Potential role of hippocampal neurogenesis in spinal cord injury induced post-trauma depression
2024-01-24YingMaYueQiaoXiangGao
Ying Ma ,Yue Qiao ,Xiang Gao
Abstract It has been reported both in clinic and rodent models that beyond spinal cord injury directly induced symptoms,such as paralysis,neuropathic pain,bladder/bowel dysfunction,and loss of sexual function,there are a variety of secondary complications,including memory loss,cognitive decline,depression,and Alzheimer’s disease.The largescale longitudinal population-based studies indicate that post-trauma depression is highly prevalent in spinal cord injury patients.Yet,few basic studies have been conducted to address the potential molecular mechanisms.One of possible factors underlying the depression is the reduction of adult hippocampal neurogenesis which may come from less physical activity,social isolation,chronic pain,and elevated neuroinflammation after spinal cord injury.However,there is no clear consensus yet.In this review,we will first summarize the alteration of hippocampal neurogenesis post-spinal cord injury.Then,we will discuss possible mechanisms underlie this important spinal cord injury consequence.Finally,we will outline the potential therapeutic options aimed at enhancing hippocampal neurogenesis to ameliorate depression.
Key Words:antidepressants;chronic pain;depression;exercise;hippocampal neurogenesis;inflammation inhibition;neuroinflammation;physical activity deficits;social isolation;spinal cord injury
Introduction
Spinal cord injury (SCI) is a highly disabling injury,which usually occurs in the most active years of a person’s life.The incidence and etiologies of SCI vary throughout the world(Kang et al.,2018).In the United States,the incidence of SCI is approximately 54 cases per million people,or approximately 18,000 new cases each year.Vehicle crashes are currently the leading cause,followed by falls,violence,and sports (Golestani et al.,2022).To date,the number of individuals who survive SCI ranges from 247,000 to 358,000 in USA (NSCISC,2019).SCI is a devastating condition with heavy financial,social,and personal burdens.It is a costly condition to treat in the United States,with over $4 billion spent annually on medical treatment alone for acute SCI and management of chronically debilitated patients (Lo et al.,2021).The average lifetime medical costs for a 25-year-old SCI victim is $1.5–4.7 million depending on severity (Raymonda et al.,2022).SCI patients suffer a variety of primary disorders,such as motor and sensory dysfunctions,neuropathic pain,spasticity,and neurogenic bladder and sexual dysfunction.They also endure many secondary complications,such as neurological and psychological disorders,cognitive deficits,and mood disturbances,especially as the average age of persons living with SCI is continuously increasing with around 80% of them being over 40 years old (Anderson et al.,2007;Shin et al.,2013;Craig et al.,2017;Barbiellini Amidei et al.,2022).These primary and secondary complications of SCI drastically decrease the patient’s quality of life (Jensen et al.,2012;Hachem et al.,2017).
Depression is one of the secondary complications that are highly prevalent after SCI in both model animals (rodent) and human patients (Kalpakjian et al.,2009;Luedtke et al.,2014;Maldonado-Bouchard et al.,2016).Depression occurs in 9.8%to 38% of SCI patients,which is higher in female patients than in male patients,and is the third most frequently reported medical complication after infections and pressure sores following SCI (Furlan et al.,2005;Placeres and Fiorati,2018).Depression in individuals after SCI has been associated with various causes such as the natural responses of catastrophic events,the interventions used during resuscitation and stabilization,the intensive care unit unpleasant environment,chronic pain,sleep and sensory deprivation,medications,and preexisting medical comorbidities (Sun et al.,2016;Saurí et al.,2017).Depressive disorders in turn promote the debilitating effects of these physical complications.For example,depression after SCI worsens motor functional recovery,gastrointestinal symptoms,and others (Ng et al.,2005;Abdul-Sattar,2014;Zhang et al.,2018b).Thus,SCI induced depression significantly affects the daily life quality of patients.
To date,a certain amount of researches has been conducted on depression after SCI,yet the molecular mechanism remains largely unknown.Adult hippocampal neurogenesis has been shown to play a critical role in the development of depression and is required for anti-depressant effect of antidepressant drugs (Costa et al.,2015;Eliwa et al.,2017;Park,2019;Gomes-Leal,2021;Malberg et al.,2021).It has also been confirmed that after peripheral neuropathy and persistent pain,the increased depression is associated with impairment of neurogenesis in the hippocampus (Mutso et al.,2012;Dellarole et al.,2014;Dimitrov et al.,2014;Apkarian et al.,2016).These findings suggest that the clinical symptoms of SCI-induced depression may come from the impaired neurogenesis in the hippocampus after SCI.To further augment our understanding,we reviewed currently published original papers related to adult hippocampal neurogenesis after SCI.We will first discuss the associations between the alteration of hippocampal neurogenesis and SCI.Next,we will explain the possible mechanisms after SCI that have an impact on depression and hippocampal neurogenesis.Third,we will point out potential therapeutic options that might increase hippocampal neurogenesis for depression alleviation.A better understanding of how SCI impaired hippocampal neurogenesis and contributed to emotional deficits,such as depression will make it conceivable to modulate hippocampal neurogenesis as a tool to reduce the rate of hippocampus associated neurological disorders within SCI patients.
