Metabolic reprogramming of the inflammatory response in the nervous system: the crossover between inflammation and metabolism
2024-01-24JesusAmoAparicioCharlesDinarelloRubenLopezVales
Jesus Amo-Aparicio ,Charles A.Dinarello ,Ruben Lopez-Vales
Abstract Metabolism is a fundamental process by which biochemicals are broken down to produce energy (catabolism) or used to build macromolecules (anabolism).Metabolism has received renewed attention as a mechanism that generates molecules that modulate multiple cellular responses.This was first identified in cancer cells as the Warburg effect,but it is also present in immunocompetent cells.Studies have revealed a bidirectional influence of cellular metabolism and immune cell function,highlighting the significance of metabolic reprogramming in immune cell activation and effector functions.Metabolic processes such as glycolysis,oxidative phosphorylation,and fatty acid oxidation have been shown to undergo dynamic changes during immune cell response,facilitating the energetic and biosynthetic demands.This review aims to provide a better understanding of the metabolic reprogramming that occurs in different immune cells upon activation,with a special focus on central nervous system disorders.Understanding the metabolic changes of the immune response not only provides insights into the fundamental mechanisms that regulate immune cell function but also opens new approaches for therapeutic strategies aimed at manipulating the immune system.
Key Words:central nervous system;fatty acid oxidation;glycolysis;inflammation;macrophage;metabolism;microglia;neurodegeneration;oxidative phosphorylation
Introduction
Metabolism and inflammation are two fundamental processes that intricately interact,exerting profound effects on health,disease,and overall well-being.Metabolism refers to the elaborate network of biochemical reactions within cells to convert nutrients into energy and essential molecules necessary for various cellular functions.On the other hand,inflammation is the body’s natural response to harmful stimuli,such as infection,injury,or toxins,and involves a complex cascade of immune reactions aimed at resolving the threat and facilitating tissue repair.Although these two processes regulate specific physiological responses,it is now evident that they are closely interconnected and can influence each other (Bernier et al.,2020;Larabee et al.,2020).
Immune cell subsets,such as macrophages,microglia,lymphocytes,and dendritic cells,are highly dynamic and energy demanding.They require specific metabolic pathways to fuel their diverse functions,ranging from proliferation and differentiation to cytokine production and effector responses.The importance of the metabolic status of immune cells is particularly relevant in the context of disease.Recent advances in technologies like metabolomics,genomics,and transcriptomics have revealed that the metabolic state of immune cells can influence their fate and functions and that dysregulation of immune cell metabolism can be linked to various disease conditions such as autoimmunity,infectious diseases,cancer,metabolic disorders,and neurological diseases (Bernier et al.,2020;Mitra et al.,2022).
The objective of this review is to explore the interplay between metabolism and inflammation in the central nervous system.We provide insights about (i) how immune cells change their metabolism upon activation and (ii) how immune cell functions may differ during disease depending on their metabolic state.We provide details about how metabolic reprogramming can be targeted in various conditions to prevent neurological decline,including in Alzheimer’s disease(AD),Parkinson’s disease (PD),stroke,spinal cord injury,and aging.Unraveling the complexities of how metabolism and inflammation interact may pave the way for developing innovative treatments aimed at targeting the metabolic vulnerabilities of immune cells,restoring balance,and improving health.
Search Strategy
We performed a literature review in PubMed from inception to July 2023 using “metabolism” OR “metabolic state” OR “glycolysis” OR “oxidative phosphorylation” AND“macrophages” OR “microglia” OR “immune cells” OR“immunity” OR “inflammation” OR “neuroinflammation”OR “glia” OR “macrophage polarization” OR “macrophage activation” AND “neurodegenerative disease” OR “Alzheimer’s disease” OR “Parkinson’s disease” OR “ischemia” OR “spinal cord injury” OR “aging.” The results were further filtered by title and abstract of articles in a preliminary screening,and articles that were not highly relevant to the topic or were deemed unnecessary were deleted.
The Warburg Effect
In a typical resting cell,glucose enters glycolysis and is converted into pyruvate,which can follow two distinct routes.In the presence of oxygen,pyruvate is decarboxylated by pyruvate dehydrogenase into acetyl-CoA,which is then oxidized in the tricarboxylic acid (TCA) cycle to produce adenosine triphosphate (ATP).This pathway is also called the Krebs cycle or citric acid cycle.Through the electron transport chain (ETC),this oxidative phosphorylation (OXPHOS)process yields 33.45 molecules of ATP per molecule of glucose (Desousa et al.,2023).However,if oxygen is limited or absent,the cell can divert pyruvate away from OXPHOS in the mitochondria,enabling ATP generation during low oxygen conditions.In this case,pyruvate is reduced to form lactate by lactate dehydrogenase (LDH) in the cytosol,generating two ATP molecules per one molecule of glucose (Desousa et al.,2023).Although glycolysis is less efficient than OXPHOS in terms of ATP production,it is 10 to 100 times faster,allowing cells to perform energy-intensive processes such as proliferation and migration (Zanotelli et al.,2021).
In rapidly dividing cells,such as tumor cells,the metabolic profile changes to meet the increased demand for macromolecules and energy.This metabolic switch involves high glucose uptake and glycolysis followed by lactic acid production,even under aerobic conditions (aerobic glycolysis).This metabolic switch is called the “Warburg effect,” named after Otto Warburg,who first characterized this phenomenon in cancer cells in 1923 (Warburg et al.,1927).The high glycolytic rate and glucose dependency of cancer cells may explain their tolerance to extreme hypoxia and anoxia and their ability to compete with normal cells under normoxia.
The high glycolytic rate during the Warburg effect also satisfies the requirements for creating new building blocks for cancer cells.For example,TCA citrate is converted to acetyl-CoA via ATP-citrate lyase,which is used for fatty acid synthesis (FAS),a key component of membrane lipid synthesis (Dominguez et al.,2021).This process necessitates NADPH-reducing equivalents,which are generated through multiple steps within the pentose phosphate pathway (PPP).Additionally,the glycolytic intermediate dihydroxyacetone phosphate provides glycerol for forming triacylglycerol with fatty acids (Friedberg et al.,1987).Therefore,even in the presence of oxygen,highly active glycolysis and increased glucose uptake enhance the availability of precursors and fulfill the energetic demands for increased biomass during cancer cell proliferation.Even though this process was first described in cancer cells,similar metabolic changes are also found in immune cells during activation.
