Exploring the synergy of the eyebrain connection: neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation
2024-01-24AntaraVermaStephenAgadagbaLeanneLaiHangChan
Antara Verma,Stephen K.Agadagba,Leanne Lai-Hang Chan
The connection and interaction between the eye and the brain are crucial to understanding brain disorders (Marchesi et al.,2021).Both the eye and the brain have a limited regenerative capacity as there are few progenitor cells,and nerve cells do not replicate.Hence,neurodegeneration implicates irreversible damage to the central nervous system,as observed in several neurodegenerative diseases (Marchesi et al.,2021).The eye serves as an easily accessible extension of the brain that enables the discovery and non-invasive visualization of possible biomarkers for several neurodegenerative and neurological diseases (London et al.,2013).There has been a recent focus on exploiting the eyebrain connection for therapeutic interventions to treat such diseases.Electrical stimulation remains the oldest and most common method of neuromodulation.However,most forms of electrical stimulation are predominantly invasive(such as deep brain stimulation and motor cortex stimulation) and have numerous postsurgical complications,while other non-invasive forms(such as transcranial electrical stimulation and vagus nerve stimulation) elicit large variability in response to stimulation (Reed and Cohen Kadosh,2018).Thus,there is a need for novel and more effective non-invasive electrical neuromodulation approaches.In this regard,transcorneal electrical stimulation (TES) is an emerging technique to modulate brain networks in neurodegenerative disorders via non-invasive stimulation of the eye.Electrophysiological,molecular,and behavioral evidence strongly support TES as a neuromodulatory method for brain regions beyond the visual cortex (Yu et al.,2022).This presents a positive outlook for the future of TES and its development as a sought-after neuromodulatory technique.This article provides a summary of the eye-brain connection,highlighting its significance in neurodegenerative diseases.Furthermore,recent advancements in neuromodulation via TES,with an emphasis on electrophysiological evidence,are reported.
The eye is an anatomical extension of the brain.The retina of the eye embryonically originates from the diencephalon,which later develops into the thalamus dorsalis of the brain.The retina is a layered tissue comprising photoreceptors(rods and cones),bipolar cells,horizontal cells,amacrine cells,and specialized neurons called retinal ganglion cells.Axonal projections from retinal ganglion cells form the optic nerve,which connects to the visual cortex and thalamus via synapses.Rod and cone cells generate electrical impulses upon photoreception,which cascade through the retinal cell groups,and are ultimately relayed to the primary visual cortex for processing(Figure 1A;Marchesi et al.,2021).As a key part of the central nervous system,the retina displays various similarities to the brain (London et al.,2013).For example,the blood-retina barrier is identical in structure and function to the bloodbrain barrier,and the immunological architecture of the eye is similar to that of the brain.