Search Strategy
Studies cited in this review were all-year publications that were searched on the PubMed,Web of Science,and Google Scholar databases using one or some combination of the following keywords: spinal cord injury (SCI),depression,hippocampal neurogenesis,physical activity deficits,social isolation,pain,neuroinflammation,exercise,antidepressant,and inflammation inhibition.These searches were performed between April and July 2023.
Adult Neurogenesis in the Hippocampus
Before 1961,the existence of neurogenesis was hard to imagine in the postnatal central nervous system,until the evidence of neurogenesis was reported in the brain of a 3-dayold rat injected with thymidine-H3by radio autography (Altman and Das,1965).By labeling uridine in the synthesizing DNA with BrdU,and other thymidine analog exogenous compounds(e.g.,IdU,CldU,and EdU) neurogenesis now is easy to be revealed and confirmed by immunostaining techniques (Kuhn et al.,1996).Thus,with different experimental paradigms,including single or multiple injections and different survival times,thymidine analog exogenous compounds can be used to monitor the process of adult neurogenesis withtime course analysis,such as the proliferation capabilities of adult neural stem/progenitor cells,survival of new neurons,neural cell fate decision,and neural cell migration (Cooper-Kuhn and Kuhn,2002;Wojtowicz and Kee,2006;Mamber et al.,2013;Kuhn et al.,2016).Neurogenesis is usually defined as “birth of new neurons”,the process by which neurons are generated from neural stem cells/progenitor cells.In the brain,adult neurogenesis has been found in two major areas.One area is the subventricular zone,which is placed along the wall of the lateral ventricles facing the hollow as a layer of dividing cells.Another area is the subgranular zone,which is positioned between the hilus and granule cell layer of the dentate gyrus (DG) in the hippocampus (Bond et al.,2015).The subventricular zone and subgranular zone thus called neurogenic niches grant and support neurogenesis throughout life (Matsubara et al.,2021).In fact,the process of neurogenesis in the hippocampus is a complex and multistep process that expands the pool of newborn cells available to differentiate into neurons,as shown in Figure 1.It could be generally divided into four stages identified by a set of protein markers.The first stage is a precursor cell or proliferation phase.Adult neural stem cells (NSCs) located in subgranular zone and displayed a radial glia-like morphology(Type 1) start to proliferate and produce progenitors (Type 2:Type 2a and Type 2b) which further undergo limited cycles of proliferation and differentiate into immature neurons(Type 3).The common marker protein among NSCs (Type 1) and progenitor cells (including Type 2a and 2b) is Nestin,a type VI intermediate filament protein expressed mostly in nerve cells (Michalczyk and Ziman,2005;Guerette et al.,2007).As Nestin has been down-regulated in Type 3 cells showing only little proliferative activity,among the neuronal lineage markers,DCX first appears.DCX expression extends from a late of proliferation stage,through the cell cycle exiting,to a period of postmitotic maturation that lasts 2 to 3 weeks (Rao and Shetty,2004;Couillard-Despres et al.,2005).Thus,DCX is a widely used representative marker for adult neurogenesis.The second and third stages are an early survival phase and a postmitotic maturation phase that could be merged as an early postmitotic maturation phase.After cell-cycle exit,besides DCX,the newborn neurons begin to express postmitotic markers,such as NeuN,and the transient marker calretinin (Brandt et al.,2003).The number of newborn neurons is highest at very early time points and decreases markedly within a few weeks via apoptotic process (Biebl et al.,2000;Kuhn et al.,2005).Therefore,the majority of newborn neurons are eliminated naturally before they have made functional connections or received correct dendritic input.Brain-derived neurotrophic factor(BDNF) signaling has been proven as the main suspect and the contribution of “survival factor” (Tashiro et al.,2006),yet the overall mechanistic picture is unclear.The last stage is a late survival phase or late post-mitotic maturation phase(Kempermann et al.,2015).The surviving new neurons go through morphological and functional maturation,and then integrate into the hippocampal circuitry.After fully integrating into the existing network,the new neurons switch their calcium-binding protein from calretinin to calbindin(Brandt et al.,2003).Then,within the next few weeks,new neurons become electrophysiologically identical to their older neighbors (Vadodaria and Jessberger,2014).Generally,adult neurogenesis in the hippocampus is composed of NSC proliferation,differentiation,immature neuron survival and maturation,and mature neuron integration.
Many marker proteins are widely used for monitoring the different stages of neurogenesis.As mentioned before,three markers are typically used in neurogenesis research,which are marked by red boxes inFigure 1.Nestin is a common marker for NSCs and neural progenitors in cell proliferation studies.Dcx is a marker for immature neurons,which is broadly used to detect the alteration of neurogenesis in adults.It is worth noting that the change in DCX does not always reflect the change in neurogenesis.The change of neurogenesis (net neurogenesis) is determined by whether an appropriate number of new functional mature neurons are generated.NeuN (expressing in early and late postmitotic maturation phase neurons) therefore is used as the one of benchmarks for mature neurons and net neurogenesis.In summary,the use of BrdUs in combination with immunostaining for neuronal stage/type-specific markers allows the process of adult neurogenesis to be studied by detecting and monitoring the proliferation of NSC/progenitors,the characterization of the newborn cell phenotype,and the functional integration of newborn neurons at different time points.