Metabolic Changes in Activated Macrophages and Microglia
Immune cells have a crucial function in maintaining homeostasis and defending against invading pathogens.In normal resting conditions,peripheral macrophages and resident microglia generate ATP via glucose metabolism through glycolysis,TCA,and ETC (Lynch,2020).Through these metabolic pathways,cells obtain the energy and resources required to perform their activity in the absence of pathogens or damage.In macrophages and microglia,this resting state is often referred to as M0 (Sica and Mantovani,2012).However,these cells have remarkable plasticity and can alter their phenotype in response to the environment.The term polarization is used to refer to the process by which macrophages and microglia obtain different and,even opposites,functions in response to the environment.Traditionally,two archetypical polarization states have been described.These states were named M1 and M2,based on the Th1 and Th2 polarization of T lymphocytes(Gordon and Martinez,2010).The M1/M2 dichotomy is an experimental construct that was initially described usingin vitrosystems (Martinez et al.,2009).However,in vivo,cells are stimulated by multiple factors,leading to a broad spectrum of intermediate phenotypes.Therefore,cells can express different,even opposite,markers at the same time.In those conditions,the terms M1 and M2 became simplistic and should be used only for explanatory purposes (Orecchioni et al.,2019).
Challenging macrophages and microglia with the canonical Th1 cytokine interferon γ (IFNγ),or lipopolysaccharide (LPS),triggers the M1 (classic) polarization in which cells secrete proinflammatory cytokines (tumor necrosis factor,interleukin (IL)-1β,IL-12,and IL-23),cytotoxic mediators (inducible nitric oxide synthase and nitric oxide),and extracellular matrix-degrading enzymes (matrix metalloproteinases).On their surface,they express phagocytic receptors (CD16/32) and costimulatory molecules (CD86) (Orecchioni et al.,2019).Therefore,they are referred to as pro-inflammatory cells.Conversely,exposition to the prototypical Th2 cytokines IL-4 or IL-13 induces M2(alternative) polarization in which cells are characterized by reduced levels of pro-inflammatory cytokines (tumor necrosis factor,IL-1β,IL-2,IL-8,IL-12),high production of anti-inflammatory cytokines (IL-10 and transforming growth factor β),defective nuclear factor-κB activation,and upregulation of arginase 1.On their surface,they show increased expression of CD204 and CD206 receptors (Orecchioni et al.,2019).M2 macrophages and microglia also release brain-derived neurotrophic factor,which plays an essential role in promoting neurogenesis and repairing damage (Miao et al.,2018).M2 cells are,therefore,referred to as anti-inflammatory cells.
M1 macrophages are essential for the first lines of defense against pathogens by phagocytizing microorganisms and killing them intracellularly.On the other hand,M2 macrophages can also fight infections by generating a fibrous capsule to sequester parasites (Orecchioni et al.,2019).Classically,M1 macrophages and microglia have been associated with detrimental effects of the inflammatory response,mostly at chronic stages,since they secrete cytotoxic factors that can damage healthy neighboring tissue (Orecchioni et al.,2019).Conversely,M2 macrophages and microglia have been associated with beneficial effects at chronic stages since they produce factors involved in the healing processes (Orecchioni et al.,2019).
Due to their unique functions,these polarization states are characterized by different energy and precursor demands,which translate into different metabolic pathways.After becoming activated,M1 macrophages and microglia switch from resting OXPHOS to aerobic glycolysis similarly to tumor cells during the Warburg effect.M1 macrophages are glycolytic and use glucose as their primary energy source (Galvan-Pena and O’Neill,2014;Liu et al.,2021).In M1 macrophages,aerobic glycolysis involves increasing glucose uptake and converting pyruvate to lactate.At the same time,the activities of the TCA and ETC are attenuated (West et al.,2011).Accumulation of the TCA intermediate succinate is associated with the stabilization of hypoxia inducible factor (HIF) 1α and the induction of the pro-inflammatory cytokine IL-1β (Liu et al.,2021).The PPP activity is increased,generating the NADPH of the NADPH oxidase,which is essential for ROS and RNS production (Aktan,2004).The main goal of all these metabolic changes is to provide the cell with rapid energy and reducing equivalents required for bactericidal activity (Figure 1).As happened in the Warburg effect,glycolysis is less efficient in energy production,but it allows rapid ATP generation for the first stages of the inflammatory response.

Figure 1 |Metabolic changes of macrophages and microglia during polarization.
In contrast,M2 macrophages obtain most of their energy from fatty acid oxidation (FAO) and OXPHOS metabolism,which can be sustained longer (Galvan-Pena and O’Neill,2014;Liu et al.,2021).Following activation,M2 macrophages induce the expression of components of the ETC for OXPHOS and drive the pyruvate into the Krebs cycle.The PPP is also diminished in M2 macrophages (Liu et al.,2021).OXPHOS is a more efficient form of energy production,but it is slower than glycolysis and intermediates are not accumulated for pro-inflammatory cytokine production.Glutamine uptake and oxidation (glutaminolysis) is also increased,fueling the TCA.Energy from the ETC is used to sustain long-term activities related to wound repair (Kotwal and Chien,2017;Figure 1).
In summary,the distinct metabolic pathways of M1 and M2 macrophages reflect their functional phenotypes and play a critical role in inflammation and tissue repair.The switch between glycolytic M1 and oxidative M2 macrophages istightly regulated and involves changes in gene expression,as explained in the following section.
Importance of Hypoxia Inducible Factor-1α in Triggering the Metabolic Switch in Microglia and Macrophages
As exemplified with cancer cells,hypoxia and metabolism are inherently linked.Hypoxia is a main component of solid tumors,primarily due to insufficient vascularization (Emami Nejad et al.,2021).By translocating to the nucleus,the HIF pathway allows cells to adapt to hypoxic conditions and is a critical transcriptional regulator of inflammation (Nagao et al.,2019).
