Biomaterials and tissue engineering in traumatic brain injury: novel perspectives on promoting neural regeneration
2024-01-24ShihongZhuXiaoyinLiuXiyueLuQiangLiaoHuiyangLuoYuanTianXuChengYaxinJiangGuangdiLiuJingChen
Shihong Zhu ,Xiaoyin Liu ,Xiyue Lu ,Qiang Liao,Huiyang LuoYuan TianXu ChengYaxin Jiang,Guangdi Liu,Jing Chen
Abstract Traumatic brain injury is a serious medical condition that can be attributed to falls,motor vehicle accidents,sports injuries and acts of violence,causing a series of neural injuries and neuropsychiatric symptoms.However,limited accessibility to the injury sites,complicated histological and anatomical structure,intricate cellular and extracellular milieu,lack of regenerative capacity in the native cells,vast variety of damage routes,and the insufficient time available for treatment have restricted the widespread application of several therapeutic methods in cases of central nervous system injury.Tissue engineering and regenerative medicine have emerged as innovative approaches in the field of nerve regeneration.By combining biomaterials,stem cells,and growth factors,these approaches have provided a platform for developing effective treatments for neural injuries,which can offer the potential to restore neural function,improve patient outcomes,and reduce the need for drugs and invasive surgical procedures.Biomaterials have shown advantages in promoting neural development,inhibiting glial scar formation,and providing a suitable biomimetic neural microenvironment,which makes their application promising in the field of neural regeneration.For instance,bioactive scaffolds loaded with stem cells can provide a biocompatible and biodegradable milieu.Furthermore,stem cells-derived exosomes combine the advantages of stem cells,avoid the risk of immune rejection,cooperate with biomaterials to enhance their biological functions,and exert stable functions,thereby inducing angiogenesis and neural regeneration in patients with traumatic brain injury and promoting the recovery of brain function.Unfortunately,biomaterials have shown positive effects in the laboratory,but when similar materials are used in clinical studies of human central nervous system regeneration,their efficacy is unsatisfactory.Here,we review the characteristics and properties of various bioactive materials,followed by the introduction of applications based on biochemistry and cell molecules,and discuss the emerging role of biomaterials in promoting neural regeneration.Further,we summarize the adaptive biomaterials infused with exosomes produced from stem cells and stem cells themselves for the treatment of traumatic brain injury.Finally,we present the main limitations of biomaterials for the treatment of traumatic brain injury and offer insights into their future potential.
Key Words:bioactive materials;biomaterials;exosomes;neural regeneration;scaffolds;stem cells;tissue engineering;traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a serious medical condition that can have detrimental effects on both the patient and society at large.TBI can be caused by various factors such as falls,car accidents,sports-related injuries,and/or acts of violence.There are several harmful effects of TBI.First,TBI can cause a range of physical disabilities including paralysis,loss of sensation,and impaired coordination and balance.Second,TBI can also cause cognitive impairments such as memory loss,difficulty concentrating,and impaired judgment (Fure et al.,2021;McGeary et al.,2022).These limitations and impairments may significantly affect a person’s capacity to carry out everyday tasks and participate in social interactions.Third,TBI can lead to certain emotional and behavioral changes such as depression,anxiety,aggression,and impulsivity,which can strain relationships with family and friends and lead to social isolation and other negative outcomes (Czeiter et al.,2020;Tarudji et al.,2021).Finally,TBI can result in significant economic costs including medical expenses,lost productivity,and long-term care needs,which can be a burden on individuals,families,and society as a whole (Lu et al.,2021).
TBI not only results in neural injury,which is always followed by a number of complex and neuropsychiatric symptoms such as seizures,memory loss,insomnia,nervousness,and depression but also damages the brain and triggers cerebral edema,ischemia,and inflammation that disrupt homeostasis and induces unusual biochemical,metabolic,circadian,and neural circuit changes (Campos-Pires et al.,2020;Killgore et al.,2020;Rowell et al.,2020;Mahncke et al.,2021;Roquilly et al.,2021).Moreover,because of the intricate histological and anatomical structure,cellular and extracellular environment,and insufficient capability of the central nervous system (CNS) for healing by endogenous cells,injured brain tissue and function can be difficult to repair.In addition,neuroinflammation,glial scar formation,and neurodegeneration in secondary injuries after TBI may characterize CNS damage (Lassarén et al.,2021).The slow infiltration of macrophages can make the region of the injury take a long time to heal (Ma et al.,2020a).
Following TBI,neurogenic inflammation may disrupt metabolic balance through biological stress,excitatory toxicity,and cell activation (Anderson et al.,2020).Proinflammatory conditions are characterized by an increase in cytokines,such as interleukins,tumor necrosis factor-α,and reactive oxygen species (ROS),which reduce healthy cell function while keeping tissues responsive (Ashina et al.,2020a;Bernard et al.,2022).Astrocyte reactivity is an important component in the vast majority of events that occur during CNS injury.However,there are dual aspects to astrocyte reactivity.On the one hand,activated astrocytes can result in the formation of glial scars,which help protect neurons by monitoring immune reactions and blood flow and preventing further damage.On the other hand,it can also cause blood-brain barrier (BBB)disruptions,persistent inflammation,neural toxic effects,and cell death in nearby areas (Galarza et al.,2020).Additionally,the activation of astrocytes may provide a significant therapeutic obstacle for CNS tissue restoration (Maas et al.,2022).
Based on these mechanisms,various therapeutic strategies have been developed;for example,traditional treatment methods such as drugs and surgery and emerging treatment methods such as tissue engineering (TE) and regenerative medicine,including bioactive materials,growth factors,scaffolds,stem cells,and stem cell-derived exosomes.However,traditional treatments can only relieve the discomfort and prevent the aggravation of the disease,they cannot improve prognosis (Nemkova,2022).Biomaterials have demonstrated benefits in promoting brain growth,preventing the creation of glial scars,and offering a proper biomimetic neural microenvironment.For instance,stem cell-loaded bioactive scaffolds can offer a biocompatible and biodegradable environment (Tuladhar et al.,2020).Additionally,stem cell-derived exosomes combine the benefits of stem cells,reduce the chance of immune rejection,synergistically work with biomaterials to enhance their biological functions,and exert stable functions (Ghosh et al.,2020).As a result,they help TBI patients heal from their injuries by encouraging angiogenesis and nerve regeneration(Ma et al.,2020b;Fletcher et al.,2021).Therefore,by contrast,the emerging treatments overcome the limitations and have broad application prospects in the field of nerve regeneration.In this review,based on the mechanisms of TBI and the properties of various biomaterials,we discuss the role of biomaterials and TE in regulating biochemical stress,anti-neuroinflammation,neuroprotection,and neural and vascular regeneration in TBI patients (Figure 1).

Figure 1 |Tissue engineering and different types of biomaterials in TBI treatment.
Search Strategy
Studies cited in this review were obtained based on a PubMed search and were published between 2018 and 2023.The following search words appearing in the Title/Abstract were used: therapeutic strategies,neuroinflammation,and biochemical stress.Additionally,the following MeSH search terms were used: biocompatible materials,tissue engineering,nerve regeneration,tissue scaffolds,stem cells,exosomes,neuroprotection,and brain injuries.Identified articles were screened by title and abstract and articles that were inconsistent with our inclusion criteria were excluded from further analysis.
The Therapeutic Strategies and Challenges of Traumatic Brain Injury
Drugs,surgery,cell-based therapy,exosomes,and TE scaffolds are the most common treatment methods (Bailes and Borlongan,2020;Nemkova,2022;Zhu et al.,2022).Traditional therapeutic approaches slow the advancement of injury,while medications have thus far mostly focused only on pain management (Ashina et al.,2020b;Nilsson et al.,2020).The creation of a biomaterial that can effectively encourage neural stem cells (NSCs) to multiply and differentiate is therefore urgently needed (Li et al.,2019;Lainé et al.,2022).The complexity of the spatial organization and structure of CNS tissue,post-mitotic state of native cells,multiple routes of damage,and restricted window of potential for treatment are just some of the factors that hinder the validity of CNS treatments (Li et al.,2020a;Liao et al.,2021;Kolias et al.,2022).
A novel strategy for the regeneration of injured tissue and organs has evolved as a result of the advancement and growth of TE and regenerative science,surpassing the drawbacks of conventional therapies (Ghosh et al.,2020;Qian et al.,2021;Hasanzadeh et al.,2023).For example,multifunctional scaffolds that carry therapeutic compounds and cells,as well as hydrogels with fine material biochemical adaptations and the ability to carry cells,could be used to transport cells,therapeutic molecules,and proteins for the treatment of damaged brain tissue (Basit et al.,2021;Roh et al.,2023).Cyclosporine A can preserve the integrity of mitochondrial function,which might be a promising neuroprotective therapeutic strategy,but it has limited access to brain targets(Tuladhar et al.,2020).Surprisingly,a lipoprotein-biomimetic nanocarrier loaded with cyclosporine A in the core was proven to accomplish medication delivery that targeted the mitochondria and the TBI damage location (Sun et al.,2022b),which could effectively improve mitochondrial dysfunction,substantially ameliorate neural inflammation,minimize damage to neurons,and restore memory loss after TBI (Chen et al.,2020b).