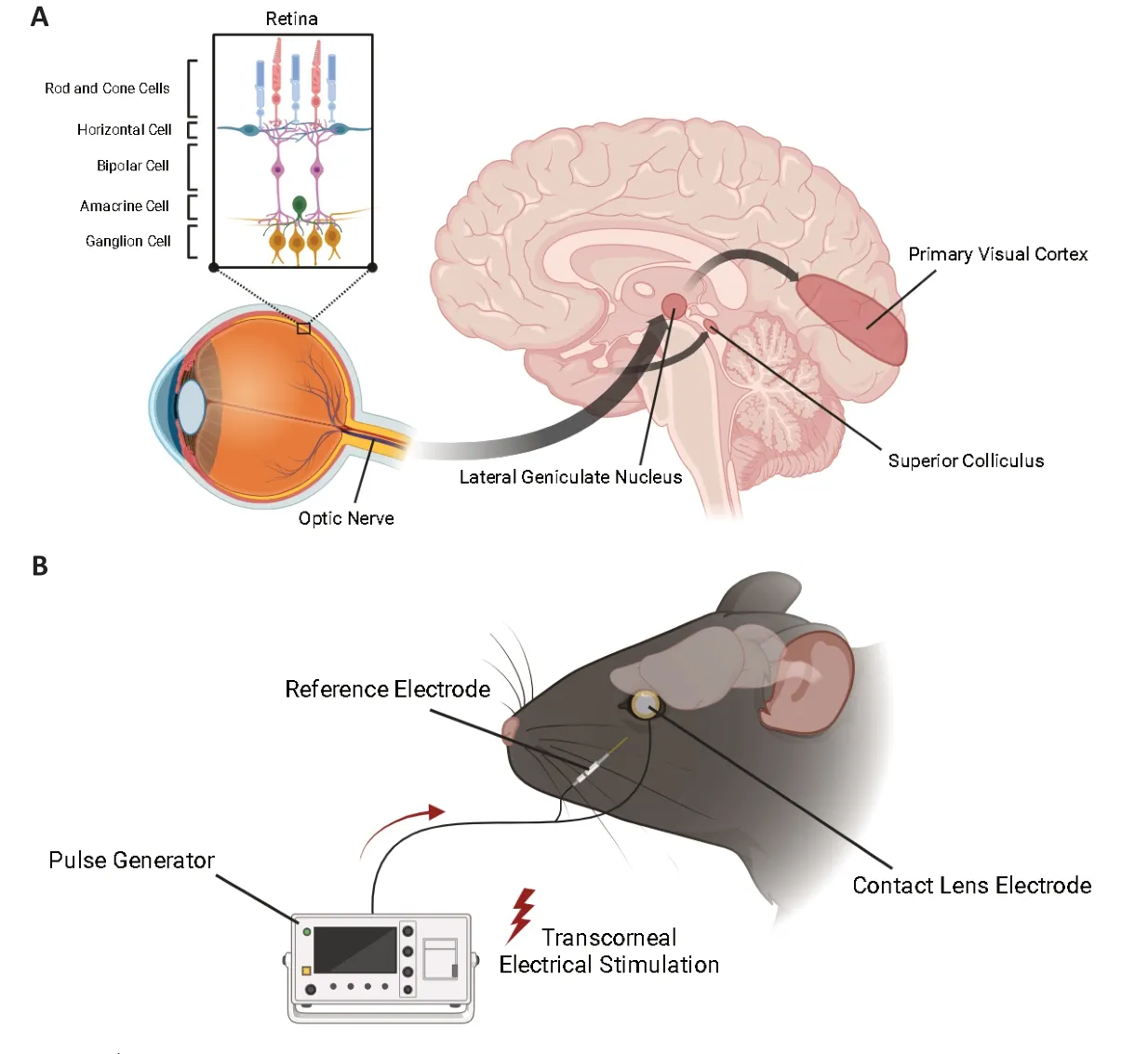
Figure 1 |The eye-brain connection and transcorneal electrical stimulation set-up.
Utilizing the eye as a window to the brain is a wellestablished paradigm.For decades,developmental neurobiologists have targeted the retinofugal pathway to study neural development and regeneration.This intrinsic connection between the eye and the brain has allowed researchers to discover a wide range of ocular biomarkers for neurodegenerative diseases (London et al.,2013).For example,Alzheimer’s disease (AD) is characterized by a variety of ocular structural and functional changes like retinal thinning,choroidal thinning,disrupted pupillary response and eye-gaze patterns,and reduced visual acuity(Majeed et al.,2021).Many of these indicators have also been reported in Parkinson’s disease,stroke,multiple sclerosis,schizophrenia,and mild cognitive impairment (London et al.,2013).Several eye-related diseases also display characteristics of neurodegenerative disorders,which further reinforce the eye-brain connection.For instance,in glaucoma,axonal atrophy patterns mimic those observed in AD and Parkinson’s disease.In age-related macular degeneration,the amyloid beta deposits seen in drusen bodies are identical to the amyloid beta plaques observed in AD.Furthermore,neurodegeneration is a defining feature of diabetic retinopathy,and a reduction in gray matter composition is correlated with retinitis pigmentosa (Marchesi et al.,2021).
The easy accessibility of the eye makes it possible to adopt a variety of non-invasive measurements to estimate neural integrity.Typical examples of such measurements include fundus imaging,which allows the visualization of retinal vascular topography;color Doppler imaging,which provides flow and velocity profiles for major arteries in the eye;the Heidelburg retinal flowmeter,which enables hemodynamic analysis;retinal oximetry,which estimates oxygen levels in arterioles and venules;Goldmann applanation tonometry,which measures intraocular pressure;electroretinography,which assesses electrical activity in retinal neurons in response to visual stimulus;and optical coherence tomography and optical coherence tomography angiography,which capture high-resolution images of ocular and oculovascular structures (Guidoboni et al.,2020).Indeed,such measurements show alterations in many neurological disorders and enhance their clinical diagnosis and evaluation.Researchers continue to direct a strong focus toward ocular diagnoses of neurodegenerative diseases based on the existing clinical evidence.
