysis marks the beginning of neuronal death and acceleration of dementia.Despite significant efforts in the investigation of AD-related pathophysiology of astrocytes,mostly astroglial reactivity in the context of neuroinflammation,little is known about molecular mechanisms of astrocytic pathophysiology,including the alterations of ER-mitochondrial interaction.
To study AD-related astrocytic cell pathology we have generated and characterized partially immortalized hippocampal astroglial cell lines from non-transgenic and 3×Tg-AD mice (WT-iAstro and 3Tg-iAstro cells,respectively).3Tg-iAstro cells express human amyloid precursor protein and produce elevated levels of both Aβ40and Aβ42(Gong et al.,2023).Furthermore,they reproduce the transcriptome profile of primary 3xTg-AD hippocampal astrocytes (Rocchio et al.,2019).A comprehensive investigation of 3Tg-iAstro cells suggests numerous alterations in cellular homeostasis.3Tg-iAstro cells show profound bioenergetic deficits including reduced glycolytic and oxidative ATP production.Basal oxygen consumption and respiratory reserve are severely impaired with a significant increase in reactive oxygen species levels in 3Tg-iAstro cells (Dematteis et al.,2020).Alterations of cellular proteostasis include a reduction of global protein synthesis in concomitance with chronic low-grade ER stress/unfolded protein response and impairment of autophagosomal protein degradation.Furthermore,the expression of constitutive proteasomal complex subunits was reduced,while β2i and β5i subunits were strongly upregulated,suggesting activation of immunoproteasome (Dematteis et al.,2020;Tapella et al.,2022;Gong et al.,2023).At a functional level,the homeostatic functions of 3Tg-iAstro cells were tested inin vitromodels of the blood-brain barrier and angiogenesis.In the first case,3Tg-iAstro cells were unable to maintain endothelial integrity and reduced levels of cell adhesion proteins (Kriaučiūnaitė et al.,2020).In the second case,3Tg-iAstro cells,plated together with pericytes and endothelial cells in 3D co-culture in Matrigel,were unable to allowin vitrotubulogenesis as opposed to the effect of WTiAstro cells.Analysis of the secretome suggests that 3Tg-iAstro cells lack neurogenic/neuroprotective factors,adhesion molecules,and components of the extracellular matrix.Finally,analysis of Ca2+homeostasis suggests that 3Tg-iAstro cells have increased ATP-stimulated Ca2+signals in the cytosol and higher steady-state ER Ca2+levels.However,ATP-induced Ca2+transient in the mitochondrial matrix was reduced,suggesting impairment of ERmitochondrial Ca2+flux (Dematteis et al.,2020).Analysis of ER-mitochondria interaction using splitgreen fluorescent protein contact site sensor (SPLICS)showed a strong increase in the interaction at the distance of 8–10 nm between ER and mitochondria and a non-significant increase at a distance of 40–50 nm,suggesting a general shortening of the ERmitochondria distance and the extension of the interface between organelles (Dematteis et al.,2020).Interestingly,the overexpression of a~10 nm-length synthetic ER-mitochondrial linker (10 nm-EML) in WTiAstro reproduces cell dysfunctions found in 3Tg-iAstro cells such as defects of protein synthesis,including phosphorylation of eukaryotic initiation factor 2α,proteasome remodeling and inability of 10 nm-EMLoverexpressing WT-iAstro cells to allow pericyte/endothelial tubulogenesis in 3D co-culture (Tapella et al.,2022).Thus,a shortening of the ER-mitochondrial distance appears to have a causal effect on AD-related cell dysfunction (Dematteis et al.,2020;Tapella et al.,2022;Figure 1A).