Role of Hippocampal Neurogenesis in Depression
Accruing evidence indicates that hippocampal neurogenesis may have distinct physiological roles in hippocampusdependent functions such as cognition and mood regulation.Adult neurogenesis is critical not only in distinguishing highly similar events or environments (Clelland et al.,2009;Nakashiba et al.,2012;Bekinschtein et al.,2014),but also in forgetting and memory clearance,especially the bad ones(Akers et al.,2014;Epp et al.,2016).Adult neurogenesis is also highly sensitive to environments and pathological conditions.In model animals,physical and psychological stresses impaired the process of adult neurogenesis and further augmented the symptoms of disorders (Coe et al.,2003;Mirescu et al.,2004).Anxiety and depression have been linked to the alteration of adult hippocampal neurogenesis (Eisch and Petrik,2012).It has been reported that adult neurogenesis is required for some antidepressants to perform their beneficial effects via 5HT1A receptors (Micheli et al.,2018).All these findings suggest that normal adult hippocampal neurogenesis is critical for mental health and its impairment may lead to mental disorders,such as depression.Indeed,in human patients,the reduction of hippocampal volume and adult hippocampal neural progenitors is documented in depression (Campbell et al.,2004;Lucassen et al.,2010).Antidepressant treatments in major depressive disorder patients increase the number of adult neural progenitors in the DG and the volume of DG (Anacker et al.,2011;Boldrini et al.,2012).Although no consensus has been reached with some reports showing no change or necessity of adult hippocampal neurogenesis in the effects of antidepressants (Holick et al.,2008;Huang et al.,2008),it is reasonable to speculate that the reduction of hippocampal neurogenesis is responsible for the depression and the increase in adult hippocampal neurogenesis may mediate the effect of antidepressants.Interestingly,patients undergoing cancer treatments,in which dividing cells including adult neural stem cells in the hippocampus are eliminated by irradiation,experience depression and cognitive impairment (Pereira Dias et al.,2014).
Change of Hippocampal Neurogenesis after Spinal Cord Injury
Only a handful of studies have been conducted to address whether SCI alters hippocampal neurogenesis (Table 1).The controversy existed,though the majority of reports demonstrated that SCI,especially at moderate and severe levels impaired the hippocampal neurogenesis.However,the relationship between depression and the change of hippocampal neurogenesis post-SCI was not widely addressed.The hippocampal neurogenesis was examined in diverse time points post SCI different from lab to lab in total 14 research studies (Felix et al.,2012;Franz et al.,2014;Wu et al.,2014,2016;Jure et al.,2017,2020,2021;Dehler et al.,2018;Zangbar et al.,2020,2021;Brakel et al.,2021;Liu et al.,2021;Kalkhoran et al.,2022;Xi et al.,2023).Though,it can summarize the impact of SCI on hippocampal neurogenesis in the acute phase (within 2 weeks) and chronic phase (over 5 weeks) post injury,it is hard to compare the results between the studies due to inconsistent experimental settings.On top of it,different animal models were used by different labs,making it even harder to compare with each other (Table 1).Among all the assessments,the alteration of immature neurons detected by Dcx immunostaining was generally examined.The increase of Dcx-positive cells in DG acutely after SCI was reported only by Dehler et al.(2018) in thoracic transection mouse model,while others showed no change(Franz et al.,2014) or decrease of Dcx signals in the acute phase in different SCI models (Felix et al.,2012;Jure et al.,2020;Zangbar et al.,2021).By BrdU incorporation,Dehler et al.(2018) traced the process of neurogenesis after SCI in the acute phase.They found that the proliferation of NSC was enhanced.This might be the reason for the increase of Dcx-positive immature neurons and eventually lead to the increase of net neurogenesis (Increase of NeuN/BrdU doublepositive mature neurons).However,Jure et al.(2017) reported totally opposite observation in thoracic compression mouse model.In this model,SCI impaired hippocampal neurogenesis in the acute phase with a decrease of NSC proliferation,Dcxpositive immature neuron,and NeuN-positive mature neuron number.The results from Zangbar et al.(2020,2021) and Felix et al.(2012) supported the findings of Jure et al.(2017),while Franz et al.(2014) did not detect any change of hippocampal neurogenesis even in severe SCI of thoracic contusion model.These contrary results suggested that SCI of different animal models might impact the hippocampal neurogenesis distinctly in the acute phase.In the chronic phase,SCI impaired or had no effect on hippocampal neurogenesis.May also due to the difference in the animal injury model,Dehler et al.(2018)claimed that SCI did not affect hippocampal neurogenesis in the chronic phase,while others demonstrated SCI reduced the neurogenesis in the hippocampus in a severity manner(Jure et al.,2017).Comparing to moderate and severe SCI,mild SCI had no obvious impact on animals behaviorally and histologically,including neurogenesis in the hippocampus(Wu et al.,2014,2016;Jure et al.,2017).While moderate and severe SCI significantly impaired the hippocampal neurogenesis reflected by a decrease of Dcx-positive immature neuron number and NeuN-positive mature neurons (Wu et al.,2014,2016;Jure et al.,2017,2021;Zangbar et al.,2020;Brakel et al.,2021;Liu et al.,2021;Kalkhoran et al.,2022;Xi et al.,2023).Among all the reports,only Dehler et al.(2018),Wu et al.(2014),Liu et al.(2021),and Brakel et al.(2021) linked the change of hippocampal neurogenesis caused by SCI to depressive-like behavior.Besides the neurogenesis,gliogenesis was also examined by cell type specific markers in some of the studies.Newly generated astrocytes were identified by colocalization of BrdU and GFAP in seven studies (Felix et al.,2012;Franz et al.,2014;Wu et al.,2016;Jure et al.,2017,2020;Dehler et al.,2018;Xue et al.,2019).The proliferation of oligodendrocytes and glial progenitor cells was determined by co-labeling BrdU with anti-adenomatous polyposis coli and chondroitin sulfate proteoglycan in one study (Franz et al.,2014).Activated microglia were detected by co-localization of BrdU with ionized calcium binding adaptor molecule 1 or OX42 (Franz et al.,2014;Wu et al.,2016;Xue et al.,2019;Jure et al.,2020).Generally,listed studies demonstrated the changes in hippocampal neurogenesis and gliogenesis after SCI.Though the changes were varied and the consensus was not met,the SCI-induced different responses of hippocampal neurogenesis might rely on injury site,severity,and/or time windows after injury.