The introduction of new biomolecules or mediators in certain prospective animal studies on CNS restoration has shown enhanced neural network repair,but when similar mediators were utilized in human clinical investigations of CNS regeneration,they showed poor regenerative potential.The challenges of transferring these therapies to clinical use are likely dependent on the intricate and unknown essential molecular and cellular functions,as well as the healing pathways,physical structure,and pathophysiological characteristics of the CNS (Jiang et al.,2021).Additionally,the BBB presents a significant obstacle,as it is a highly selective semipermeable membrane that limits the number of potential biomolecules for therapeutic use.
In conclusion,the only goals of conventional therapies are to reduce discomfort and halt the spread of disease.By efficiently promoting the proliferation and differentiation of neurons,fresh strategies for neural regeneration have been developed as a result of the development of TE and biomaterials,which go beyond the restrictions of conventional therapies.Furthermore,these innovative methods also offer a platform for creating effective treatments for nerve injuries,with the potential to restore nerve function,enhance patient outcomes,and lessen the need for drugs and invasive surgical procedures,which is accomplished by combining biomaterials,stem cells,growth factors,and scaffolds.The use of biomaterials in the field of nerve regeneration is promising because of their advantages in promoting neural development,preventing the growth of glial scars,and providing an appropriate biomimetic neural milieu (Figure 2).
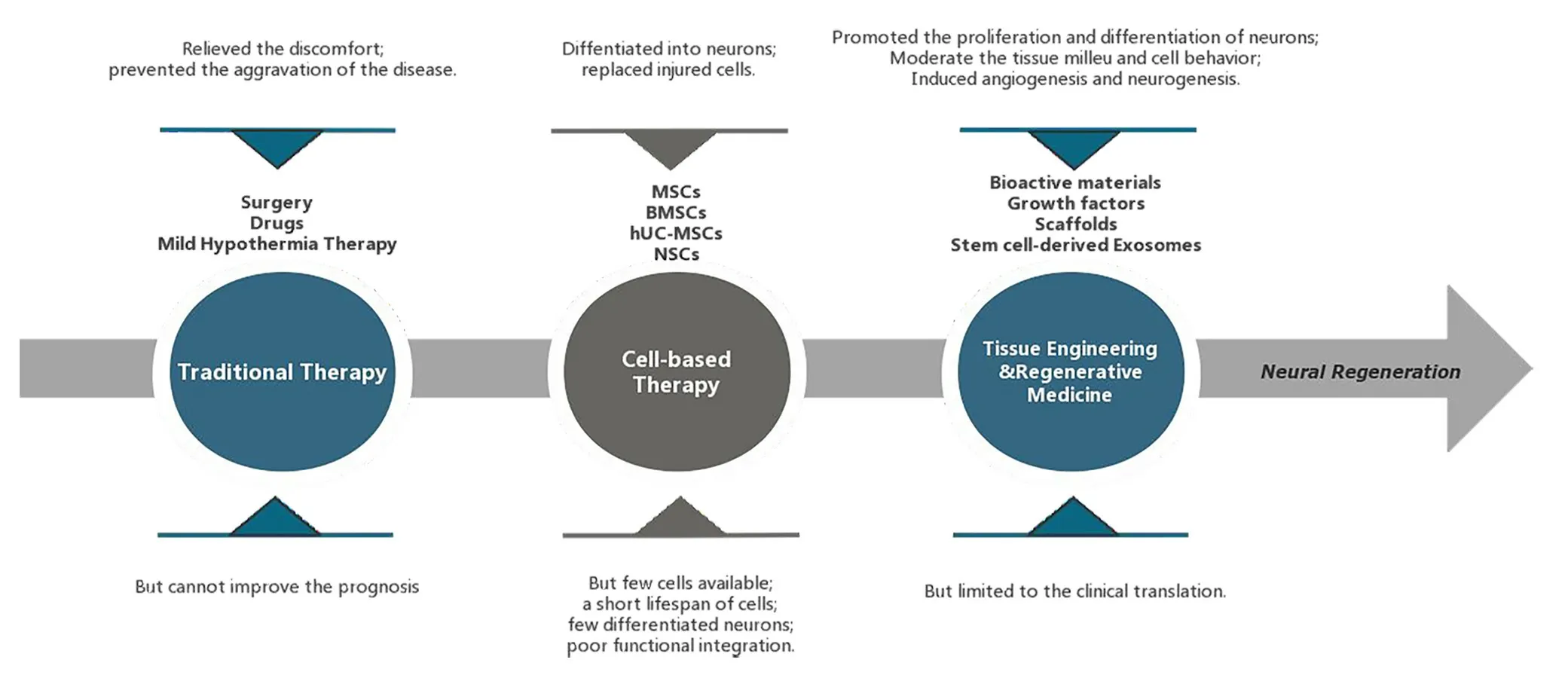
Figure 2 |The development and characteristics of traumatic brain injury (TBI)treatment strategies.
Natural and Synthetic Biomaterials
The interaction between immune cells and biomaterials remains a challenge in tissue regeneration.The application of stem cells in therapy to treat a variety of illnesses has shown promise,but their low retention rate,viability,and differentiation have limited their efficacy.To overcome these drawbacks,biomaterials can serve as carriers and regulators to amplify the benefits of stem cells for healing (Jeong et al.,2021;Thomas et al.,2021).Effective biomaterials must regulate the tissue microenvironment and cell behavior to promote neural regeneration (Tuladhar et al.,2020;Shen et al.,2022).Adaptive biomaterials can also respond to unfavorable chemicals in the milieu to improve the viability and functionality of host cells,creating an adjustable regenerative physiological environment (Jiang et al.,2021).Collagen,gelatin,laminin,silk fibroin,alginate,and other injectable hydrogels based on polysaccharides,such as chitosan and hyaluronic acid (HA),are examples of natural substances and derivates used in neural applications (Liu et al.,2020b;Nguyen et al.,2020;Lainé et al.,2022).These materials and derivatives aim to mimic extracellular matrix (ECM) properties such as viscoelasticity,biological degradation,and compatibility (Lim et al.,2020).
A potential hydrogel for neural TE was the methylcellulose/agarose composite thermosensitive hydrogel,a synthetic biomaterial that maintains the survival of neuronal cells.Another thermosensitive hydrogel was poly(N-isopropylacrylamide) (PNIPAAm),which might combine with polyethylene glycol to ensure excellent cell attachment and maintain growth factor production throughout protracted CNS treatment (Hasanzadeh et al.,2023).
Physical hydrogels are simple to produce and have low cytotoxicity,but they frequently become unstable in physiological settings and do not immediately react (Khan et al.,2022;Liang et al.,2023).Chemical hydrogels have had deleterious effectsin vivobecause of the chemical interactions,while having outstanding mechanical characteristics and remarkable stability (Finbloom et al.,2020;Townsend et al.,2020).Synthetic biomaterials used to create hydrogels showed great stability but low biological activity and compatibility (Macks et al.,2022;Park et al.,2022).Natural biomaterial-based hydrogels are distinguished by their excellent biocompatibility and biodegradability and low cytotoxicity,but their potential for use in medicine was constrained by the absence of physical rigidity (Xin et al.,2023).In light of these benefits,hybrid hydrogels assembled from both synthetic and natural components might provide a more promising avenue for therapeutic applications in CNS regrowth and engineering (Man et al.,2021;Park et al.,2021;Asghari et al.,2022).In summary,a potential biomaterial that helps to promote neurite outgrowth and 3D cell culture should have the best possible biodegradability,stability,biocompatibility,and mechanical properties (Bartlett et al.,2020;Ye et al.,2021;Table 1).