HIF is a heterodimeric protein composed of an alpha and beta subunit (Wang et al.,1995).Three isoforms of the oxygen-sensitive alpha subunit have been identified.HIF-1α is ubiquitously expressed in several cell types (Cramer et al.,2003;Walmsley et al.,2005).HIF-2α expression,on the other hand,is limited to certain cells,including myeloid cells (Talks et al.,2000).HIF-3α is the most recently discovered isoform,and its expression is largely restricted to tissues where control of neo-angiogenesis is tightly regulated (Makino et al.,2001).HIF-3α is thought to operate as a dominant negative inhibitor of HIF-1α and HIF-2α since it lacks the transactivation domain required to initiate transcription (Moniz et al.,2014).
The stability of the alpha subunit is the primary regulator of HIF signaling.In normoxia,HIF-1α is rapidly hydroxylated and marked for continuous degradation,resulting in low basal levels (Huang et al.,1996).Hypoxic conditions result in an attenuation of HIF-1α hydroxylation.As a result,HIF-1α accumulates and translocates to the nucleus where it induces the transcription of hypoxia response elements (Corcoran and O’Neill,2016).
Due to low oxygen levels in tumor cells,HIF-1α binds to hypoxia response elements on target genes,such as the glucose transporter GLUT1 and glycolytic enzymes (Chen et al.,2001).HIF-1α induces the expression of LDH,which catalyzes lactate production from pyruvate (Cui et al.,2017).This enzyme inhibits the conversion of pyruvate to acetyl-CoA,hampering the activity of the TCA cycle.HIF-1α also increases the expression of pyruvate dehydrogenase kinase(Kim et al.,2006),which inhibits pyruvate dehydrogenase and limits the formation of acetyl-CoA from pyruvate.Through this mechanism,HIF-1α promotes the glycolytic conversion,allowing the production of ATP in hypoxic macrophages and microglia (Figure 1).
Plenty of evidence suggests a role for the HIF-1α and HIF-2α in macrophage polarization.While HIF-1α has been linked with classical M1 macrophage activation,HIF-2α has been linked to an M2 phenotype (Takeda et al.,2010).In macrophage activation,LPS boosts HIF-1α mRNA transcription via induction of NF-kB activity,further increasing glycolytic signaling through the HIF pathway (Rius et al.,2008;Fitzpatrick et al.,2011).IL-1β production in response to LPS treatment has been shown to be inhibited by the glycolytic inhibitor 2-deoxyglucose (2-DG),being HIF-1α part of this process (Zhang et al.,2006;Tannahill et al.,2013).Macrophages lacking HIF-1α also show decreased expression of inducible nitric oxide synthase under hypoxic conditions and after LPS administration in mice (Takeda et al.,2010).Moreover,bacterial infection induces HIF-1α expression in macrophages,while macrophages are less efficient in killing bacteria when HIF-1α is lacking (Peyssonnaux et al.,2005).
The potential role of HIF-2α in promoting the M2 phenotype remains unclear,and further research is required (Steinberger and Eubank,2023).HIF-2α has been shown to regulate the transcription of the M2 marker arginase 1 (Takeda et al.,2010).However,there are discrepancies regarding the role of HIF-2α in macrophage polarization since it also induces IL-1β production,which is associated with the M1 phenotype rather than M2 (Tannahill et al.,2013).Additionally,HIF-2α has been linked to NF-kB activity,also associated with the M1 phenotype (Fang et al.,2009).Both HIF-1α and HIF-2α isoforms appear to have some redundant and overlapping functions;however,knocking down one isoform does not seem to compensate for losing the other (Fang et al.,2009).This indicates that there are still major breaks in understanding the opposite activities of the two isoforms.
Metabolic Changes in Other Immune Cells
T-cells
During their lifetime,T-cells experience a wide range of biological activities and metabolic adjustments to meet their energy and biosynthesis requirements.First,at early phases of thymic maturation,an increase in glycolytic metabolism is indicated by the expression of glucose transporters GLUT1 and GLUT4.However,as thymocytes mature to later stages,the levels of GLUT1 and GLUT4 are significantly diminished(Swainson et al.,2005;Carbo and Guarner,2010).Naïve T lymphocytes (Tn) abandon the thymus,circulate into the bloodstream,and reach the secondary lymphoid organs,such as the spleen and lymph nodes.Peripheral Tn cells remain in a quiescent state with relatively low energetic requirements and only accumulate essential cellular building components (Jones et al.,2019).To meet their minimal energy requirements,Tn cells metabolize glucose into pyruvate,which enters the TCA cycle and undergoes OXPHOS (Nicoli et al.,2018;Jones et al.,2019).Alternatively,Tn cells may also utilize FAO to provide energy (Nicoli et al.,2018;Figure 2).
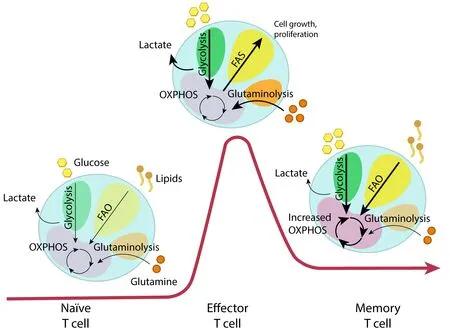
Figure 2 |Metabolic reprogramming of T-cells during the activation process.
Activation of Tn cells is produced by the binding of the T-cells receptor to the antigen peptides on the major histocompatibility complex from antigen-presenting cells(Gaud et al.,2018).Together with co-stimulatory molecules,this bind triggers multiple signaling pathways,leading to the expansion and transformation of activated T-cells into effector T-cells (Teff).To meet the energetic needs for rapid proliferation,clonal expansion,and effector functions,Teff cells are reprogrammed into anaerobic glycolysis (Pearce et al.,2009;Michalek et al.,2011;van der Windt et al.,2012).Glutaminolysis is also enhanced in Teff cells by providing intermediates for the generation of building blocks (Wang et al.,2022).This metabolic reprogramming is due to the activation of PI3K-AKT-mTOR axis and Myc signaling,which is triggered by the stimulation of the T-cells receptor and some mediators such as IL-2 (Wang et al.,2022).
The remaining population of Teff cells evolves into memory T (Tm) cells,which will respond to future challenges Tm cells have experienced in the past (Pearce et al.,2009).In contrast to the high glycolytic rate of Teff cells,Tm cells depend on OXPHOS and FAO for their long-term survival and sustain a recall response (Pearce et al.,2009;Michalek et al.,2011;van der Windt et al.,2012).Compared to Teff,Tm cells have an increased mitochondrial volume and are more dependent on mitochondrial respiration (Wherry and Ahmed,2004;Figure 2).