Table 1 |Hippocampal neurogenesis after SCI
Potential Mechanisms Underlying the Alteration of Hippocampal Neurogenesis after Spinal Cord Injury
Physical activity deficit and social isolation
Adult neurogenesis is activity-dependent and different kinds of “activity” prompt adult neurogenesis (Kempermann et al.,2015).The different activities could be put into two categories: physical activity (exercise) and environment(cognitive) stimuli,which have different impacts on the different stages of hippocampal neurogenesis: a less-specific phase of precursor cell proliferation and a specific phase of selective survival respectively (Kempermann et al.,2004,2006).Physical exercise significantly increases the proliferation of precursor cells in the hippocampus (Brown et al.,2003),while environmental stimuli have no or a limited effect on cell proliferation but facilitate the long-term survival of newborn neurons (Kempermann and Gage,1999;Kempermann et al.,2002;Kronenberg et al.,2003).However,the effects of physical exercise and environmental stimuli (environmental enrichment) are additive (Fabel et al.,2009).Hippocampal neurogenesis in adulthood has been proven to buffer stress responses and depressive behavior (Snyder et al.,2011).Lack of activities,such as in aged people,results in the decline of hippocampal neurogenesis,which may be responsible for cognitive impairment and mental disorders (Sofi et al.,2011;Huffman and Stark,2017).It has been reported that physical activity and exercise are universal depression prevention in young people (Kim et al.,2019;Pascoe and Parker,2019).SCI patients have been long ranked at the lowest end of the human fitness spectrum (Dearwater et al.,1986).In many incidences,high level muscle paralysis due to SCI makes voluntary exercise impossible or unproductive.Moreover,obstacles caused by physical,financial,architectural,and transportation issues also impede the participation in physical exercise (Cowan et al.,2013).Indeed,1 in 4 young persons with SCI fails to have satisfied fitness for essential activities of daily life (Noreau and Shephard,1995) and approximately 50% of all age patients have no leisure-time physical activity at all (Ginis et al.,2010).Lack of physical activity in SCI patients may attenuate the hippocampal neurogenesis that eventually results in depression symptoms post-trauma.Indeed,the prevalent depression post-SCI has been linked to a decrease in physical activity (Mulroy et al.,2016).Social isolation (disconnectedness) is another big issue among SCI patients.Individuals with SCI are challenged by developing and maintaining social relationships and community participation (Newman et al.,2016;Guilcher et al.,2021).Though the underlying mechanisms remain unclear,social isolation is strongly associated with the development of depression (Noguchi et al.,2021).The decline of hippocampal neurogenesis caused by inadequate cognitive stimuli due to isolation may be the one of reasons (Cinini et al.,2014;Grigoryan et al.,2022).
Chronic neuropathic pain
Chronic neuropathic pain is a prevalent secondary complication following SCI that has been suggested to play a critical role in the development of depression (Burke et al.,2017;Sauri et al.,2017).Approximately 65% of SC patients have been reported suffering persistent pain with the majority of them being in a “moderate” or “severe” level (Siddall et al.,2003;Nicholson Perry et al.,2009).Yet,whether pain directly causes depression remains under debate,persistent pain is believed to have a negative impact on hippocampal neurogenesis,related functions,and recovery.Thus it can be used as a predictor for the prognosis of cognitive function,emotional function (depression),and quality of life (Mutso et al.,2012;Grilli,2017;Somelar et al.,2021).Chronic pain post-SCI may also indirectly lead to depression by promoting immobility and sleep disturbance to downregulate hippocampal neurogenesis (Romero-Grimaldi et al.,2015).For the same reason,the absence of physical activity caused by pain could be considered another risk factor for depression whereas an appropriate amount of physical activities protect against depression (Kim et al.,2019;Pascoe and Parker,2019).The positive correlation between pain severity and the development of depression has been reported by Avluk et al.(2014).Collective data indicates that chronic pain is one of the critical factors for determining depression,and further their coexistence exaggerates the severity of both disorders(Sheng et al.,2017).Thus,more efforts should be made to understand the underlying mechanisms and to determine whether pain management would benefit emotional wellbeing (preventing depression) in SCI patients.