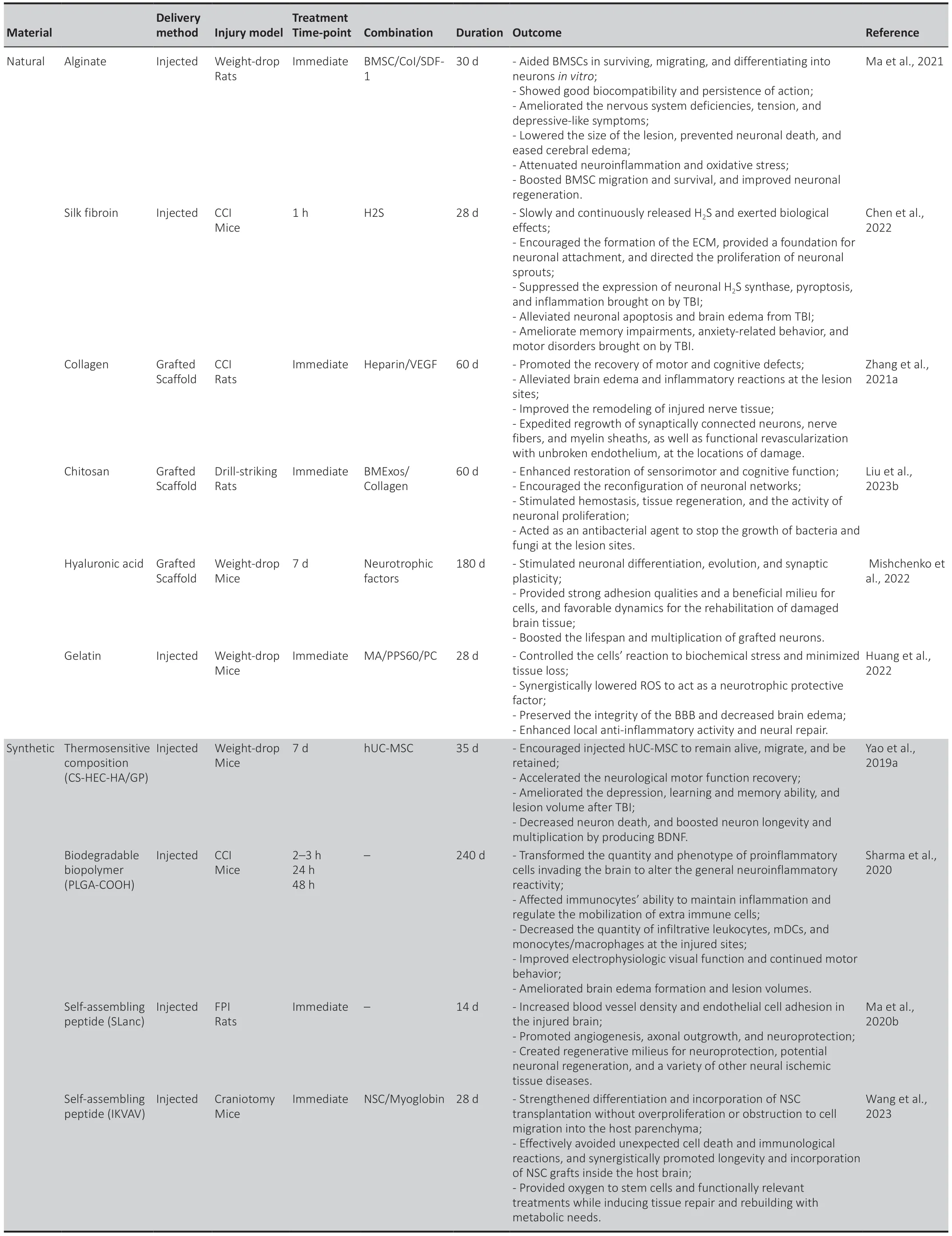
Table 1 |Natural and synthetic biomaterials and their derivatives for TBI treatment
Mechanisms and Regulation of Biochemical Stress
It is vulnerable to suffer from excitotoxicity due to the depletion of mitochondria and the disruption of mitochondrial calcium homeostasis.According to a recent study (Verma et al.,2022),PTEN-induced putative kinase 1 controlled the activity of the mitochondrial sodium calcium exchanger protein by phosphorylation,which controlled the regulation of mitochondrial calcium homeostasis and suppressed the expression of leucine-rich repeat kinase 2 mutants (G2019S or R1441C) by inhibiting mitochondrial calcium uniporter activity,reducing the Parkinson’s loss-of-function mutant,and preventing exposure to oligomeric amyloid-beta peptide(Chavez et al.,2020;Pandya et al.,2023).By normalizing mitochondrial calcium flow,a common group of methods that targeted the mitochondrial calcium homeostasis machinery could prevent excitatory mitochondrial toxicity and protect nerves (Du et al.,2022).Biomaterials combined with drugs based on the above mechanisms could be developed to prevent biochemical stress (Han et al.,2022;Mason et al.,2023).
Excessive levels of reactive oxygen species and reactive nitrogen species (ROS/RNS) by inflammatory cells can cause an oxidative microenvironment after initial brain damage caused by external stimuli (Fan et al.,2022).For patients with severe TBI,lowering ROS/RNS levels ameliorated prognosis by enhancing neuronal survival,reducing neuroinflammation,and facilitating recovery (Sun et al.,2022a).By encouraging the expression of cytokines,excessive ROS production in the milieu of brain injury might stimulate inflammation and the immune system,which can ultimately lead to neurological impairments (Takahashi et al.,2020;Waheed et al.,2022).Additionally,neuroinflammation,which is characterized by a mass of activated astrocytes and microglia in the damaged region,inhibits neuron regeneration and axon development(Zhang et al.,2020b).Therefore,it is critical to eliminate ROS,reduce neural inflammation,and accelerate neuronal regeneration following TBI (Sun et al.,2022a).
In contrast to traditional systemic drug treatment,which is currently unable to effectively deliver the medicine to the targeted region in the early stages of TBI and might instead have systemic adverse reactions owing to the existence of the BBB,gelatin methacrylate-propylene sulfide/procyanidin(GelMA-PPS/PC) has provided an immediate approach to the surface of brain tissue and concentrated on the injured region(Huang et al.,2022).As a result of the involvement of the hydrogen peroxide (H2O2)-responsive material of hydrophobic poly (propylene sulfide) 60 (PPS60),PPS60 might additionally trigger hydrogel construction to breakdown and expel the procyanidins it contains.In addition,PC could modulate the oxidative stress response in the cells,work in concert to deplete ROS,and raise the level of brain-derived neurotrophic factor (BDNF),so that could exert a neurotrophic protective function,and inhibit the expression of ionized calcium-binding adapter molecule 1 (Iba-1),glial fibrillary acidic protein,chemokine (C-X-C motif) ligand 1,tumor necrosis factor-α,interleukin-1β (IL-1β),and IL-8 to reduce inflammation(Grebenik et al.,2020).Triglycerol monostearate-loaded PC (TM/PC) was another novel ROS depletion hydrogel.According to a study,PPS120 and curcumin (Cur) might be enclosed within the hydrophobic core of TM lamellae,which could be self-assembled into a hydrogel to create a TM/PC hydrogel.Upon direct injection into the TBI wound cavity,TM decomposed,and PPS120 scavenged ROS to release Cur,thereby decreasing neural inflammation and subsequent damage spread.In addition,enhanced neuronal growth and regeneration as well as migration after TM/PC treatment could be observed by more powerful DCX expression.Moreover,a lower dichlorodihydrofluorescein fluorescence intensity after treatment showed stronger ROS-scavenging ability of the TM/PC hydrogel (Qian et al.,2021;Figure 3).

Figure 3 |ROS-scavenging functional hydrogels treat TBI through neuroprotection,pro-nerve regeneration,and anti-neuroinflammatory effects.
In a recent study,Zhang et al.(2022b) developed a novel injectable,ROS-scavenging,and biocompatible gallic acidconjugated gelatin/oxidized dextran hydrogel to provide protection from the harmful microenvironment by neutralizing free radicals and aid in neural healing by suppressing oxidative stress and inflammation.
In addition to being safe for neurons under physiological conditions,carbogenic nanozymes that are tiny multienzyme mimics also showed powerful scavenging capacity for redundant ROS and RNS,thereby offering neuroprotection(Mu et al.,2019;Zhang et al.,2021d;Tarudji et al.,2023).Moreover,the carbogenic nanozyme could decrease the level of matrix metalloproteinases in TBI patients,contributing to effective repair of BBB disruption and a reduction in cerebral edema (Zhai et al.,2021).In addition,nanozymes could reduce proinflammatory immune responses by limiting the proliferation of microglia and astrocytes,which could result in strong neuronal inflammation.The nanozyme could also increase the activity of superoxide dismutase and decrease the amount of H2O2and the expression levels of lipid peroxidation and glutathione disulfide (GSSG),which could alleviate severe oxidative stress (He et al.,2020).Additionally,the nanozyme might efficiently boost the capacity of spatial memory.Carbon dots (CDs),a new type of carbon-based nanomaterial,has recently emerged as a valid new method to permeate the BBB and enter the brain parenchyma (Zhang et al.,2022c).Given their vast and unique surface area and abundance of functional groups,carbon dots offer the benefits of outstanding solubility,superior biocompatibility,simple functionalization,and tremendous drug-loading ability to deliver therapeutic medicines.More significantly,carbon dots can maintain or even increase the biological activity of their precursors (Ouyang et al.,2020).
Strategies for Anti-Neuroinflammation and Neuroprotection
The prevention of neuroinflammation and pyroptosis was thought to be a potential therapeutic method for TBI.In recent studies,a surface-fill hydrogen-sulfide (H2S)-releasing silk fibroin (SF) hydrogel (H2S@SF hydrogel) was shown to be neuroprotective following TBI (Liu et al.,2023e).On the one hand,the H2S@SF hydrogel most significantly inhibited the activation of H2S synthase,and the expression of neuronal NOD-like receptor protein 3,gasdermin D,cysteinyl aspartate specific proteinase-1 (caspase-1),apoptosis-associated speck-like protein containing CARD,IL-1β,and tumor necrosis factor-α,and inhibited TBI-induced pyroptosis and necroptosis-related protein (receptor interacting protein-1;Wehn et al.,2021;Chen et al.,2022;López-Preza et al.,2023).H2S@SF hydrogel,on the other hand,appeared to slow the decrease in the level of glutathione following moderate TBI,demonstrating that it might protect against mitochondrial dysfunction and oxidative stress in this scenario (Kwiecien,2021).In addition,H2S@SF could reduce neuroinflammation by inhibiting the proliferation and activation of reactive astrocytes and activated microglia,and the expression of glial fibrillary acidic protein and Iba-1 in these glial cells (Zhang et al.,2022a;Figure 4).