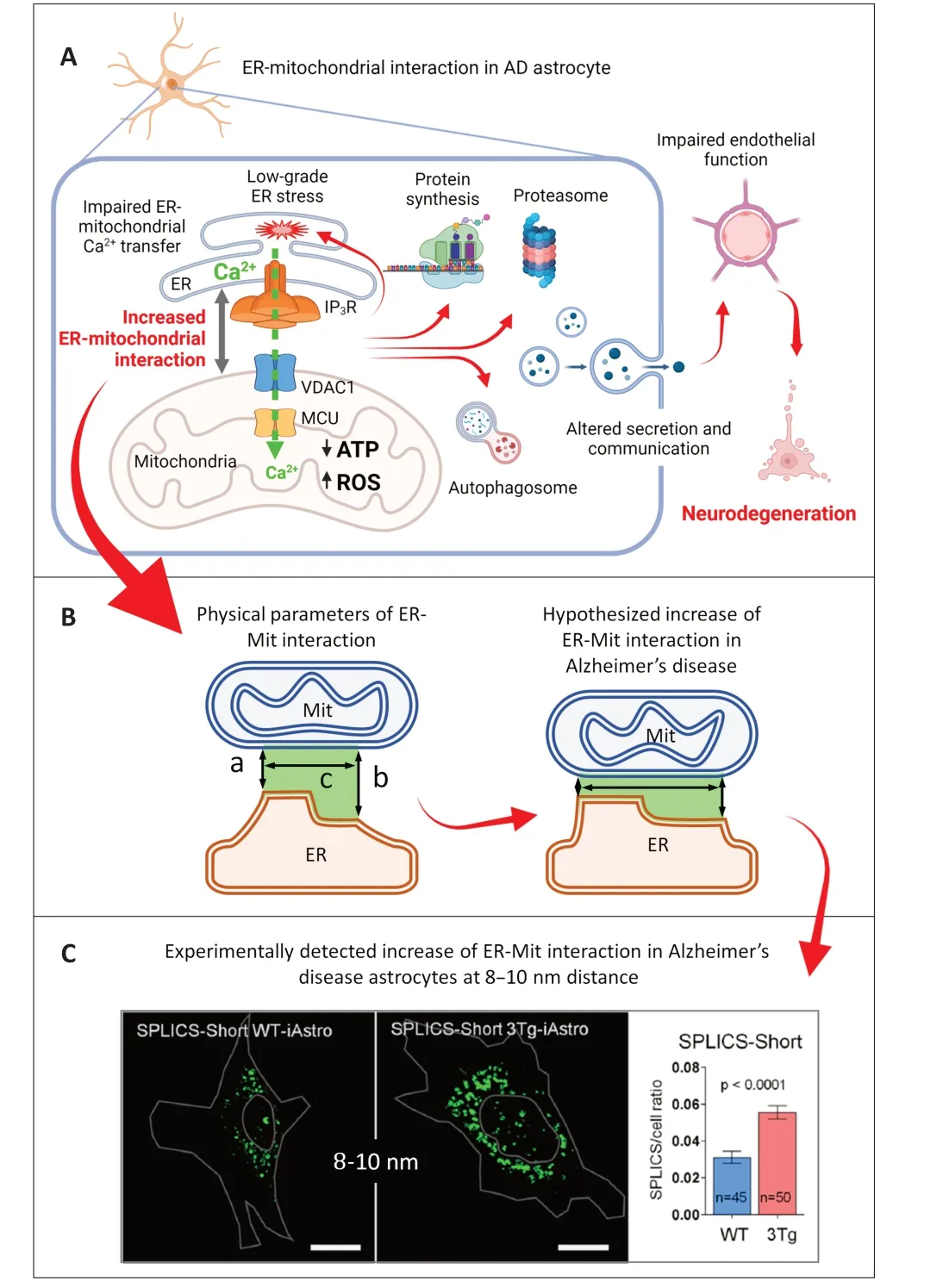
Figure 1 |Hypothesized mechanisms of the ER-mitochondrial interaction alterations in AD.
However,one could find it contradictory that an increased ER-mitochondrial interaction produced reduced ER-mitochondrial Ca2+transfer as an increased interaction generally reflects an increased ER-mitochondrial interface and,therefore,increased Ca2+flux between two organelles.To solve this apparent contradiction,the physical parameters of ER-mitochondrial interaction need to be defined(Giacomello and Pellegrini,2016),and the functional topology of the ER-mitochondria Ca2+transferring complex needs to be considered in the context of these parameters.
Physical parameters of MERCS define ERmitochondrial Ca2+ transfer:MERCS are morphofunctional units composed of the ER membrane and the outer mitochondrial membrane (OMM)juxtaposed to each other at a distance between about 5–80 nm.MERCS are highly organized and dynamic structures held by protein tethers (Lim et al.,2021).There are three key physical parameters of MERCS:(i) the transversal distance between ER membrane and OMM at a certain interaction site (also known as gap size);(ii) the length of the ER-OMM interface,which can be measured as a general parameter at a certain range of transversal distances;and (iii) the number of contact sites (Figure 1B).The functional meaning of holding the MERCS at different gap sizes is defined by the processes occurring at MERCS,which require a certain distance range.Thus,it is thought that phospholipid biosynthesis occurs at a short distance of <10 nm;autophagosome formation requires a 40–50 nm range;ribosomal protein synthesis was thought to occur at about 50–80 nm.For ER-mitochondrial Ca2+transfer,a distance between 15 and 25 nm was suggested (Csordás et al.,2010;Giacomello and Pellegrini,2016).The minimal distance is hypothesized based on steric hindrance imposed by the IP3R-Grp75-VDAC1 super complex,whose assembly would be impossible at a distance of less than 15 nm.
Therefore,the inhibition of ER-mitochondrial Ca2+flux in 3Tg-iAstro cells can be explained by the excessive shortening of the gap between the organelles below the average of 15 nm and the reduction of the number of functional Ca2+transferring IP3R-Grp75-VDAC1 supercomplexes (Figure 1C).
While theoretical considerations help to explain experimental results,a lack of methodological tools leaves an issue of the correct quantification of ERmitochondria interaction (and,potentially of all interorganellar and inter-membrane contacts) open.In the last part of this contribution,we discuss available and desirable tools for the investigation of inter-organellar contact sites.