Neuroinflammation: microglia,astrocyte,and proinflammatory cytokines
SCI not only activated microglia,the resident immune cells of the central nervous system (CNS) that are responsible for phagocytosis,nurturing neural cells and tissue repair during infections and injury (Rock et al.,2004;Lenz and Nelson,2018) in injury site (Zhou et al.,2018),but also in multiple brain regions,including the hippocampus (Felix et al.,2012;Wu et al.,2014;Jure et al.,2020;Mandwie et al.,2022).After SCI,the increase of activated microglia in the hippocampus is in a severity-dependent manner (Jure et al.,2017).Wu et al.(2014) reported that SCI initiated a chronic brain neurodegenerative response,likely related to delayed,sustained induction of M1-type microglia,which resulted in cognitive deficits and physiological depression.This phenomenon has been confirmed by Jure et al.(2017),which could explain the post-traumatic cognitive deficits of hippocampal-dependent tasks in rodents and even in human with SCI experience.In the hippocampus,microglia are critical for neurogenesis and neuronal differentiation (Appel et al.,2018).In non-SCI injury models,microglia activation in the hippocampus impaired NPC proliferation and hippocampal neurogenesis (Stefani et al.,2018).After SCI,initially,microglia are activated for neuroprotection,yet excessive activation leads to aberrant behaviors including in declined hippocampal neurogenesis that is associated with cognitive and emotional deficits.Thus,it has been reported that microglial depletion after SCI mitigated the symptoms of depression and cognitive dysfunction (Li et al.,2020b).Microglia activation also has a great impact on the activity and proliferation of astrocytes(Zhang et al.,2010;Burda and Sofroniew,2014).After SCI,the increase of reactive astrocytes was observed across the brain including hippocampus in both acute and chronic phases (Jure et al.,2020).Though the role of astrocytes in adult hippocampal neurogenesis remains elusive,it may assist in integration of newborn hippocampal neurons into the existing neural network (Krzisch et al.,2015).The activation of microglia and astrocyte may also be responsible for the increase of pro-inflammatory cytokine expression in the brain,such as interleukin (IL)-1β,IL-6,and tumor necrosis factor alpha (TNF-α) (Wu et al.,2014;Jure et al.,2020),which may have contribution to the reduction of adult hippocampal neurogenesis as well.Pro-inflammatory cytokines have been associated with a reduction in hippocampal neurogenesis in many pathologies and diseases.Chronic IL-1β overexpression leads to neurodegeneration and memory deficit (Hewett et al.,2012) via STAT3 signal pathway mediated astrogliosis to hamper neuronal differentiation (Chen et al.,2013).On the other hand,significantly reducing IL-1β increases hippocampal neurogenesis (Ryan et al.,2013).Chronic IL-6 overexpression impairs hippocampal neurogenesis,while transient increase of IL-6 enlarges the NSC population (Storer et al.,2018).TNF-α via activating nuclear factor-κB signaling also reduces NSC proliferation leading to the hippocampal neurogenesis impairment (Chen and Palmer,2013).Taken together,proinflammatory cytokines induced by SCI are likely to have a negative impact on the hippocampal neurogenesis at the different stages,and therefore affect neurological functions differently.It remains unclear whether local increase of pro-inflammatory molecules from activated astrocytes and microglia in the hippocampus is the only way responsible for the reduction of neurogenesis.Actually,an alternative hypothesis is that molecules,such as chemokines transported anterogradely from the injury site to the hippocampus directly reduce neurogenesis.At meantime,they also activate microglia and astrocyte.For example,cysteine-cysteine chemokine ligand 21 produced in lumbar dorsal horn neurons around the SCI lesion site was also found in the thalamus,cerebral cortex,and hippocampus at later time points posttrauma (de Jong et al.,2005;Zhao et al.,2007;Wu et al.,2016).Further study demonstrated that cysteine-cysteine chemokine ligand 21 was transported throughout neuronal processes into presynaptic structures (de Jong et al.,2005).Yet,the functional role of these chemokines needs to be determined.