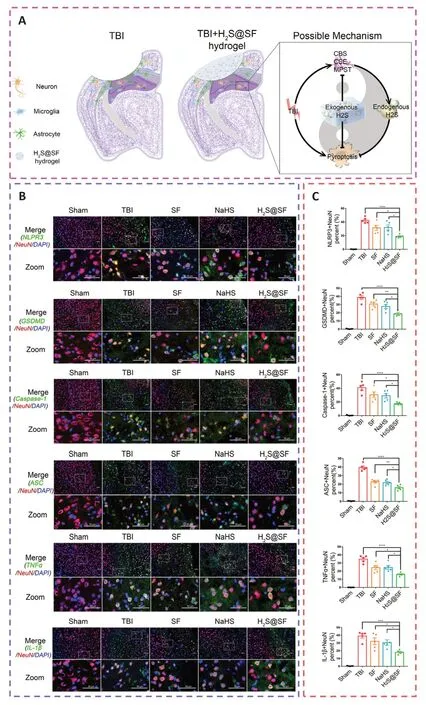
Figure 4 |H2S-releasing SF hydrogel (H2S@SF hydrogel) inhibits neuronal pyroptosis and inflammation,and exerts a neuroprotective impact by inhibiting the expression of pyroptosis-related proteins,H2S synthase,and proinflammatory factors.
Bioactive multifunctional nanocomposites (ANGMnEMNPs-Cur [AMEC]) can regulate antioxidation and anti-neuroinflammation for targeted TBI therapies by integrating the neuroprotective drug Cur with angiopep-2 functionalities and manganese-doped eumelanin-like nanoparticles (Fyfe,2020;Sun et al.,2022a).Mechanistically,angiopep-2 could bind to low-density lipoprotein receptorrelated protein-1 and help cross the BBB into TBI lesions and enhance drug accumulation (Bechinger et al.,2023).Melanin-like nanoparticles had powerful and extensive scavenging capabilities against an array of ROS (Zinger et al.,2021a).Cur,a specific kind of plant polyphenol,offered several pharmacological benefits,including antioxidative,anti-inflammatory,and antiapoptotic effects (Dong et al.,2022).The functional moieties of Cur and eumelanin,when combined,work cooperatively to increase the effectiveness of AMEC at the targeted site.This was accomplished by reducing oxidative stress,preventing neurological inflammation by converting M1 macrophages to M2 macrophages,and promoting neuronal regeneration (Hong et al.,2020;Wu et al.,2021;Figure 5).

Figure 5 |By preventing the appearance of activated astrocytes and microglia,reducing inflammatory cytokine levels,and activating M1-to-M2 macrophage reprogramming,AMEC significantly reduces oxidative stress,attenuates neural inflammation,and prevents the subsequent spread of damage following TBI.
Immunomodulatory nanoparticles (IMPs) are highly negatively charged,500 nm-diameter particles composed of the biodegradable biopolymer carboxylated poly(lactic-co-glycolic)acid (PLGA-COOH) (Sharma et al.,2020;Xu et al.,2022).The quantity of proinflammatory cells that infiltrates the brain and their phenotype can both be changed by IMPs.To alter the general neuroinflammatory reaction,IMP treatment drastically reduced the quantity of monocytes/macrophages,myeloid dendritic cells,and permeating leukocytes in the region of injury (Blanco-Ocampo et al.,2020).This had an effect on these cells’ ability to endure inflammatory reaction and guide the recruitment of extra immune cells.According to a study,R2* sequences were used to assess brain edema at 24 hours,whereas ventricular volume was used to assess intracranial pressure because brain edema caused ventricular compression and thus elevated intracranial pressure.The R2* hyperintensity volume was smaller in the IMP treatment group than in the vehicle treatment group,and the ventricular volume was larger in the IMP treatment group than in the vehicle treatment group,indicating that IMPs were effective in reducing brain edema and significantly reduced the intracranial pressure,thereby preserving brain tissue and significantly maintaining the anatomic and neurologic function(Sharma et al.,2020;Figure 6).
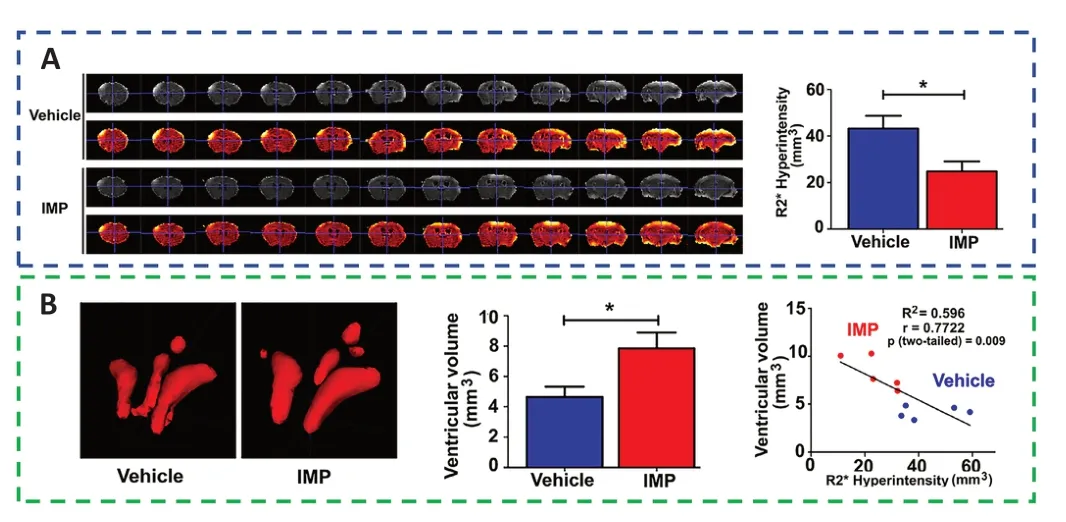
Figure 6 |IMPs might ameliorate acute brain edema and lower intracranial pressure to provide neuroprotection.
Following TBI,ferroptosis was confirmed by iron buildup,higher transferrin expression,decreased glutathione peroxidase activity,lipid ROS accumulation,and shrinking mitochondria (Rui et al.,2021;Cheng et al.,2023;Li and Jia,2023;Yang et al.,2023a).An unusually excessive iron buildup in chronic TBI patients might be present,and this was strongly connected with cognitive deficits (Xie et al.,2019;Wang et al.,2022a).Transferrin was essential for the induction of ferroptosis and might reduce cell death and lipid ROS accumulation when it was knocked out (Jia et al.,2023).When siramesine and lapatinib were used as treatments,the overexpression of ferroportin could lower ROS and cell death,while ferroportin knockout could increase ROS and cell death(Cheng et al.,2022).A significant regulator of ferroptosis was glutathione peroxidase 4,which could catalyze the decrease in lipid peroxides (Fang et al.,2023).In addition,ferrostatin-1 inhibited cell death,reduced glutamate-induced ferroptosis and iron accumulation,and attenuated neuronal degeneration(Ge et al.,2022).Ferrostatin-1 treatment might lessen neuronal cell loss and enhance cognitive and motor function over the long run.
Promotion of Neuroregeneration and Revascularization
Neuronal damage occurs immediately after TBI and is further aggravated in the chronic phase,leading to progressive neuronal death.Neuroplasticity goes some way toward minimizing the consequences of neuronal damage after TBI,but given that most such patients are adults,a critical question is whether it is possible to generate new neurons in the adult brain.Therefore,to prevent and improve this outcome,it is crucial to confirm the presence of adult neurogenesis.
For decades,it has been believed that neurogenesis occurs only during embryonic development and that new neurons do not appear in the adult brain (Ramon y Cajal and May,1928;Ming and Song,2005).Altman et al.,however,disproved this theory in 1962 by proving that the adult rodent brain could reproduce new neurons (Altman and Das,1965;Altman,1969).This occurrence of neurogenesis in the adult rodent brain was further evidenced in the 1990s with the fast development of new technologies,including 5-bromo-2′-deoxyuridine labeling and antibody-based biomarker detection approaches (Ming and Song,2005;Jurkowski et al.,2020).In the adult rodent brain,subventricular zone astrocytes were discovered to be NSCs around the year 2000 (Doetsch et al.,1999);in subsequent animal studies,Lindvall and Kokaia (2015)found the feasibility of stimulating endogenous neurogenesis in the subventricular zone to promote neural repair in the striatum and cortex.
In the course of progress or following injury and the onset of disease,BDNF,which exclusively integrates with the tyrosine kinase B (TrkB) receptor,can stimulate neuronal differentiation,evolution,and synaptic plasticity in the CNS (Edelbrock et al.,2018;Fletcher et al.,2021;Zhao et al.,2021).It has been reported that a supramolecular peptide amphiphile (PA)nanostructure incorporated in a BDNF mimetic sequence could activate the BDNF receptor TrkB and its downstream signaling pathways in primary cortical neurons (Mishchenko et al.,2022;Wu et al.,2023b).Next,the activated TrkB signaling pathways increased neuronal maturation.Then,the BDNF@PA nanostructures encouraged cell infiltration and increased functional maturation (Wu et al.,2023a).In summary,the BDNF-mimetic PA nanostructure produced an extremely bioactive matrix that could activate the TrkB receptor and its subsequent signaling cascades,which was necessary for cell viability,development,and functional maturation in 2D cultures,and could stimulate neuron infiltration in 3D while boosting maturity and functional electrical activity,which aided in the promotion of the regeneration or integration of transplanted cells in the CNS (Nam et al.,2020;Yin et al.,2020a;Liu et al.,2023b,d).