As it takes place with macrophages,changes in metabolism can alter the phenotype and function of T-cells.For example,Th1 cells,which produce IFNγ and activate macrophages to eliminate intracellular pathogens (Geginat et al.,2014),rely mainly on glycolysis and some OXPHOS with high expression of GLUT1 (Michalek et al.,2011;Ray et al.,2015;Rashida Gnanaprakasam et al.,2018).Deficiency of LDHA has shown reduced IFNγ levels under Th1 conditions (Peng et al.,2016).Another study proved that under defective Th1 conditions,T-cells fail to upregulate glycolysis and OXPHOS (Kolev et al.,2015).In contrast,Th2 cells,which protect against extracellular pathogens by secreting IL-4,IL-5,and IL-13 cytokines (Jones et al.,2019),predominantly utilize aerobic glycolysis and express higher levels of GLUT1 than any other Teff cells (Stark et al.,2019).Studies have demonstrated that treatment with the glycolysis inhibitor 2-DG impaired Th2 differentiation (Yang et al.,2016;Jones et al.,2019).Finally,T helper 17 (Th17) cells,involved in protection against extracellular bacteria and fungi,rely on glucose and glutamine uptake (Michalek et al.,2011;Ray et al.,2015).Regulatory T cells (Treg) have immunosuppressive capacities and exhibit both OXPHOS and FAO to maintain function (Berod et al.,2014;Ray et al.,2015).
B-cells
Like T-cells,B cells show increased glycolysis after activation following B-cell receptor engagement or cytokine stimulation(Doughty et al.,2006;Dufort et al.,2007).Although it has been found that B-cells increase both oxygen consumption rate and glycolysis rate upon antigen challenges,they polarized towards a more glycolytic metabolism (Caro-Maldonado et al.,2014).Glycolysis is used to support B-cell growth or protect B-cell from apoptosis stimulation (Doughty et al.,2006;Dufort et al.,2007).Indeed,inhibition of glycolysis suppresses B-cell proliferation and antibody production (Caro-Maldonado et al.,2014).In the case of long-lived memory B-cells,metabolic studies revealed that they rely on mitochondrial metabolism.This is sustained by increased respiratory fuels,such as pyruvate (Lam et al.,2016).In this line,inhibition of pyruvate imports by knocking down mitochondrial pyruvate carrier 1 (MPC1) or mitochondrial pyruvate carrier 2 (MPC2)is expected to reduce long-lived memory B-cells and antibody secretion (Corcoran and Nutt,2016;Lam et al.,2016).
Dendritic cells
Dendritic cells are antigen-presenting cells.As happened with macrophages and microglia,metabolic reprogramming accompanies dendritic cell development,activation,and differentiation (Krawczyk et al.,2010;He et al.,2019).This reprogramming is tightly regulated by AMP-activated protein kinase (Krawczyk et al.,2010).Resting dendritic cells rely predominantly on FAO and OXPHOS as the main sources of energy production and switch to glycolysis upon TLR activation(Krawczyk et al.,2010).This provides the energy required to support their motility,migration,and pro-inflammatory phenotypes.Blockade of glycolysis by glucose restriction or 2-DG is unable to support dendritic cell shape and motility(Guak et al.,2018).
Astrocytes
In resting conditions,astrocytes maintain a metabolism based on glycolysis and lactate production with minimal reliance on mitochondrial oxidation (Hyder et al.,2006).Astrocytederived lactate provides an oxidable energetic substrate to fuel neurons (Magistretti and Allaman,2015).Therefore,astrocytes process glucose for their own demands and provide energetic support to neurons (Mergenthaler et al.,2013).Unlike microglia,reactive astrocytes show no significant changes in their metabolic profile in reactive conditions.However,an increase in glycolytic activity has been described(Zamanian et al.,2012;Liddelow et al.,2017).Astrocytes exposed to LPS showed increased production of ATP through glycolysis coupled with reduced OCR (Ferrick et al.,2008).The same results were obtained after exposure to beta-peptide with increased lactate production (Allaman et al.,2010).Attenuated astrocytic glucose uptake leads to reduced lactate production and intellectual disabilities that can be restored by lactate supplementation (Zhang et al.,2022).
Contribution of Lipids to Inflammation
Lipids play a crucial role in cell function,participating in the synthesis of biomass products and activating signaling pathways related to normal cell activity.However,they are also used as an alternative fuel source (Cockcroft,2021).FAO is a fundamental catabolic pathway for energy production (Houten et al.,2016).First,long-fatty acids are converted to fatty-acyl-CoAs in the cytosol.Then,fatty acyl-CoA are transported by the carnitine shuttle system into the mitochondria,where they undertake different steps to generate acetyl-CoA,which can be further oxidized by the TCA.The reduced electron carriers FADH2 and NADH from FAO and TCA deliver electrons to the ETC,producing high levels of ATP (Figure 1).In humans,FAO accounts for more than half of the ATP production (Houten et al.,2016).
Abnormal lipid metabolism has been associated with irregular immune responses,leading to atherosclerosis,diabetes,obesity,and cancer (Mathis and Shoelson,2011).On the other hand,pro-inflammatory signaling can regulate lipid metabolism (Okoro,2021).Although there is mounting evidence of a connection between lipid metabolism and inflammation,the molecular mechanisms involved are still poorly understood.
Macrophages and microglia express numerous receptors to monitor and sense alterations in the lipid content of the environment (Hickman et al.,2013).These receptors participate in lipid sensing,uptake,and signaling to regulate cellular functions.The triggering receptor expressed on myeloid cells 2 (TREM2),CD300f,and CD36 are examples of lipid receptors expressed on macrophages and microglia(Figure 1).TREM2 recognizes various ligands,including lipids,to induce their phagocytosis (Wang et al.,2015b;Nugent et al.,2020).TREM2 also recognizes ApoE,the brain’s predominant carrier of cholesterol transport (Figure 1).Targeting ApoE has been demonstrated to change the phenotype of macrophages and microglia.For example,increasing expression or activation of ApoE impairs pro-inflammatory microglial function and suppresses T-cell proliferation (Krasemann et al.,2017).In the same way,deleting ApoE in mice results in the accumulation of cholesteryl ester in microglia,which may dampen mitochondrial OXPHOS (Nugent et al.,2020).This leads to a metabolism shift towards glycolysis and pro-inflammatory conditions,as explained before.