Potential Therapeutic Intervention
Anti-depressants
Mood disorders,such as depression have detrimental effects on SCI patients,thus mood stabilization and good mental health maintenance is a key for recovery and rehabilitation.General anti-depressant drugs can be used to treat SCImediated depression to improve mood status and enhance the quality of life post-injury,and therefore reduce the risk of delirium and suicide (Bayoumi et al.,2019).Additionally,some antidepressant medications,such as amitriptyline (tricyclic antidepressants) and venlafaxine (serotonin-norepinephrine reuptake inhibitor) have been shown to also treat SCI pain effectively (Richards et al.,2015;Tate et al.,2015).Since pain and depression are often comorbidities in SCI patients and their coexistence exaggerates the severity of both,it is reasonable to believe that anti-depressants working on both pain and depression may show additive effect on SCI patients.Indeed,some reports demonstrated an increased effectiveness of tricyclic antidepressants on severe depression in SCI patients compared to those without SCI (Kim et al.,1977).However,the efficacy of different antidepressants in SCI pain still needs to be determined.For example,Duloxetine (serotonin-norepinephrine reuptake inhibitor)was reported to alleviate dynamic and cold allodynia but did not affect tactile or pressure pain (Vranken et al.,2011).Beyond neurotransmitter regulation,accumulative data indicate that the effect of antidepressants also relies on their ability to induce hippocampal neurogenesis in rodents as well as in non-human primates (Perera et al.,2011;Mateus-Pinheiro et al.,2013).Fluoxetine is one of the promising anti-depressants.It may be through multiple ways to augment hippocampal neurogenesis for the relief of depression in SCI patients.Fluoxetine is a selective serotonin reuptake inhibitor by blocking the reabsorption (reuptake) of serotonin into neurons to make more serotonin available to improve the transmission of messages between neurons.Serotonin receptors are known to contribute to motor function recovery after SCI,thus fluoxetine has been reported to improve motor function reflected by an increase in Basso,Beattie,and Bresnahan scale post-trauma (Murray et al.,2010).Better motor function recovery means better hippocampal neurogenesis.Meanwhile,high-dose and chronic fluoxetine treatment was also reported to induce the production of neurotrophic factors,such as BDNF to increase hippocampal neurogenesis following SCI (Engesser-Cesar et al.,2007).Further,X-irradiation of a mouse brain with a restricted region in the hippocampus prevented the hippocampal neurogenesis and behavioral effects of mentioned two classes of antidepressants (Santarelli et al.,2003;Liu et al.,2015).Taken together,antidepressants may directly and/or indirectly enhance hippocampal neurogenesis to treat depression.
Exercise and social interaction
Physical deconditioning and decrease of hippocampal neurogenesis after SCI has been linked to post-trauma depression.Exercise,known to promote neuroplasticity and functional connectivity (Gomez-Pinilla et al.,2002;Thomas and Gorassini,2005),enhance hippocampal neurogenesis (Liu et al.,2015),reduce susceptibility to depression (Stanton and Reaburn,2014;Kim et al.,2019;Pascoe and Parker,2019),and improve psychological wellbeing (Callaghan et al.,2011),therefore,is a potential treatment to alleviate the depression caused by SCI.Indeed,SCI patients with higher physical activity via exercise have better mental health with lower depression,anxiety,higher social support scores,and better quality of life (Hicks et al.,2003;Martin Ginis et al.,2010;Kim et al.,2020).Exercise reverses the reduction of neurogenesis after SCI possibly through augmented hippocampal BDNF level (Hotting et al.,2016;Liu and Nusslock,2018),lowered pro-inflammatory cytokine expression,and increased antiinflammatory cytokine expression (Petersen and Pedersen,2005;da Silva Alves et al.,2013;Dallagnol et al.,2017).Voluntary (running wheel) or force exercise (treadmill) in mice and aerobic exercise in humans increase BDNF expression in the hippocampus (Kim et al.,2015;Luo et al.,2019).BDNF binds to tropomyosin receptor kinase B of hippocampal neurons that serves as a docking site for numerous signaling pathways (Phillips et al.,2014) to regulate many features of hippocampal neurogenesis,such as NSCs proliferation,differentiation,newborn neuron maturation,and survival(Acheson et al.,1995;Lee et al.,2002;Bartkowska et al.,2007;Li et al.,2008;Waterhouse et al.,2012) to augment net hippocampal neurogenesis.Exercise may also enhance hippocampal neurogenesis via epinephrine and adiponectin(Ahmadiasl et al.,2003;Yau et al.,2014).Thus,targeting epinephrine and adiponectin may represent a promising therapeutic treatment for depression as well.On the other hand,autonomous exercise after SCI remains a challenge,especially for persons with severe or high cervical injuries.It would be great to clinically test whether new technologies that can stimulate muscles provide some of the benefits associated with exercise (Ragnarsson,2008).Meanwhile,passive range of motion exercises (environmental stimuli)with a partner/caregiver,maintaining and increasing social interaction improve quality of life and decrease neurological deficits via augmented hippocampal neurogenesis,which may produce the same impact as regular physical exercise(Monteiro et al.,2014;Venna et al.,2014).
Analgesia for chronic neuropathic pain
Chronic neuropathic pain is a critical factor for post-SCI depression,and their coexistence exaggerates the severity of both disorders (Sheng et al.,2017).Thus,reducing pain with analgesic drugs may attenuate the development of depression.However,extensive experimental data have been accumulated on the negative impact of morphine and other opiates,which exert a deleterious effect on hippocampal neurogenesis (Eisch et al.,2000;Bortolotto and Grilli,2017).Recent work demonstrated an association between prolonged opiate use and the risk of new-onset depression (Scherrer et al.,2016).On the other hand,other analgesic drugs,such as the α2δ ligands pregabalin/gabapentin (Valente et al.,2012) and acetyl-l-carnitine (Cuccurazzu et al.,2013)have been shown to significantly promote hippocampal neurogenesis in preclinical studies.Importantly,both drugs exert antidepressant activity in rodents where depressivelike behavior was induced by chronic restraint or chronic mild stress and in depression patients (Pae,2009;Nasca et al.,2013).Together,analgesic drugs that promote hippocampal neurogenesis could represent the first option therapy since they have multiple beneficial effects on emotional and cognitive aspects in chronic pain states.