Since many neurodegenerative diseases are often associated with retinal degeneration,it stands within reason that modulating neuronal activity in the retina may have therapeutic impacts on the brain.Neuromodulation involves altering neuronal activity by targeting specific regions of the body using electrical,magnetic,optogenetic,thermal,chemical,or mechanical stimulation (Luan et al.,2014).Among these,electrical stimulation is the most extensively studied modality.Invasive electrical stimulation in the form of deep brain stimulation,retinal and cochlear implants,and intraspinal microstimulation is carried out using surgically implanted electrodes in the body cavity.While its positive effects have been reported in patients with AD and Parkinson’s disease,there are several postsurgical complications related to safety such as infection,intracranial hemorrhage,seizures,and cognitive impairment.Transcranial electrical stimulation is non-invasive and includes transcranial direct current (tDCS),transcranial alternating current,and transcranial random noise stimulation techniques (Reed and Cohen Kadosh,2018).TES and tDCS are both non-invasive neuromodulation techniques used for various purposes.However,TES could be a feasible alternative to tDCS because of its added advantages.While tDCS has been widely studied for its effects on cognitive and motor functions,as well as psychiatric disorders TES offers specific advantages over tDCS particularly when targeting visual functions and related neural circuits.For example,TES directly stimulates the retina and related neural circuits,making it more effective for treating visual disorders such as optic neuropathy and retinitis pigmentosa (Lee et al.,2021).Moreover,repetitive TES has been reported to induce long-lasting improvement in visual acuity,quick contrast sensitivity function,and/or Goldmann visual fields in patients with retinitis pigmentosa (Bittner and Seger,2018).
Recent efforts have ventured into using the eyebrain connection beyond diagnostics,and toward therapeutics.In this regard,TES modulates neuronal networks in the brain by activating the retinofugal pathway.The TES set-up is relatively simple: It involves placing an active electrode on the corneal surface and inserting a reference electrode underneath the skin,in close proximity to the eye.A pulse generator connected to the active electrode controls current delivery.The stimulation current amplitude,stimulation frequency,pulse shape/pulse duration,and repetition periods can be adjusted easily and varied based on each subject and pathological condition (Figure 1B).TES has neuroprotective effects,promotes neuronal regeneration and neuroplasticity,induces functional improvements in the retina,delays photoreceptor and retinal ganglion cell loss,inhibits apoptosis,and exerts anti-depressant-like effects (Miyake et al.,2007;Sergeeva et al.,2015;Agadagba et al.,2022;Yu et al.,2022).
There has been a palpable shift in exploring the neuromodulatory effects of TES in the context of neurodegenerative and psychiatric diseases.Several studies have shown the promising effects of TES in enhancing functional connectivity and modulating brain oscillations,promoting antidepressant-like effects,and reversing cognitive dysfunction in various rodent models of human neurodegenerative and neurological diseases(Agadagba et al.,2022;Yu et al.,2022).TES reduced hippocampal plaque deposition in male 5XFAD mice,a model of AD.It also improved postsynaptic function by upregulating postsynaptic protein 95 in the hippocampus of male 5XFAD mice.Prolonged TES increased theta-gamma cross-frequency coupling,network coherence,and functional and directional connectivity in the primary visual cortex and prefrontal cortex of retinal degeneration (rd) mice.The effects of 7-day prolonged TES were maintained for up to 2 weeks poststimulation,hinting at the long-lasting neuromodulatory impact of TES.In another study,TES elicited protective effects on the visual pathway of rd Long Evans rats.The same study reported upregulation of Ki67 and Nestin,common markers of neuronal progenitor cells,in the hippocampus of TES-treated animals(Yu et al.,2022).In another experiment with chronic unpredicted stress rats,TES restored locomotor activity and exerted anxiolytic effects on stressed rats.TES downregulated the proapoptotic BAX protein in the hippocampus and amygdala,and increased synaptophysin expression and protein kinase A activity,which are crucial to presynaptic function and synaptic plasticity (Yu et al.,2022).The hippocampus and amygdala are both functionally connected to the visual and retinotectal pathways.These results represent only a fraction of the neuromodulatory effects of TES.While these molecular and behavioral results provide compelling evidence for the therapeutic capability of TES,further analysis of electrophysiological data unveils relevant information about the neuromodulatory mechanisms of TES.Interestingly,TES excites both visual and non-visual regions of the brain (the primary visual cortex and the prefrontal cortex,respectively).An earlier electrocorticography study of awake TES-treated rd mice reported a significant increase in the spontaneous absolute power of theta (5–10 Hz),alpha (10–15 Hz),and beta (15–30 Hz) band oscillations in the primary visual cortex.Furthermore,low-frequency TES in anesthetized rd mice elicited a significant increase in the spontaneous absolute power of medium,high,and ultra-high gamma oscillations in the prefrontal cortex.These changes are often associated with enhanced cognition.The authors also observed increased gamma coherence in the primary visual and prefrontal cortices of TEStreated rd animals,which suggested increased inter-cortical synchronization and advanced neural processing.A follow-up study revealed TES associated with increased theta-gamma coupling and enhancement of functional and directional connectivity between the visual and non-visual regions of the brain (Agadagba et al.,2022).The results from the aforementioned studies suggest the downregulation of maladaptive spontaneous neuronal firing commonly observed in neurodegenerative diseases,modulation of visual perception,strengthening of cognitive functions,and neuroplastic interactions in the neurons.