CD300f is an immune receptor that shares many lipid ligands with TREM2.The lack of CD300f impedes the adoption of the Warburg effect in microglia isolated from mice challenged with systemic LPS,suggesting that this immune receptor plays a key role in regulating microglia metabolism (Lago et al.,2020).Indeed,RNAseq analysis from microglial isolated from mice treated with systemic LPS revealed that genetic deletion ofCd300fresulted in downregulation of several pathways related to glycolysis,gluconeogenesis,FAO,and TCA,indicating that the lack of CD300f results in metabolic dysfunction.In accordance,CD300f KO microglia isolated from LPS-treated mice showed reduced basal mitochondrial and glycolytic activity,and the glycolytic shift induced by several mitochondrial stressors was not observed in the lack of CD300f (Lago et al.,2020).
CD36 is another lipid-sensing receptor densely expressed in macrophages and microglia with roles in metabolism and immunity (Chen et al.,2022).Upon binding lipid-related ligands such as long-chain FAs or oxidized LDL,CD36 mediated lipid uptake but impairs FAO and OXPHOS.This leads to fatty acid accumulation and foam cell formation (Rahaman et al.,2006;Stewart et al.,2010;Chen et al.,2019).These foam cells are usually found on atherosclerotic plaques,and they rely on glycolysis as the main energy source with the corresponding production of pro-inflammatory cytokines and mitochondrial ROS production (Chen et al.,2019).
Normally,lipids accumulate in the cell in the form of lipid droplets (LDs) (Figure 1).LDs are small,spherical,and dynamic organelles found in almost every cells of the body (Cohen,2018).They are composed of a neutral lipid core formed by triglycerides and cholesterol esters,which are surrounded by a phospholipid monolayer with associated proteins (Welte and Gould,2017).LDs play a critical role in a range of cellular processes,including energy storage,membrane synthesis,and the regulation of cellular metabolism.
The phospholipid monolayer surrounding the neutral lipid core of LDs is similar in composition to cellular membranes.Therefore,LDs can serve as a reservoir of membrane lipids that can be mobilized when new membranes are needed,like in phagocytosis (Cohen,2018).However,one of the primary functions of LDs is to store excess energy in the form of triglycerides.Adipocytes,which are specialized cells found in adipose tissue,are the primary cells responsible for lipid storage in the body.LDs in adipocytes can reach sizes of up to several microns in diameter,and they can occupy up to 95%of the cytoplasmic volume of these cells (Walther and Farese,2012).When energy is required,the stored triglycerides in LDs are hydrolyzed by lipases to release fatty acids and glycerol.These products can then be oxidized in mitochondria to produce ATP,which is the primary energy currency of the cells.The same can be found in macrophage foam cells (Robichaud et al.,2021).
Although LDs have traditionally been seen as inert storage organelles,recent studies have suggested that they respond to physiological stressors and are involved in the pathogenesis of various metabolic diseases (Welte and Gould,2017;Jarc and Petan,2019;Monson et al.,2021a).Under normal conditions,LDs are observed at low levels,but they tend to accumulate following insults (Monson et al.,2021b).For this reason,LDs are studied as a landmark of inflammation.
Neurodegenerative diseases and aging are examples of LDs increased biogenesis (Liu et al.,2015b,2017;Marschallinger et al.,2020).The transition from resting to activated phenotypes in immune cells requires a re-distribution of nutrients into different metabolic pathways to support functional changes and LDs play a main role in this process(Caputa et al.,2019).Catalysis of LDs can generate lipids that can be used as substrates for critical metabolic processes or be utilized for synthesizing of membranes,inflammatory mediators,or signaling to the nucleus (Jarc and Petan,2020).As explained before,activated immune cells switch their metabolic state to a higher reliance on aerobic glycolysis(O’Neill et al.,2016).On the other hand,cells with respiratory roles tend to use lipids from LDs for FAO and energy production (O’Neill et al.,2016).For these reasons,M1 macrophages are characterized by an increase in the synthesis of FA and cholesterol (Hsieh et al.,2020).Lipogenesis in M1-polarized macrophages is essential to support their inflammatory and phagocytic roles,which require intensive remodeling of the plasma membrane,ER,and Golgi apparatus(Figure 1).They also require access to precursors to produce bioactive lipids (Everts et al.,2014).For this reason,the formation of LDs is increased through the upregulation of LDs-associated proteins and the induction of ER stress.In contrast,M2 macrophages,which rely on FAO and OXPHOS,do not accumulate LDs to the same extent.The breakdown of lipids for metabolic consumption and the cholesterol efflux is favored,preventing the accumulation of LDs (Huang et al.,2014;Figure 1).Moreover,impairment in LDs turnover mechanism has been shown to promote pro-inflammatory phenotype in murine macrophages (Liu et al.,2015a) and impair neutrophil differentiation (Riffelmacher et al.,2017).
Metabolic State of Immune Cells Regulates NLRP3 Activation
NLR family pyrin domain containing 3 (NLRP3) is an essential component of the innate immune system responsible for detecting and responding to various danger signals and pathogens.It plays a central role in initiating the formation of a macromolecular complex named inflammasome,which facilitates the activation of caspase-1,which catalyzes the processing of IL-1β and IL-18 (Guo et al.,2015).These cytokines are crucial in promoting inflammation and immune responses,including those affecting the central nervous system (Amo-Aparicio et al.,2022,2023).Glycolysis,OXPHOS,and FAO have a direct impact on the priming and activation of the NLRP3 inflammasome.For instance,in the case of glycolysis,blockade of hexokinase 1 and pyruvate kinase M2 (PKM2),which catalyze the first and last stages of glycolysis respectively,limits canonical NLRP3 activation in bone marrow-derived macrophages (BMDMs) (Xie et al.,2016).However,it has also been shown that HK2 inhibition by bacterial peptidoglycans boosts inflammasome activation(Wolf et al.,2016).Disruption of glycolytic flux triggers the NLRP3 inflammasome in mouse BMDMs,suggesting that NLRP3 can detect changes in the glycolytic flux (Sanman et al.,2016).