Inflammation inhibition
Posttraumatic neuroinflammation is another possible mechanism underlying the reduction of hippocampal neurogenesis among SCI patients that results in depression(Maldonado-Bouchard et al.,2016;Troubat et al.,2021).Microglia,as the major cellular component of the innate immune system in the CNS,play multidimensional roles in the response to CNS trauma (Muzio et al.,2021).Overwhelmed microglia with excessive activation produce high levels of pro-inflammatory and cytotoxic mediators,referred to as neuroinflammation that exacerbate damage and hamper CNS recovery (Plemel et al.,2014).Chronic neuroinflammation is able to continue for months to years after trauma(Ramlackhansingh et al.,2011;Smith et al.,2013),leading to progressive neuronal cell loss,neurodegeneration,and eventually neurological disorders.Accumulative data suggest that chronic inflammation may be a critical pathogenic mechanism in neurodegenerative disorders such as Alzheimer’s disease (Eikelenboom et al.,2010).Neuroinflammation in aged animals has a negative impact on hippocampal neurogenesis and inflammatory blockade restores adult hippocampal neurogenesis (Monje et al.,2003;Kohman and Rhodes,2013).Chronic brain neuroinflammation has been reported after SCI that is linked to progressive neurodegeneration and reduction of neurogenesis in the brain regions,such as hippocampus,contributing to cognitive impairment (Wu et al.,2014;Allison et al.,2017).Based on recent experimental works,a legitimate hypothesis is that SCI-induced brain neuroinflammation is responsible for the reduction of hippocampal neurogenesis that leads to post-trauma depression.Suppressing inflammation during chronic SCI is thus vital to improving cognition and alleviating depression.For example,NADPH oxidases (NOX2) has been reported to play an important role in microglial activation and the production of pro-inflammatory factors that contribute to prolonged neuronal cell loss and related neurological dysfunction after trauma (Cooney et al.,2014;Khayrullina et al.,2015;Taylor and Tse,2021).Constitutive depletion or systemically inhibition of NOX2 significantly reduced motor dysfunction and had a greater overall improvement in functional recovery after moderate contusion SCI (Sabirzhanov et al.,2019) and enhanced hippocampal neurogenesis following traumatic brain injury (Wang et al.,2018).Several more anti-inflammatory therapeutics have been shown to improve hippocampal neurogenesis as well.Acute treatment with methylprednisolone,an anti-inflammatory agent against the cytotoxic effects of pro-inflammatory cytokines (Sloka and Stefanelli,2005),increases both the number and migration of newborn neurons in DG after transient cerebral ischemia.Erythropoietin,another hinder of pro-inflammatory cytokines(Peng et al.,2020),also increases the number of newborn hippocampal neurons and improves locomotor recovery after SCI (Zhang et al.,2018a).Minocycline,an anti-inflammatory and antibiotic agent,mitigates age-related decline of spatial learning and increases the number of newborn neurons in the hippocampus (Kohman et al.,2013).Chondroitinase ABC,which degrades pro-inflammatory CSPGs,improves locomotor recovery and prevents social defeat-induced persistent stress and memory loss following SCI (Garcia-Alias et al.,2011;Riga et al.,2017).Baicalin,an anti-inflammatory and immunomodulatory agent,shows an anti-depressant effect via promoting hippocampal neurogenesis in a chronic corticosterone-induced mouse model of anxiety/depression(Li et al.,2020a).The listed reagents all positively impact hippocampal neurogenesis.Meanwhile,some treatments also improve locomotor function that will lead to the increase of physical activity,thereby likely benefiting hippocampal neurogenesis and cognitive function.Though,none of the listed drugs has been tested on SCI patients to directly improve post-trauma depression or hippocampal neurogenesis,they are potentially anti-depressants and more experimentation is required in the context of SCI.Directly targeting CNS resident microglial populations also has been considered as potential therapeutics for various neurodegenerative diseases (Subramaniam and Federoff,2017) and neurotrauma(Scott et al.,2021).Unfortunately,to date,most approaches either have no specificity or only have modest effectiveness in eliminating adult microglia in the brain.For example,the genetic deletion of CX3CR1,a microglial chemokine receptor,does promote functional recovery after SCI,but this receptor is also highly expressed in infiltrating macrophages (Donnelly et al.,2011;Freria et al.,2017).Therefore,the functional recovery after SCI cannot be simply attributed to the deletion of resident microglia.To overcome this flaw,transgenic mice (CX3CR1CreER/+:R26iDTR/+) were generated to allow only the depletion of resident microglia in the CNS but not blood CX3CR1-positive cells (Peng et al.,2016).Microglia rely on colony-stimulating factor 1 receptor (CSF1R) signaling for survival in the brain (Elmore et al.,2014).Thus,administration of CSF1R antagonists induces the rapid and continued removal of nearly all microglia from the CNS without affecting peripheral macrophage populations and overall health (Elmore et al.,2014).Elimination of microglia using this method is beneficial in multiple CNS disease models associated with neuroinflammation (Dagher et al.,2015;Acharya et al.,2016;Walter and Crews,2017),suggesting that CSF1R antagonists can be effective therapeutically treatment for various CNS disorders.Another potential therapeutic intervention for increasing hippocampal neurogenesis,reducing inflammation,and improving locomotor function is the application of mesenchymal stem cell derived extracellular vesicles (MSCEV).MSCEVs have been shown to improve locomotor recovery after SCI via suppressing the neurotoxic reactive astrocytes and shifting microglial polarization from pro-inflammatory to anti-inflammatory profiles (Liu et al.,2019,2020).MSCEVs also reduce neuron apoptosis and increase locomotor recovery through the Wnt/β-catenin signaling pathway,which is essential to hippocampal neurogenesis (Qu et al.,2013;Li et al.,2019).The prompted hippocampal neurogenesis by MSC-EVs improves cognitive impairments in diabetes and Alzheimer’s disease (Nakano et al.,2016;Reza-Zaldivar et al.,2019).However,in the context of SCI,the effect of MSC-EVs in human patients remains to be determined.