HA,a natural polymer,is the major substance of the brain ECM,with the advantages of a lower cytotoxicity level,a flexible biostructure,and a controllable rate of biodegradation(Li et al.,2020b;Yang et al.,2023b).Because of the high hydrophilicity of HA,scaffolds made on its foundation could offer strong adhesion qualities for cells and provide a beneficial milieu for them (Ngo et al.,2020;Satish et al.,2021).Glial-cell-derived neurotrophic factor (GDNF) can boost the lifespan and multiplication of grafted neurons,promote the renewal of nerves,and offer favorable dynamics for the rehabilitation of damaged brain tissue (Selvakumar et al.,2020;Torres-Ortega et al.,2022;Wang et al.,2022b).GDNFloaded scaffolds were better than BDNF in that they sustain long-term memory while also reducing intense neurological impairments and noticeable alterations in motor and orienting-exploratory activity (Mishchenko et al.,2022).The 3D extrusion printing technique has the advantages of rapid fabrication and faster deposition.Therefore,the implantation of 3D-printed HA hydrogel scaffolds impregnated with the neurotrophic factor GDNF can be a promising therapeutic strategy for morphological and functional nerve tissue restoration after TBI (Rouleau et al.,2020).
Angiogenesis initiates a vascular network in the growing body that results in the growth and maturation of the CNS(Ma et al.,2020b).Neogenesis/maturity,permeability,and the capacity to revascularize therapeutic regions of ischemia are crucial for the development of angiogenesis.SLanc,an angiogenic self-assembling peptide hydrogel,could accelerate endothelial cell attachment and angiogenesis by greatly increasing the recruitment of von-Willebrand factor,which plays an important role in CNS neuroprotection and angiogenesis (Ma et al.,2020b;Figure 7).The most important angiogenesis-inducing growth factor,vascular endothelial growth factor (VEGF),is associated with increased BBB opening,brain edema,and neurological impairments.In a recent study,a VEGF-containing hydrogel was injected directly into the region of damage to stimulate the creation of a vascular and neuronal structure that led to behavioral improvement (Zhang et al.,2021a).In the injured area,HA gel and high cluster VEGF might encourage the development of a strong,mature,and well-established vascular bed and accelerate axonal extention alongside these blood vessels.Additionally,this hydrogel could encourage the inward migration of embryonic neurons from the subventricular zone,decrease the activation of microglia and the extent of the reactive astrocyte boundary,and change the surrounding periinfarct tissue (Ma et al.,2020a).Meanwhile,numerous studies have proven that heparin exerted anti-inflammatory effects by blocking leukocyte adhesion to the vessel wall and stabilizing chemokine and growth factor gradients (Schirmer et al.,2020;Zhang et al.,2021a;Liu et al.,2022b).VEGF delivery was related to increased inflammation,which could weaken the efficacy and strengthen the adverse effects.Surprisingly,the proinflammatory action of VEGF could be counteracted by bare heparin particles,which also provided a pro-repair milieu that promoted the proliferation of fresh neural tissue (Jiang et al.,2020;Figure 8).Hence,the combination of the rapid development of a neurovascular network in the damaged location and the control of responses to inflammation,scars,and NSC in the neighboring brain position might pave the way for a new approach in the field of neural healing following TBI(Nih et al.,2018).
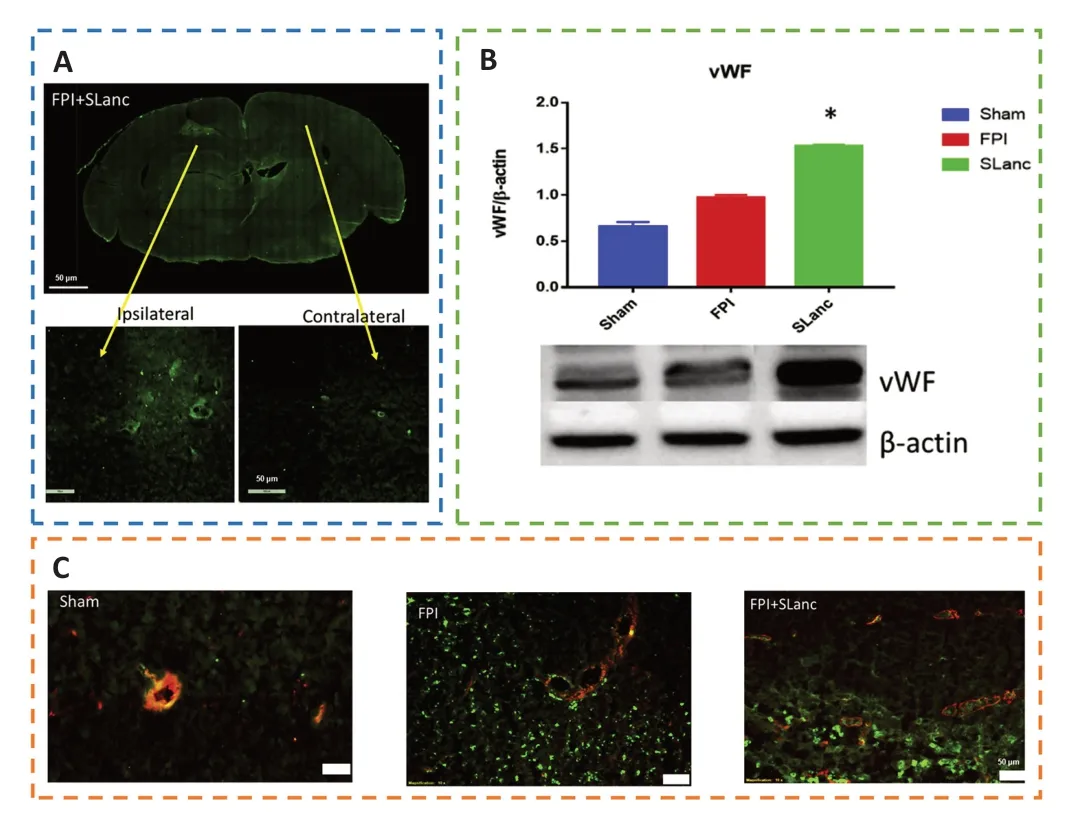
Figure 7 |SLanc,an angiogenic self-assembling peptide hydrogel,accelerates ischemic tissue revascularization by improving endothelial cell adhesion and angiogenesis.
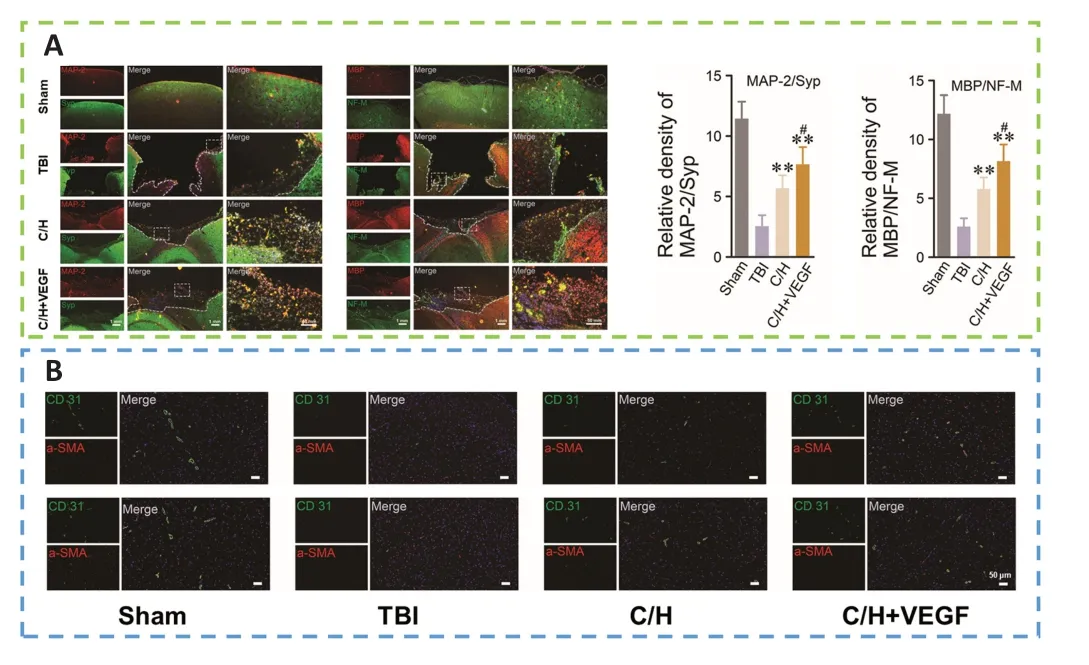
Figure 8 |C/H scaffolds combined with VEGF can promote the regeneration of axons and myelin sheaths and promote angiogenesis after TBI.
Biomaterial Scaffolds Carried with Stem Cells
Mesenchymal stem cells (MSCs) are capable of differentiating into neurons,replacing injured cells,and secreting paracrine hormones,making them a viable therapy for brain injury (Xu et al.,2020a;Sharma et al.,2021;Viet et al.,2022;Table 2).However,there has been little success and many problems with MSCs,namely limited cell availability,short cellular lifespan,few differentiated neurons,and poor functional integration (Bonsack et al.,2020;Ruppert et al.,2020;Jha et al.,2021;Pischiutta et al.,2021).Thus,encapsulation of transplanted cells with biomaterials might provide a suitable milieu for the growth and proliferation of these cells,protect them from immune rejection,and improve cell engraftment and survival during transplantation of injured brain lesions(Sultan et al.,2021;Figure 9).