Several other electrophysiological effects of TES have been explored.The electrophysiological effects of TES are not limited to the occipital and prefrontal cortices.TES also affects the inferior temporal and parahippocampal gyri.In another study,visually evoked potentials recorded in TES rats with optic nerve injury showed restoration of nerve function and protected retinal axons from imminent degeneration (Miyake et al.,2007).Sergeeva et al.(2015) reported that chronic alternating current TES can reduce cortical “idling”by modulating electroencephalographic power to normal values in an optic nerve crush model of rats.Poststimulation visually evoked potentials from optic nerve crush rats also demonstrated the neuroprotective effects of TES,which supports previous findings.Electrophysiological evidence reinforces the neuromodulatory capacity of TES to excite various regions of the brain and to exert long-term positive effects on neural integrity via the eye-brain connection.The eye-brain connection is integral to our understanding of retinal biomarkers of neurological diseases,as well as the invention and optimization of novel therapeutics for these conditions.At present,the vital implications of TES as a noninvasive neuromodulatory tool strengthen the rationale of utilizing it as a treatment for neurodegenerative and neurological diseases.There is a strong need to study and delineate TES mechanisms to optimize its therapeutic effects in target brain regions.The added benefit of non-surgical neuromodulation and flexibility in stimulation parameters allow individual and conditional tailoring of TES,making it an increasingly popular modality.There is considerable electrophysiological,molecular,and behavioral evidence to support future research into TES as a tool to modulate brain function.Research on TES must gain momentum so that it can be translated into preclinical and human models.
This work was supported by grants from City University of Hong Kong,China(Project No.SRG-Fd 7005632,SRG-Fd 7005854,and SIRG 7020058)(to LLHC).
SKA is employed by Centre for Eye and Vision Research Limited(CEVR).The remaining authors declare that the work was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Antara Verma,Stephen K.Agadagba,Leanne Lai-Hang Chan*
Department of Electrical Engineering,City University of Hong Kong,Kowloon,Hong Kong Special Administrative Region,China (Verma A,Agadagba SK,Chan LLH)
Centre for Eye and Vision Research Ltd.,Hong Kong Special Administrative Region,China(Agadagba SK)
Pennsylvania State University,University Park,PA,USA (Verma A)
*Correspondence to:Leanne Lai-Hang Chan,PhD,leanne.chan@cityu.edu.hk.
https://orcid.org/0000-0002-9542-6666
(Leanne Lai-Hang Chan)
Date of submission:May 22,2023
Date of decision:September 14,2023
Date of acceptance:November 24,2023
Date of web publication:January 8,2024
https://doi.org/10.4103/1673-5374.392877
How to cite this article:Verma A,Agadagba SK,Chan LLH(2024)Exploring the synergy of the eyebrain connection:neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation.Neural Regen Res 19(10):2097-2098.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Cecilia Perin,University of Milan,Italy;Alfred Stett,Okuvision GmbH,Germany.
Additional file:Open peer review reports 1 and 2.
杂志排行
中国神经再生研究(英文版)的其它文章
- Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice
- Conformational dynamics as an intrinsic determinant of prion protein misfolding and neurotoxicity
- Pathogenic contribution of cholesteryl ester accumulation in the brain to neurodegenerative disorders
- Cognition and movement in neurodegenerative disorders:a dynamic duo
- Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease: searching far and wide
- Music as medicine for traumatic brain injury: a perspective on future research directions