Conclusions and Perspectives
SCI-induced post-trauma depression is consistently reported in human patients and studies of experimental animal models.Some preclinical works have been done trying to understand the underlying mechanisms.The changes in hippocampal neurogenesis have been observed and speculated to induce depression post-trauma.However,the full picture of the connection between the alteration of hippocampal neurogenesis and SCI-induced depression remains elusive with many questions that need to be addressed.For example,hippocampal neurogenesis has not been examined in all different kinds of SCI animal models to address whether the change of neurogenesis (increase,decrease,or no change) is injury-type related.The blanks inTable 1need to be fulfilled.Inconsistent in experimental settings and the SCI animal model among different research groups is a big challenge for getting a consensus answer (Table 1).Researches with systematically studied the effect of SCI on each step of hippocampal neurogenesis at different time points post-injury are urgently need to address: (1) whether an injury has an impact on NSCs,newborn immature neurons,newborn mature neurons separately,or affects all of them;(2) what type of NSCs (type1,2 or 3) is affected;(3) the change of immature newborn neuron number is due to differentiation or survival,and more.
Meanwhile,what causes the change of hippocampal neurogenesis after SCI is another big question yet no clear answers.Data from limited SCI studies and other types of trauma suggests that physical activity deficits,social isolation,chronic pain,and neuroinflammation mutually have major contributions (Figure 2).Therefore,targeting them with drugs or interventions augments the hippocampal neurogenesis and shows promising results on depression alleviation in both preclinical and clinical studies.
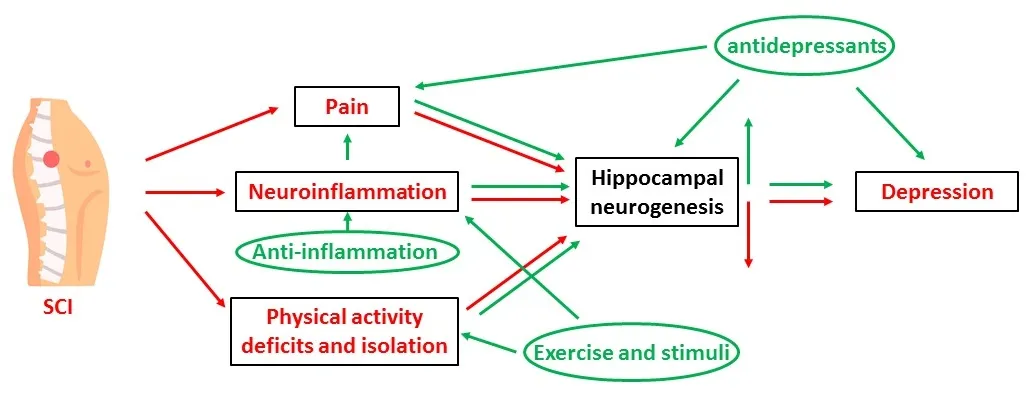
Figure 2 |Schematic drawing of possible mechanisms underlying SCI-induced depression and potential treatments.
In summary,depression after SCI may reflect a combination of biochemical,physiological,and behavioral alternations induced by injury.Growing evidence indicates the associated functional changes in the hippocampus.Further preclinical studies should focus on these important issues in addressing injury mechanisms and therapeutic approaches to mitigate SCI-induced depression.
Acknowledgments:The authors would like to express their gratitude to Dr.Jinhui Chen(MD &PhD,Senior Associate Professor,Academy of Pharmacy,Xi’an Jiaotong-Liverpool University,China)for his assistance with an earlier version of the manuscript.
Author contributions:Original draft preparation:YM and YQ;conceptualization,preparation of original draft manuscript and figures,review,and editing:XG.All authors have read and agreed to the published version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Kiralyn Brakel,Texas A&M University,USA.
Additional file:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice
- Conformational dynamics as an intrinsic determinant of prion protein misfolding and neurotoxicity
- Exploring the synergy of the eyebrain connection: neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation
- Pathogenic contribution of cholesteryl ester accumulation in the brain to neurodegenerative disorders
- Cognition and movement in neurodegenerative disorders:a dynamic duo
- Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease: searching far and wide