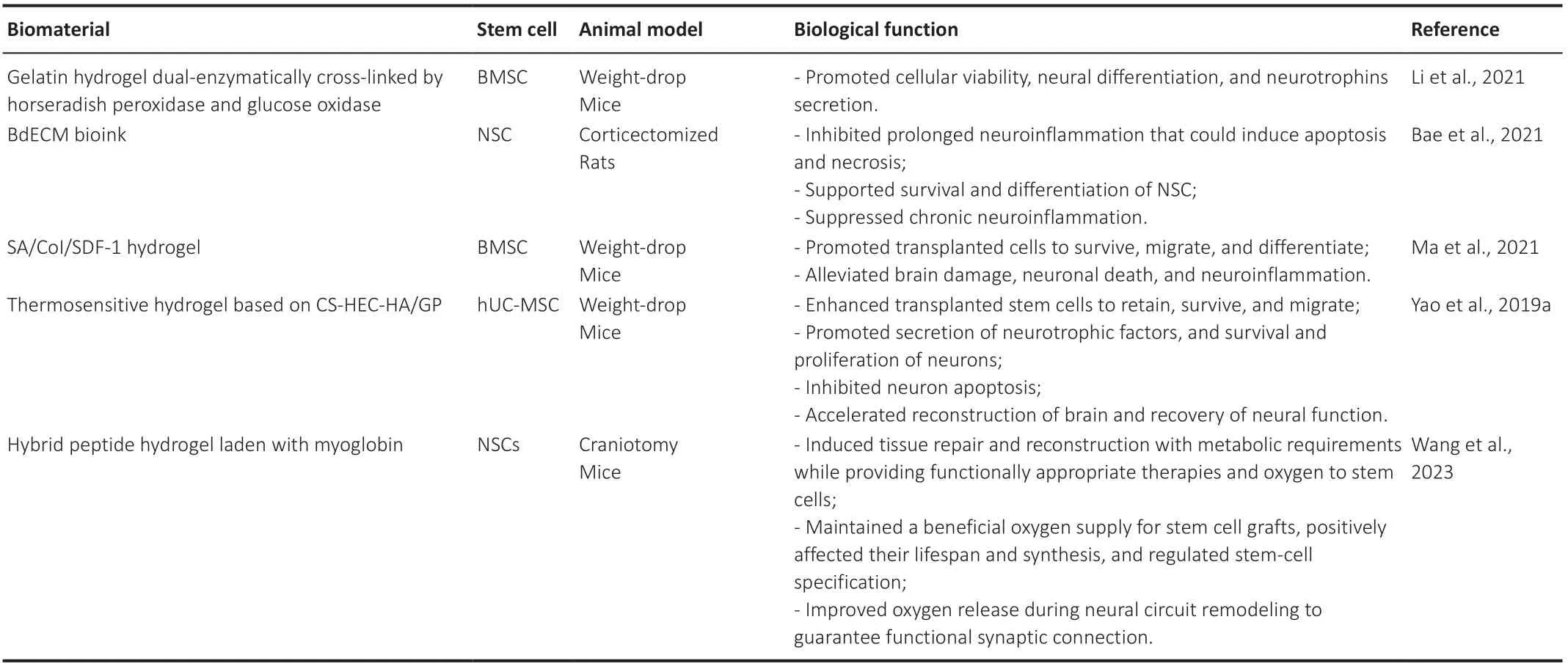
Table 2 |Applications of stem cells combined with biomaterials in therapy
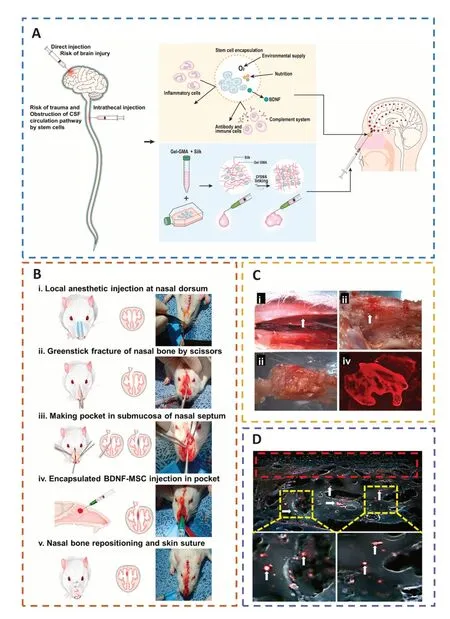
Figure 9 |The encapsulation of BDNF-MSCs in biomaterials can protect transplanted cells from immune rejection,offer a favorable milieu,and improve the survival rate of transplanted cells at the injured site.
In the study by Ma et al.(2021),the longevity,attraction,and proliferation of stem cells were all regulated by stromal cell-derived factor-1 (SDF-1) integrating with its receptor(chemokine C-X-C motif receptor 4,CXCR4).As a result,they created a hydrogel composed of sodium alginate (SA),collagen type I (CoI),and SDF-1,which was infused with bone marrowderived mesenchymal stem cells (BMSCs;Addington et al.,2015).The SA/CoI/SDF-1 scaffold could maintain a milieu with biological compatibility and degradability for BMSCs to survive,migrate,and differentiatein vitro,while also being able to steadily release SDF-1 (Guo et al.,2020;Liu et al.,2023a).The synthetic scaffold could significantly alleviate motor and cognitive impairment and lessen tension and depressive-like behaviors when it was infused with BMSCs (Hu et al.,2020).Additionally,the BMSC/SA/CoI/SDF-1 scaffold could facilitate BMSC migration in lesions and partially boost neurogenesis by triggering the FAK/PI3K/AKT pathway (Wang et al.,2020a),which was regulated by SDF-1/CXCR4 and could contribute to the reduction in brain damage,neuronal death,and neural inflammation (Ma et al.,2021).In addition,the hydrogel cross-linked by 0.1 U/mL glucose oxidase and 0.5 U/mL horseradish peroxidase exerted superior cytocompatibility and a low immune response,which could further promote the therapeutic effect of GH/BMSC scaffolds (Li et al.,2021).Under 3D culture conditions,neuronal differentiation and neurotrophin secretion might be significantly improved by a dual-enzymatically cross-linked injectable gelatin hydrogel laden with BMSCs (Yao et al.,2019b).The GH/BMSC hydrogel might control the inflammatory response,restrain apoptosis,encourage neurogenesis,and increase the plasticity of neurological function (Cozene et al.,2021;Yuan et al.,2021).
To improve the rate of cell engraftment and survival,some scholars developed a new thermosensitive hydrogel based on chitosan,hydroxyethyl cellulose,HA,and β-glycerophosphate(CS-HEC-HA/GP),which possessed better biocompatibility with human umbilical cord mesenchymal stem cells (hUC-MSCs) and a faster gelation process than traditional hydrogel (Yao et al.,2019a;Yea et al.,2020).In comparison to BMSCs,hUC-MSCs offered the benefits of abundant sources,simple accessibility,few negative outcomes for the donor,low requirement for consistency in human leukocyte antigen matching,and less danger of viral infection following transplantation (Chen et al.,2020a;Barretto et al.,2021).CS-HEC-HA/GP hydrogel-laden hUC-MSCs had the ability to enhance the reservation,survival,and transfer of encapsulated hUC-MSCs (Sultan et al.,2021).Moreover,this synthesized hydrogel could possibly secrete neurotrophic factors and suppress apoptosis to promote the subsistence and multiplication of endogenous neurons (Torres-Ortega et al.,2022;Zhang et al.,2023),and expedite the rebuilding of brain tissue and recovery of neural function in TBI patients (Liu et al.,2020a,2023b;Bamshad et al.,2023).Jiang et al.(2021) reported that compared with the simple stem cell (SC) or collagen/SF (CS) scaffold,the implantation of CS combined with the SC (CB) scaffold showed better cortical integrity and motor function and more regeneration of nerve fibers (Figure 10).