Measuring the ER-mitochondria interaction:searching far and wide:The three most used methods to assess ER-mitochondrial interaction include transmission electron microscopy,colocalization of fluorescent proteins (Co-FP),and complementation methods.Nowadays,transmission electron microscopy remains the most accurate method to measure physical parameters of interorganellar interaction.Two main disadvantages of transmission electron microscopy are (i) significant labor time,especially when 3D reconstruction is needed;and (ii) impossibility of live imaging due to fixation of samples.
The Co-FP method exploits the co-expression of two (or more) fluorescent proteins with different spectral properties,subsequent optical imaging,and estimation of a degree of the overlap between colors.Co-FP method requires widely available imaging setups and is suitable for both fixed samples and live imaging.The principal drawback of the Co-FP method is its inability to provide quantitative information about physical parameters such as the length of the interface in relation to the gap between ER membrane and OMM.In spite of its poor precision,Co-FP is,arguably,the most widely used method to estimate ER-mitochondria interaction.
The complementation (also called proximity-driven)methods rely on the reconstitution of an optical signal in the sites of interaction at a defined distance.Among others,these methods include proximity ligation assay,Forster-or bioluminescent resonance energy transfer-based methods,and methods based on the reconstitution of the fluorescent signal upon complementation of two or more parts of an enzyme or a fluorescent protein.The last development of these methods is represented by SPLICS sensors based on the inability of green fluorescent protein to form mature chromophores without a portion of the protein.Removal of the last 17 amino acids forming the last 11thβ-sheet from green fluorescent protein prevents the chromophore from maturation.When targeted to different organelles,SPLICS reconstitutes bright fluorescence only when the membranes are opposed at a specific distance defined by rigid α-helical spacers.Currently,SPLICS are available for distances of 8–10 nm (SPLICS-Short) and 40–50 nm (SPLICS-Long) (Poggio et al.,2022).Among the advantages of SPLICS sensors are their ease of use and compatibility with live and time-lapse imaging.The principal disadvantage is that SPLICS sensors measure only one single distance defined by the length of spacers,while the information about other distances in the specimen is not available.
Further work is needed to develop polychromatic contact site sensors simultaneously reporting a range of distances,possibly spanning from 5 to 50 nm.Such sensors should be able to reveal MERCS dynamics in a wide temporal range,e.g.,MERCS remodeling during fast signaling events as well as during chronic physiopathological cell remodeling.Last,it is desirable that the future contact site sensors would work with optical as well as biochemical assays suitable for high throughput methods to enable drug screening assays.
Final remark:Dysregulation of the ER-mitochondria interaction is an emerging phenomenon in many diseases including AD.Molecular mechanisms and physical parameters of such dysregulation are poorly investigated.It is emerging that the information about the dynamic change of the parameters such as the transversal distance (or a range of distances),in relation to the interface length and number of contacts,between the organelles is important compared with a mere “strength” of the interactions.Easy-to-use and accessible methods need to be developed for fast and quantitative measurement of these MERCS parameters to be suitable for both single-cell analysis and high throughput screening.
This work had the following financial supports:GD is a recipient of the EMBO Short-Term Fellowship(grant 9584);LT is a recipient of a fellowship from the CRT Foundation(1393-2017);a grant FAR-2019 from The Università del Piemonte Orientale(to DL).
Giulia Dematteis*,Laura Tapella*,Dmitry Lim*
Department of Pharmaceutical Sciences,Università del Piemonte Orientale “Amedeo Avogadro”,Novara,Italy
*Correspondence to:Giulia Dematteis,PhD,giulia.dematteis@uniupo.it;Laura Tapella,PhD,laura.tapella@uniupo.it;Dmitry Lim,PhD,dmitry.lim@uniupo.it.
https://orcid.org/0000-0002-6317-3182(Giulia Dematteis)
https://orcid.org/0000-0002-8159-1628(Laura Tapella)
https://orcid.org/0000-0002-4316-2654(Dmitry Lim)
Date of submission:October 8,2023
Date of decision:November 22,2023
Date of acceptance:December 6,2023
Date of web publication:January 8,2024
https://doi.org/10.4103/1673-5374.392880
How to cite this article:Dematteis G,Tapella L,Lim D(2024)Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease:searching far and wide.Neural Regen Res 19(10):2103-2104.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Timothy E.Kennedy,McGill University,Canada;Patricia Gomez-Suaga,King’s College London,UK.
Additional file:Open peer review reports 1 and 2.
杂志排行
中国神经再生研究(英文版)的其它文章
- Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice
- Conformational dynamics as an intrinsic determinant of prion protein misfolding and neurotoxicity
- Exploring the synergy of the eyebrain connection: neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation
- Pathogenic contribution of cholesteryl ester accumulation in the brain to neurodegenerative disorders
- Cognition and movement in neurodegenerative disorders:a dynamic duo
- Music as medicine for traumatic brain injury: a perspective on future research directions