In the case of TCA,intermediates perform important roles in cell signaling,including the activation of the NLP3 inflammasome (Martinez-Reyes and Chandel,2020).For example,experiments in mouse BMDMs lacking IRG1,which catalyzes the TCA intermediate citrate into itaconate,reducing the TCA cycle efflux,revealed lower NLRP3 activation(Lampropoulou et al.,2016).Moreover,mouse macrophages exposed to high concentrations of itaconate also resulted in NLRP3 activation (Swain et al.,2020).Succinate,one of the intermediates of the TCA,has also been proposed to stabilize the HIF-1α,resulting in IL-1β expression through NLRP3(DeBerardinis et al.,2007;Tannahill et al.,2013).Conversely,hydrolysis of HIF-1α by prolyl hydroxylase generates succinate as a product,and it is impaired during macrophage activation(Tannahill et al.,2013).Itaconate also impairs the conversion of succinate to fumarate in the TCA by blocking succinate dehydrogenase and taking succinate away from the TCA cycle (Lampropoulou et al.,2016).Finally,fumarate can block gasdermin D,required for pyroptosis (Humphries et al.,2020),while itaconate activates it (Qin et al.,2020).
FAS and FAO can also regulate the NLRP3 inflammasome.Carnitine palmitoyltransferase 1A,the enzyme regulating the FA entry into the mitochondrial matrix for FAO,enhances NLRP3 activation (Moon et al.,2016).On the other hand,the downregulation of this enzyme and inhibition of FAO diminishes NLRP3 activation.FAS also interacts with NLRP3 activation.Fatty acid synthase is upregulated in proinflammatory macrophages and is essential for NLRP3 inflammasome priming (Moon et al.,2015).Moreover,it has been demonstrated that pharmacological inhibition of the NLRP3 inflammasome disturbs LDs formation (Tian et al.,2019).
Immune Metabolic Reprogramming in the Brain:Neuroimmune Metabolism
Despite comprising only 2% of the body weight,the human brain utilizes approximately 20% of the body’s glucose and oxygen to satisfy its high metabolic demands during neural activity (Magistretti and Allaman,2018).This microenvironment metabolically unique is maintained through a strict regulation of energy substrates,including glucose,fatty acids,and amino acids.
The blood-brain barrier (BBB) regulates the transfer of energy sources from the blood to the brain parenchyma.Glucose,the brain’s primary substrate,is transported from the blood into the brain through specialized GLUT transporters(Mergenthaler et al.,2013;Jurcovicova,2014).The passage of fatty acids through the BBB is debated,with some publications suggesting diffusion and others supporting protein-mediated transport (Romano et al.,2017).Various amino acids and its metabolic subproducts,such as glutamate,glycine,and GABA,are tightly regulated because they are used for synaptic communication between neurons (Schousboe,2019).Astrocytes,components of the BBB,play a crucial role in maintaining the brain’s metabolic requirements.Astrocytes provide lactate from stored glycogen and rapidly uptake glutamate for its conversion to glutamine (Belanger et al.,2011).
Several studies have revealed that microglia possess transporters and enzymes that could potentially facilitate the metabolism of glucose,amino acids,and FA (Bennett et al.,2016).Microglia demonstrate transcription of multiple isoforms of glucose transporters (GLUTs),including GLUT3,which is also elevated in neurons.Notably,among the components of the central nervous system,microglia are the unique expressers of the hexose transporter GLUT5,which exhibits a strong affinity for fructose (Payne et al.,1997).However,it remains uncertain whether GLUT5 plays a role in microglial functions,given the limited availability of fructose in the brain under normal physiological circumstances (Hwang et al.,2017).Through glycolysis,FAO,and glutaminolysis,microglia obtain all the sources and intermediates to perform OXPHOS.
Alzheimer’s disease
Recent studies highlight the role of microglial metabolism in AD.Several lines of evidence describe that exposure to amyloid beta peptide (Aβ) triggers metabolic changes in microglia,like those observed with damage-associated molecular patterns and pathogen-associated molecular patterns stimuli.Increased glycolysis was observed in culture microglia exposed to IFNγ+Aβ (McIntosh et al.,2019),LPS +Aβ (Rubio-Araiz et al.,2018),as well culture microglia from APP/PS1 mouse model of AD (Holland et al.,2018).Therefore,Aβ led microglia to a more glycolytic state (Baik et al.,2019).This correlates with cytokine release,which is blocked by 2-DG.Longer exposition to Aβ makes microglial tolerant and deprived of the metabolic changes necessary for their function(Baik et al.,2019).Therefore,microglia became less sensitive to local damage.Notably,the addition of IFNγ worked as a“metabolic booster,” restoring mTOR signaling and glycolysis and rescuing some of the microglial functions in 5XFAD mice(Baik et al.,2019).These data suggest that restoring metabolic functions in microglia represents a therapeutic target.
Mutations in TREM2 are a risk factor for AD pathogenesis,but the definitive mechanism is unknown.Microglial cells with mutations in TREM2,which also recognizes Aβ aggregates,are unable to undergo a metabolic reprogram to glycolysis,which ultimately obstructs the phagocytic activity required to remove amyloid aggregates (Piers et al.,2020).Gene expression analysis in 5xFAD mouse models of AD revealed the absence of TREM2 not only reduced mTOR signaling but also avoided the required increase in HIF-1α accumulation and glycolysis activity.Furthermore,TREM2 deficiency translates into metabolic defects characterized by deficient autophagy,altered mTOR signaling,and reduced ATP production (Ulland et al.,2017).Moreover,the addition of Dectin-1,which mimics TREM2 signaling,rescued the protective microglial functions,such as aggregation around amyloid plaques (Ulland et al.,2017).
Wide analysis of AD patients revealed that reduced glucose consumption in the brain is a component of AD (Daulatzai,2017;Butterfield and Halliwell,2019).Low glucose availability in the AD brain can be due to vascular changes (Nortley et al.,2019),insulin resistance (Neth and Craft,2017),or downregulation of GLUTs (Shah et al.,2012).This not only affects the overall brain energetics but also affects microglia function by preventing regulatory signals associated with glycolytic intermediates during activation.