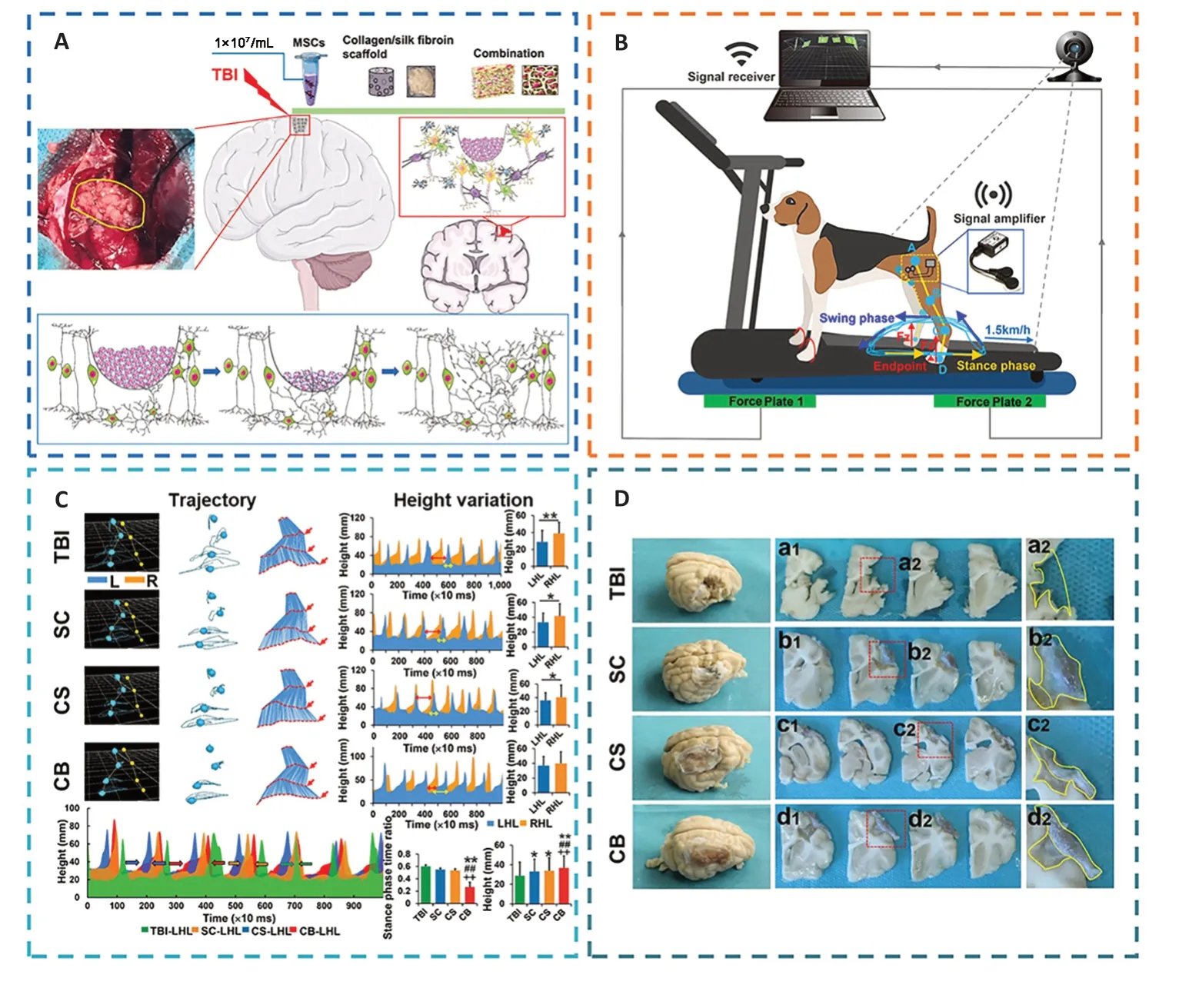
Figure 10 |The implantation of regenerative complexes could strengthen the reconstruction of neural networks and the recovery of motor function after TBI.
However,typically,the effectiveness of existing stem cell treatment is limited in its ability to address problems including poor implanted stem cell survival and ineffective differentiation into targeted cells (Alvarado-Velez et al.,2021).Bae et al.(2021) developed a brain-derived decellularized extracellular matrix (BdECM) bioink.With the use of BdECM,a manufactured defect-fit stem cell carrier might improve NSC differentiation capacityin vitroand the ability of implanted NSCs to survivein vivo(Bae et al.,2021).Additionally,by extending cell behavior on tissue-specific materials into the brain,the BdECM bioink could increase grafted NSC survival and development into mature neurons while maintaining grafted NSCs on the site without volumetric loss (Hu et al.,2023).Moreover,the BdECM bioink had the ability to perform an anti-neuroinflammation function for a longer period oftime,which could cause cell death and necrosis at a secondary damage region from TBI,promote the long-term viability and neuronal differentiation of implanted cells,and dampen persistent neuroinflammation (Damian et al.,2021).
Bioactive Effects of Biomaterial Scaffolds Carried with Exosomes
In recent studies,stem cell-derived exosomes have been considered an alternative to exogenous stem cells for a wide range of tissue regeneration owing to their advantages such as immunity from T-cell and NK cell attack and their roles as paracrine messengers that regulated the microenvironment to trigger tissue regeneration and promote angiogenesis(Ghosh et al.,2020;Williams et al.,2020;Bambakidis et al.,2022;Zhuang et al.,2022).Liu et al.(2023c) reported a new therapeutic strategy for integrating bone marrow mesenchymal stem cell-derived exosomes (BME) into HA collagen hydrogels (DHC-BME).BME could combine the advantages of stem cells and avoid the risk of immune rejection,but persistent delivery or difficulty focusing at the lesion site were limitations (Zhang et al.,2021f).HA could promote neurodevelopment and inhibit glial scars,thereby constructing an ideal biomimetic neural microenvironment for cell adhesion,growth,and differentiation,but a single collagen hydrogel had the tendency to shrinkin vivo,destabilizing the ecological niche of cell growth (Zinger et al.,2021b).The synergy of BME and DHC could enhance the biological functions of both and exert a stable function,such as promoting the differentiation of NSCs into neurons and oligodendrocytes,while inhibiting astrocyte differentiation.DHC-BME could therefore stimulate vascular and neuronal development,from endogenous NSC attraction to neuronal differentiation and vascularization (Mu et al.,2022),collaboratively foster axon regeneration,myelin renewal,synaptic formation,and even neural structure restoration,and could ultimately encourage the rebuilding of nerve function following TBI (Xu et al.,2020b;Figure 11).
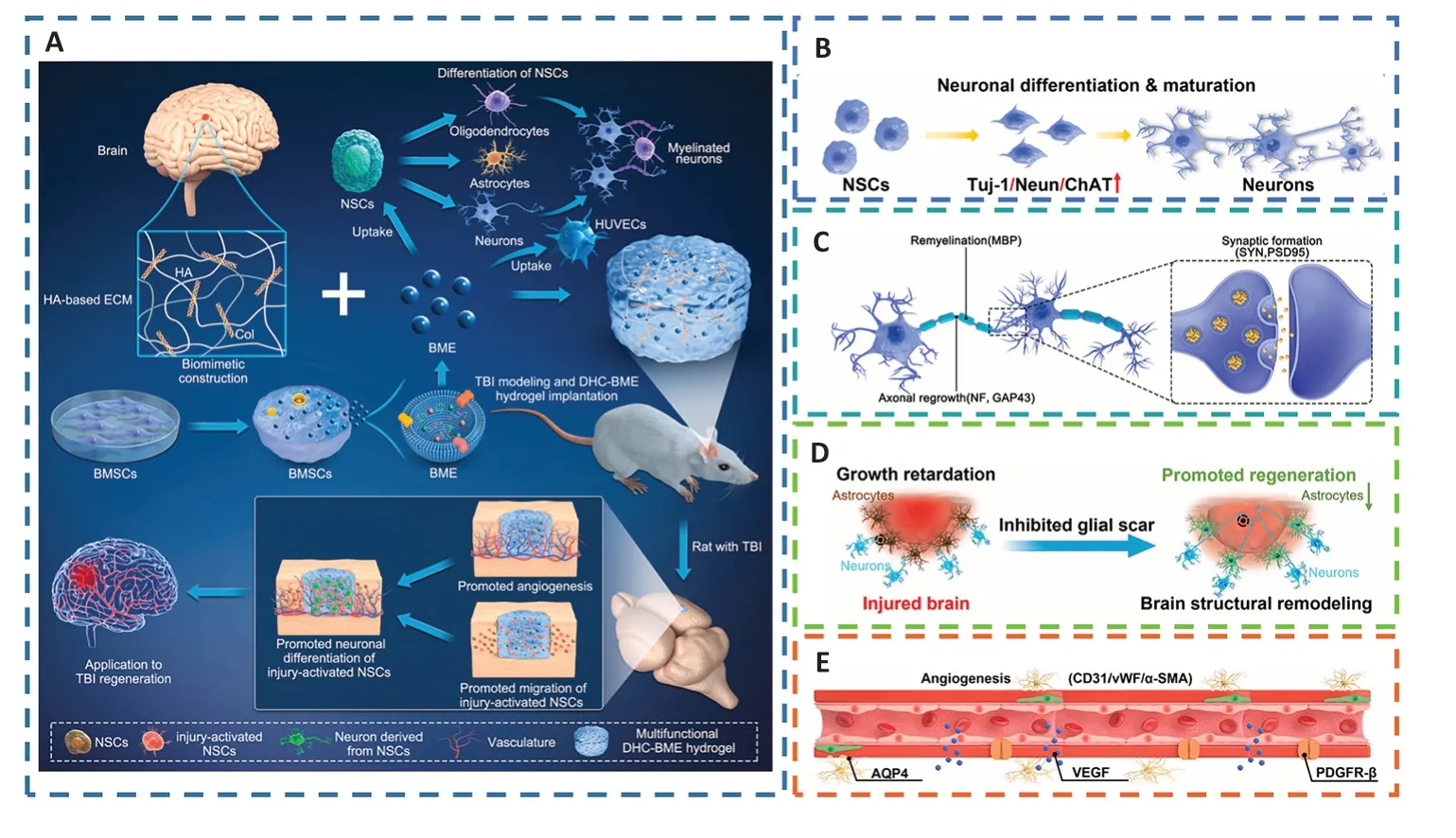
Figure 11 |DHC-BME synergistically promotes the recovery of neural structure and function and angiogenesis after TBI.
Microglia and astrocytes actively participate in a number of disease events in the CNS.Numerous regulatory substances are produced by activated astrocytes that affect CNS immunity and microglia (Chen et al.,2020c).Microglia have a dual role: pro-inflammatory (M1-like) and anti-inflammatory(M2-like) phenotypes (Yin et al.,2020b).Exosomes and extracellular vesicles,play a key role in initiating,transmitting,and monitoring immune reactions to nearby cells through cargo proteins,RNAs,and miRNAs (Sun et al.,2020;Tonarelli and Quinn,2020).Astrocyte-derived exosomes (AS-Exos)promote the transformation of the microglial M2 phenotype,improving neuronal flexibility,immune reactions,and neuronal lifespan in a variety of pathological conditions.ASExos are rich in numerous miRNAs,of which miR-873a-5p is a highly expressed major component in TBI (Gayen et al.,2020;Wang et al.,2020b),which can significantly inhibit LPS-induced microglial M1 phenotypic transformation and exert anti-inflammatory effects by reducing extracellular regulated protein kinases (ERK) and nuclear factor-κB (NFκB) p65 phosphorylation (Long et al.,2020;Xin et al.,2023).Thus,activated astrocyte-derived miR-873a-5p can attenuate microglia-mediated nerve inflammation and neural deficits post TBI by inhibiting the nuclear factor-κB signaling pathway.Additionally,it has been demonstrated that AS-Exos drastically lowered the levels of oxidative stress and mitochondrial H2O2 in TBI patients’ hippocampal neurons by increasing the expression of antioxidant enzymes such as superoxide dismutase and catalase (Nourbakhsh et al.,2020;Zhang et al.,2021e).Furthermore,AS-Exos could reduce oxidative stress and neuronal death by triggering Nrf2/HO-1 signaling in TBI patients’ hippocampi (Salman et al.,2020;Zhao et al.,2022).
3D-printed collagen/chitosan scaffolds based on BDNFstimulated hUC-MSC-derived exosomes (3D-CC-BMExos)were proposed as a viable treatment method for TBI (Xu et al.,2020b;Yuan et al.,2020;Liu et al.,2023b).Collagen and chitosan have the advantages of outstanding biocompatibility and physical properties that enable crucial cell-cell and cellmicroenvironment interactions to support the healing of injured tissue (Zhang et al.,2021b;Hajinejad et al.,2023).Forin vivotransplantation,the use of 3D printing might further modify the compound scaffold dimensions,form,porosity ratio,and morphology (Liu et al.,2022a).Moreover,the water absorption ratio of 3D-printed scaffolds was suitable,preventing the loss of nutrients and bodily fluids as well as promoting cell proliferation and tissue regeneration(Zhou et al.,2020;Tsui et al.,2021;Zhang et al.,2021c).However,traditional 3D printing techniques might impair the biological activity of exosomes;thus,when printed at a low temperature,the composite scaffolds might very well regulate exosome release (Liu et al.,2023d).In addition,the exosomes generated by 3D-CC-BMExos tended to be more persistent,and their overall release volume was greater than that of CC-BMExos owing to the 3D printing technology’s ability to equally blend and attach the exosomes to the scaffolds (Liu et al.,2020b;Zhang et al.,2020a).Furthermore,3D-printed collagen/chitosan scaffolds that were based on NSC-derived exosomes and pretreated with insulin-like growth factor 1 (IGF-1-Exos,3D-CC-INExos) at a low temperature could also promote neural repair by inhibiting inflammation and apoptosis,and accelerating neurogenesis and angiogenesis(Liu et al.,2023d;Figure 12).
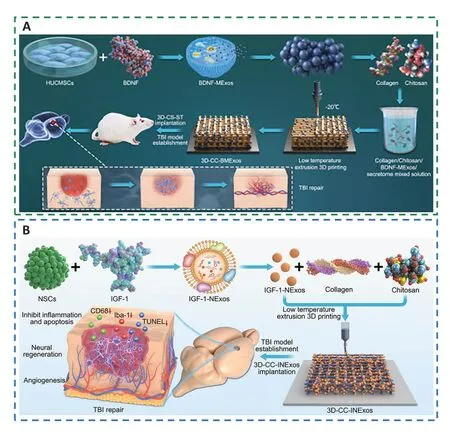
Figure 12 |3D-printed collagen/chitosan scaffolds loaded with growth factors promote neural repair and angiogenesis at a low temperature.
Limitations
This review has some limitations.First,little is known about the variety of biological roles performed by NSC-derived exosomes,and it is still unclear how these exosomes connect with recipient cells to modulate target cells.However,this undoubtedly points to a new research focus for cell-to-cell communication (Yuan et al.,2020).Besides,exosome release can be better controlled by 3D-printed composite scaffolds at low temperatures,but it is vital to determine at what temperature exosomes can be stably stored for an extended period while maintaining their integrity (Liu et al.,2023d).Additionally,it is challenging to translate exosome research into clinical applications for the treatment of diseases,as there are yet no reliable,efficient,and safe procedures for the manufacture and purification of exosomes (Zhang et al.,2021e).In summary,creating an NSC exosome amplification system without heterologous components,a unified standard for purification,and storage means of NSC exosome formation should be emphasized,and a method for singleexosome NSC analysis should be defined to better understand the characteristics and mechanisms of exosome-based therapeutics and promote the clinical translation of exosome therapy.
Conclusion and Perspective
Traditional treatments such as drugs and surgery are limited to slowing the progression of disease and relieving pain.The rise and progression of TE and biomaterials have led to novel methods for neural regeneration that can effectively promote the proliferation and differentiation of neural cells,surpassing the limitations of traditional treatments.However,the challenges in translating these therapies into clinical applications may be the complex and unclear key cellular and molecular processes and healing mechanisms,as well as the physical structure and pathophysiological features of the CNS.In addition,the BBB presents a significant obstacle,as it is a highly selective semipermeable membrane that limits the number of potential biomolecules for therapeutic use.
Tissue regeneration is hindered by limitations in the interaction between immune cells and biomaterials.Although stem cell therapy has potential for treating diseases,its effectiveness is limited by its low retention rate,viability,and differentiation.To address these issues,biomaterials can act as carriers and regulators to improve the therapeutic effects of stem cells.To ameliorate neural regeneration,effective biomaterials must moderate the tissue microenvironment and cell behavior.Additionally,adaptive biomaterials can respond to undesirable substances in the milieu to boost the lifespan and functionality of host cells,resulting in an adaptable regenerative physiological environment.Last,biomaterial design must also consider the following: 1)Physical hydrogels are easy to manufacture and have minimal toxicity to cells,but they are uncertain and sluggish to react,whereas chemical hydrogels are stable but will have negative effects on the body;2) Synthetic biomaterials possess stable and excellent properties,but their biological activity and biocompatibility are low;and 3) Natural biomaterials have superior biocompatibility,low cytotoxicity,and high biodegradability,but lack mechanical strength.In conclusion,a promising biomaterial should have optimal biodegradability,stability,biocompatibility,and mechanical quality to support neurite outgrowth and 3D cell culture.
MSCs are a promising treatment for TBI,but they remain unsuccessful because of poor cellular availability,short lifespan of cells,presence of few differentiated neurons,and poor functional integration.Surprisingly,bioactive scaffolds loaded with NSCs might supply a biocompatible and biodegradable milieu that would support NSCs to migrate,survive,and mature while alleviating neuropsychiatric symptoms.However,the current effectiveness of stem cells has certain limitations in solving the issues of transplanted stem cells having a poor survival rate and their ineffectiveness in differentiating into the corresponding cells.
Stem cell exosomes are considered an alternative to exogenous stem cell therapy.Stem cell-derived exosomes combine the advantages of stem cells and avoid the risk of immune rejection.Biomaterials can promote neural development and inhibit glial scar formation,thereby providing a good biomimetic neural microenvironment.The synergistic effect can enhance biological functions and exert a stable function,thereby inducing angiogenesis and neurogenesis and promoting the restoration of brain function in TBI patients.Furthermore,glial cell-derived exosomes can improve neuronal plasticity,immune response,and neuronal survival under various pathological conditions by promoting phenotypic transformation and exerting regulatory effects on biochemical stress and anti-neuroinflammation effects.In addition,bioactive scaffolds combined with exosomes are prospective therapeutic strategies for TBI,as compared to traditional exosome therapy,they possess better biocompatibility and mechanical properties,facilitate cell growth and neural regeneration,release exosomes in a higher total amount,and are more durable.
Author contributions:Manuscript design:JC,SZ,XiaoL,XiL;manuscript drafting:JC,SZ,XiaoL,XiL;data collection and analysis:QL,HL,YT;preparation of figures and tables,and obtaining permission to reuse images:XC,YJ,GL.All authors approved the final version of the manuscript.
Conflicts of interest:There are no actual or potential conflicts of interest.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice
- Conformational dynamics as an intrinsic determinant of prion protein misfolding and neurotoxicity
- Exploring the synergy of the eyebrain connection: neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation
- Pathogenic contribution of cholesteryl ester accumulation in the brain to neurodegenerative disorders
- Cognition and movement in neurodegenerative disorders:a dynamic duo
- Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease: searching far and wide