Parkinson’s disease
PD is also linked to metabolic changes.Indeed,risk factors for PD often involve changes in glucose transporters,metabolic enzymes,and transcription factors responsible for metabolic pathways (Dunn et al.,2014;Zhang et al.,2016;Anandhan et al.,2017).Diminished glucose metabolism within the cortex is often associated with the onset of PD (Firbank et al.,2017).Downregulation in the catabolism of branched-chain amino acids by branched-chain amino acid transferase (BCAT1) has been observed in the substantia nigra of PD patients (Yao et al.,2018).Genetic deletion ofBcat1enhances mitochondrial respiration and leads to oxidative damage in neurons through mTOR-independent pathways (Mor et al.,2020).Lastly,disruption in glycolytic enzymes (GLUT,MCT1,MCT4,LDHA,and PKM2) contribute to a hypometabolic state in the PD brain (Vallee et al.,2019;Lundquist et al.,2022).Knockdown of one of these genes also translates into functional deficits in mice models of PD (Lundquist et al.,2021).
Rotenone and tebufenpyrad,inhibitors of the mitochondrial complex I of the ETC,are common neurotoxic models of PD.Exposure of primary microglia to these neurotoxins drives mitochondria to ROS generation,activation of the NLRP3 inflammasome,and release of IL-1β (Sarkar et al.,2017).LRRK2,which drives mitochondrial fragmentation,leading to excessive mitochondrial fission and the secretion of proinflammatory cytokines (Ho et al.,2018),is one of the genes affected in familial cases of PD.Finally,TREM2 has recently been proposed to contribute to PD with numerous findings correlating loss-of-function mutations in TREM2 with increased risk of PD (Li and Zhang,2021).
The connection between metabolism and inflammation is evident in the case of PD and diabetes.Epidemiological studies have revealed a strong correlation between type 2 diabetes and increased risk and disease progression of PD (Camargo Maluf et al.,2019).Animal models for diabetes are more susceptible to neurotoxins used to mimic PD symptoms and show higher accumulation of α-synuclein,neuroinflammation,dopamine depletion,and loss of dopaminergic neurons (Wang et al.,2014).Conversely,transgenic mice overexpressing α-synuclein for PD show impairments in the insulin signaling pathway and develop insulin resistance (Gao et al.,2015).This is produced by α-synuclein,which destabilizes the insulin receptor substrate-1 (Gao et al.,2015),leading to abnormal mitochondrial metabolism and increased production of reactive oxygen species (Hong et al.,2020).Due to the relation with insulin,glucagon-like peptide-1 and gastric inhibitory polypeptide analogs,used to stimulate the release of insulin in the treatment of type 2 diabetes,are being evaluated in clinical trials as new approaches for PD.These compounds have shown neuroprotective activities by inhibiting inflammation and enhancing neuronal survival (Yang et al.,2022).
Stroke
Metabolic disorders correlate with the incidence and progression of stroke.Upon ischemic onset,brain tissues enhance their metabolic plasticity through energy metabolic reprogramming.Chemokine-like factor 1 (CKLF1) is highly expressed in the brain after cerebral ischemia (Kong et al.,2011;Wang et al.,2012) and has been found to enhance the glycolysis ability of microglia while inhibiting the OXPHOS (Ma et al.,2023).This is produced through C27,the C-terminal peptide of CKLF1,which significantly increases the expression of GLUT1 and improves glucose uptake,providing more substrates for the increased glycolysis (Ma et al.,2023).Finally,microglia unable to react to CKLF1 show metabolic and phagocytic dysfunction (Ma et al.,2023).This correlates with other publications showing that the inhibition of CKLF1 may translate into severe nerve injury and decrease the number of activated microglia (Zhou et al.,2022).
Immune-responsive gene 1 (IRG1) is a mitochondrial enzyme induced after inflammatory stimuli that has been shown to catalyze the production of itaconate by diverting cisaconitate away from TCA and,therefore,reducing OXPHOS(Lampropoulou et al.,2016).Macrophages lacking IRG1 exhibited augmented inflammatory response after LPS stimulation (Mills et al.,2018).In the case of ischemic stroke,induction of IRG1 after damage serves as an endogenous protective mechanism to restrain ischemic brain injury,allowing macrophages and microglia to respond to the new energy requirements.IRG1-null mice displayed aggravated BBB disruption,augmented microglia activation,and exacerbated ischemic brain injury (Kuo et al.,2021).Conversely,administering dimethyl itaconate to compensate for the deficiency in IRG1 led to enhanced microglial heme oxygenase-1 expression,alleviated ischemic brain injury,improved motor function,and decreased mortality (Kuo et al.,2021).
LDs accumulation in microglia is also observed after ischemic stroke (Arbaizar-Rovirosa et al.,2023).This accumulation is even higher in old mice after stroke,but also under steady conditions,and is associated with impaired phagocytic functions and exacerbated inflammatory response (Arbaizar-Rovirosa et al.,2023).Moreover,microglial renewal in old mice reduced the accumulation of LDs and improved motor function recovery after stroke (Arbaizar-Rovirosa et al.,2023).In the same way,siRNA-mediated inhibition of LDs-related proteins decreased neurological deficit scores and reduced infarct areas in rats (Liu et al.,2023).
Spinal cord injury
Recent studies suggest that myelin debris significantly affects macrophage function after spinal cord injury (SCI)(Greenhalgh and David,2014;Wang et al.,2015a).Myelinladen macrophages,referred to as foam cells,show an impaired inflammatory function including inefficient phagocytosis (Bogie et al.,2013).RNA-sequencing studies have revealed how the function of macrophages changes over the development of the inflammatory response after SCI from inflammation and migration to lipid catabolism (Zhu et al.,2017).Genetic deletion ofCd36reduced the lipid content in macrophages and improved functional recovery after SCI (Zhu et al.,2017)
The polarization state of macrophages and microglia after SCI have been extensively described (Kigerl et al.,2009).Approaches aimed at increasing the levels of antiinflammatory cytokines in the damaged area seem to exert beneficial effects on the locomotor recovery,in part,by modulating the polarization state of immune cells (Amo-Aparicio and Penas,2022).However,little is known whether the beneficial actions of some anti-inflammatory cytokines to neurotrauma are due to metabolic reprogramming of immune cells.We recently reported that administration of recombinant IL-4 or IL-13 into the lesioned spinal cord promotes similar actions on the polarization of macrophages and microglia towards the anti-inflammatory phenotype.However,IL-4,but not IL-13,improved functional and histological outcomes(Amo-Aparicio et al.,2021;Figure 3).In the search for the mechanisms underlying the different response elicited by IL-4 and IL-13,we found that IL-4,but not IL-13,shifted metabolism of microglia and macrophages from glycolytic to OXPHOS after SCI (Amo-Aparicio et al.,2021;Figure 3).Importantly,blockade of OXPHOS in macrophages stimulated with IL-4 led to deleterious effects on neurons,highlighting the importance of the metabolic shift from glycolysis to OXPHOS to mediate the protective actions of microglia and macrophages after neurotrauma (Amo-Aparicio et al.,2021).The connection between metabolism and immune cell responses after central nervous system injuries has not been fully address yet,and thus,further research is needed.
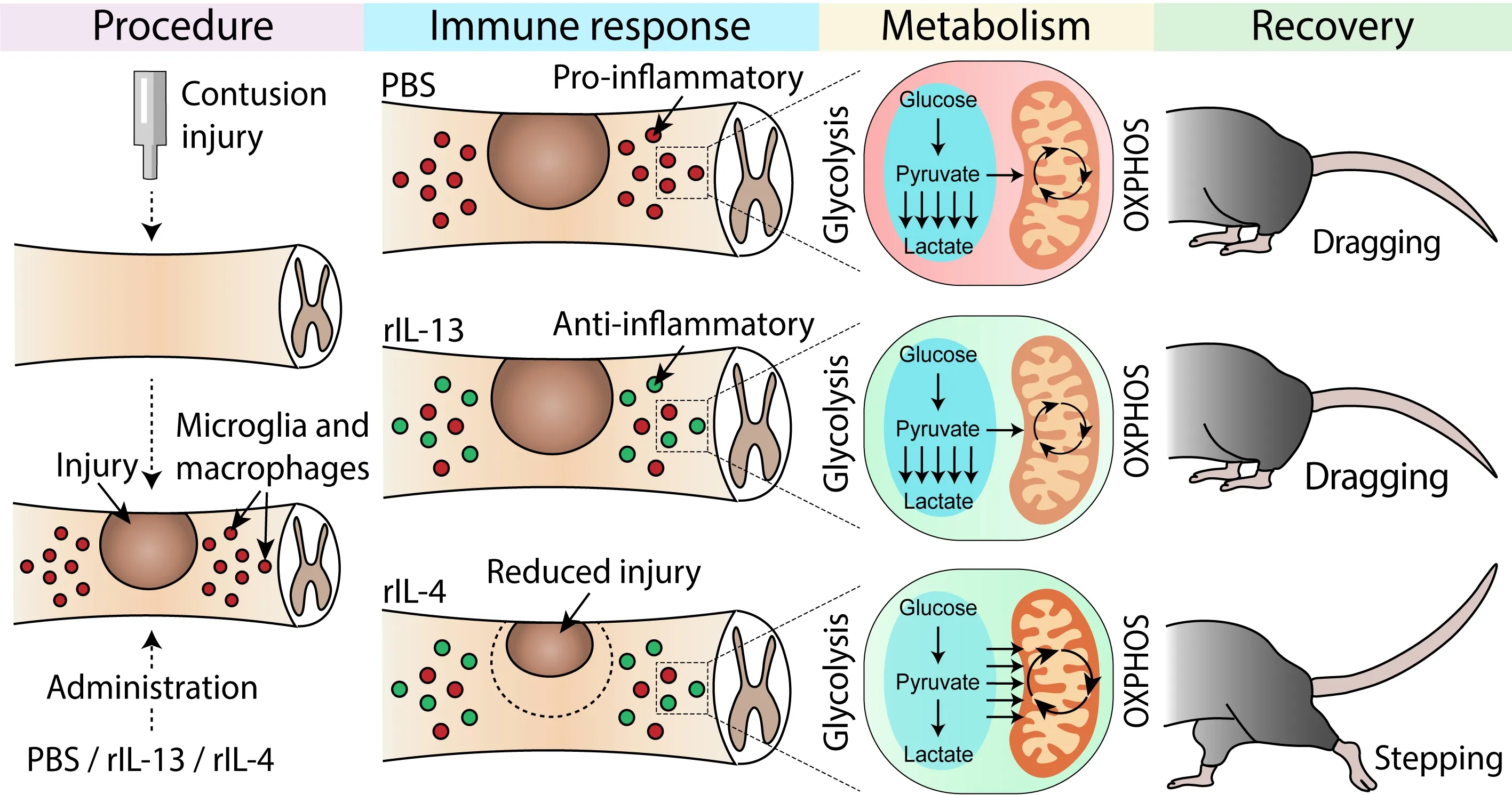
Figure 3 |Effect of metabolic reprogramming in functional recovery after spinal cord injury.
Aging
Aging is associated with a pro-inflammatory polarization of microglia (Norden and Godbout,2013),which is associated with PFKFB3 upregulation and increased glycolysis (Mela et al.,2020).However,like AD,a metabolic switch occurs in aged mice (Ivanisevic et al.,2016).This is characterized by imbalances in the cellular levels of metabolic substrates due to impaired blood flow and a reduced consumption of glucose in the brain (Mattson and Arumugam,2018).Studies in mice and humans have found that aging correlates with loss-offunction mutations in TREM2 (Nugent et al.,2020) and LDs accumulation (Marschallinger et al.,2020).This translated into defects in cellular debris clearance.
Conclusions
Metabolic reprogramming of the inflammatory response is a cutting-edge field in biomedical research.This review provides insights into how changes in inflammatory response affect metabolism and vice versa.With further research,manipulating the metabolic pathways can be used as a therapeutic target for treating a wide variety of inflammatoryrelated diseases.This is expected to revolutionize the field of immunology and open new avenues for medicine in the future.
Author contributions:Review conception and design,data search and manuscript writing:JAA,CAD,RLV.Figure preparation:JAA.All authors have read and approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no conflicts of interest.
Data availability statement:Not applicable.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice
- Conformational dynamics as an intrinsic determinant of prion protein misfolding and neurotoxicity
- Exploring the synergy of the eyebrain connection: neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation
- Pathogenic contribution of cholesteryl ester accumulation in the brain to neurodegenerative disorders
- Cognition and movement in neurodegenerative disorders:a dynamic duo
- Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease: searching far and wide
